Abstracts
PURPOSE: To investigate if diabetes mellitus may alter breaking strength (BS) and collagen content in ileum and colon anastomoses in rats. METHODS: Three-hundred Wistar rats were randomly assigned to 5 experimental groups, 60 per group: normal controls surgically manipulated (G1); normal controls submitted to ileum (G2) and colon (G3) anastomotic construction; diabetic rats submitted to ileum (G4) and colon (G5) anastomotic construction. Each group was further divided into 6 subgroups with 10 rats each for sacrifice at 0, 4, 7, 14, 21, and 30 days after surgery. All surgical procedures were performed 3 months after alloxan diabetes induction. BS was measured in all intestinal anastomoses. Fragments of ileum and colon anastomoses were taken for hydroxyproline concentration (HP) and total tissue protein (TP) dosages. RESULTS: Anastomotic BS was significantly decreased (P<0.05) in ileum and colon of G4 and G5 diabetic groups up to 7 and 14 days after surgery, respectively, compared with G2 and G3 normal control groups. Anastomotic HP and TP content did not significantly differ between diabetic and normal control operated groups in ileum or colon at all evaluation times. CONCLUSION: Experimental diabetes leads to impaired intestinal anastomotic strength during early surgical wound repair, but does not appear to be implicated with collagen synthesis capacity.
Diabetes Mellitus; Wound Healing; Ileum; Colon; Rats
OBJETIVO: Investigar se o diabetes mellitus pode alterar a força de ruptura (FR) e o conteúdo de colágeno em anastomoses realizadas no íleo e cólon de ratos. MÉTODOS: 300 ratos Wistar foram distribuídos por sorteio em 5 grupos experimentais com 60 animais cada: controle normal manipulado cirurgicamente (G1); normais controles submetidos a anastomoses no íleo (G2) e cólon (G3); ratos diabéticos submetidos a anastomoses no íleo (G4) e cólon (G5). Cada grupo foi dividido em 6 subgrupos com 10 ratos cada para sacrifícios com 0, 4, 7, 14, 21 e 30 dias após as operações. Os procedimentos cirúrgicos foram realizados 3 meses após a indução do diabetes com aloxana. A FR foi medida em todas anastomoses intestinais. Fragmentos de anastomoses do íleo e cólon foram retirados para dosagens de hidroxiprolina (HP) e proteína tecidual total (PT). RESULTADOS: A FR teve significante redução (P<0,05) nos grupos diabéticos G4 e G5, até 7 e 14 dias após a operação, respectivamente, quando comparada à observada nos grupos controles G2 e G3. Não foram observadas diferenças significantes nas dosagens de HP e PT em ratos diabéticos e controles, tanto operados no íleo como no cólon, em todos os momentos de avaliação. CONCLUSÃO: O diabetes conduz a alterações da força de ruptura de anastomoses intestinais durante a fase inicial da reparação da ferida cirúrgica, porém, este fato parece não estar relacionado à capacidade de sintetizar colágeno.
Diabetes Mellitus; Cicatrização de Feridas; Íleo; Colo; Ratos
12 - ORIGINAL ARTICLE
ALIMENTARY TRACT
Anastomotic healing in ileum and colon of alloxan-induced diabetic rats1 1 Research performed at the Experimental Laboratory, Botucatu School of Medicine, Sao Paulo State University (UNESP), Brazil.
Cicatrização de anastomoses realizadas no íleo e cólon de ratos diabéticos induzidos pela aloxana
José Lúcio Martins MachadoI; Érika Veruska Paiva OrtolanI; César Tadeu SpadellaII
IPhD, Assistant Professor, Pediatrics Surgery, Department of Surgery, Botucatu School of Medicine, Sao Paulo State University (UNESP), Brazil
IIPhD, Associate Professor, Gastroenterology, Department of Surgery, Botucatu School of Medicine, UNESP, Brazil
Correspondence Correspondence: César Tadeu Spadella Departamento de Cirurgia e Ortopedia, UNESP, Campus 18618-970 Botucatu - SP Brazil spadella@fmb.unesp.br
ABSTRACT
PURPOSE: To investigate if diabetes mellitus may alter breaking strength (BS) and collagen content in ileum and colon anastomoses in rats.
METHODS: Three-hundred Wistar rats were randomly assigned to 5 experimental groups, 60 per group: normal controls surgically manipulated (G1); normal controls submitted to ileum (G2) and colon (G3) anastomotic construction; diabetic rats submitted to ileum (G4) and colon (G5) anastomotic construction. Each group was further divided into 6 subgroups with 10 rats each for sacrifice at 0, 4, 7, 14, 21, and 30 days after surgery. All surgical procedures were performed 3 months after alloxan diabetes induction. BS was measured in all intestinal anastomoses. Fragments of ileum and colon anastomoses were taken for hydroxyproline concentration (HP) and total tissue protein (TP) dosages.
RESULTS: Anastomotic BS was significantly decreased (P<0.05) in ileum and colon of G4 and G5 diabetic groups up to 7 and 14 days after surgery, respectively, compared with G2 and G3 normal control groups. Anastomotic HP and TP content did not significantly differ between diabetic and normal control operated groups in ileum or colon at all evaluation times.
CONCLUSION: Experimental diabetes leads to impaired intestinal anastomotic strength during early surgical wound repair, but does not appear to be implicated with collagen synthesis capacity.
Key words: Diabetes Mellitus. Wound Healing. Ileum. Colon. Rats.
RESUMO
OBJETIVO: Investigar se o diabetes mellitus pode alterar a força de ruptura (FR) e o conteúdo de colágeno em anastomoses realizadas no íleo e cólon de ratos.
MÉTODOS: 300 ratos Wistar foram distribuídos por sorteio em 5 grupos experimentais com 60 animais cada: controle normal manipulado cirurgicamente (G1); normais controles submetidos a anastomoses no íleo (G2) e cólon (G3); ratos diabéticos submetidos a anastomoses no íleo (G4) e cólon (G5). Cada grupo foi dividido em 6 subgrupos com 10 ratos cada para sacrifícios com 0, 4, 7, 14, 21 e 30 dias após as operações. Os procedimentos cirúrgicos foram realizados 3 meses após a indução do diabetes com aloxana. A FR foi medida em todas anastomoses intestinais. Fragmentos de anastomoses do íleo e cólon foram retirados para dosagens de hidroxiprolina (HP) e proteína tecidual total (PT).
RESULTADOS: A FR teve significante redução (P<0,05) nos grupos diabéticos G4 e G5, até 7 e 14 dias após a operação, respectivamente, quando comparada à observada nos grupos controles G2 e G3. Não foram observadas diferenças significantes nas dosagens de HP e PT em ratos diabéticos e controles, tanto operados no íleo como no cólon, em todos os momentos de avaliação.
CONCLUSÃO: O diabetes conduz a alterações da força de ruptura de anastomoses intestinais durante a fase inicial da reparação da ferida cirúrgica, porém, este fato parece não estar relacionado à capacidade de sintetizar colágeno.
Descritores: Diabetes Mellitus. Cicatrização de Feridas. Íleo. Colo. Ratos.
Introduction
Despite the mythos that diabetic patients have poor wound healing, the real effect that diabetes mellitus has on the biological process of intestinal tissue repair is still unclear. There are many study protocols, results vary and conflict1, doubts exist whether healing changes seen in diabetic patients are a primary consequence of diabetes on the intestines or are due to imperfect collagenogenesis occurring after surgical injury2-4.
Healing of intestinal anastomoses can also be compromised by other technical, local, and systemic factors. Diabetes mellitus, however, is considered as one of these detrimental factors5, as it delays the closure and contraction of surgical wounds, decreases granulocyte and leucocyte chemiotaxis, and has a negative effect on the collagen metabolism, blood cell count and the biomechanical characteristics of surgical wounds6.
There are few long-term studies showing how diabetes does affect this process. A previous study performed in our laboratory showed that diabetes on its own cannot change healing parameters in the colon wall of uninjured alloxan-induced diabetic rats7, and suggests that injury may play an important role in the pathophysiology of intestinal healing.
The aim of this study was to correlate the breaking strength of the ileum and colon after anastomoses in chronic diabetic animals with their capacity to synthesize collagen.
Methods
Three-hundred, 3 month old male Wistar rats weighing approximately 250g each, were randomly assigned to five experimental groups with 60 animals each: G1 - normal control group surgically manipulated (sham operated); G2 and G3 - normal control groups submitted to ileum and colon anastomotic construction, respectively; G4 and G5 - diabetic rats submitted to ileum and colon anastomotic construction, respectively. Animals in the latter two groups were rendered diabetic three months before surgery by a single intravenous injection of 2% alloxan at 42 mg/kg body weight. Only rats showing severe diabetic state were included in the experiment.
Each experimental group was divided into 6 subgroups of 10 rats each for sacrifice at the beginning of the experiment, immediately after surgery (initial parameter), and 4, 7, 14, 21, and 30 days after surgery. In these periods clinical and laboratory parameters such as body weight, food and water intake, urine output, blood glucose, and urinary glucose were recorded in all rats. Breaking strength was measured in all intestinal anastomoses, and fragments of ileum and colon anastomoses were taken for hydroxyproline (HP) and total tissue protein (TP) measurements.
Surgical procedures were performed under semi-sterile conditions. Rats were anaesthetized by intraperitoneal injection of sodium pentobarbital 30mg/kg body weight. A median laparotomy of approximately 5cm was performed. After section of the terminal ileum 5cm proximal to the ileocecal valve, an end-to-end ileoileal anastomosis was constructed using a single-layer interrupted with 6-0 Ethilon sutures. A similar procedure was performed for the colonic anastomoses, 5cm proximal to the peritoneal reflection. The abodomen was closed with interrupted 5-0 Ethilon for the musculofacial and skin layers. Identical procedures, with exception of the anastomoses, were performed on the sham-operated group (G1) for both ileum and colon.
The biomechanical study used 3cm cylindrical segments of ileum and colon containing the anastomoses, fecal content was removed and segments flushed with saline. After cleaning both ileum and colon segments were immersed in warm saline (37ºC) with papaverine (250mg/dL) for 30 minutes. After rinsing with saline, segments were opened at the external mesenterial border. Breaking strength was measured using a universal mechanical testing instrument coupled to a microcomputer using Mtest version 1.0 software.
Tissue hydroxyproline (HP) concentration was measured using a modified Bergman & Loxley method8. Total tissue protein (TP) was quantified by the Lowry method9.
The study was performed after approval from Botucatu Medical School Ethics Committee for Experimental Research, Sao Paulo State University.
Data were analyzed by analysis of variance, complemented by the Dunn test, with level of significance set at p<0.05.
Results
Normal control surgically manipulated rats (G1) exhibited weight gain over the course of the experiment. In contrast, normal and anastomotic diabetic rats presented significant body weight loss up to 7 days after surgery, with subsequent complete restoration of the weight curve. However, the loss of body weight in diabetic rats was significantly higher than in normal control rats and those with ileum or colon anastomosis.
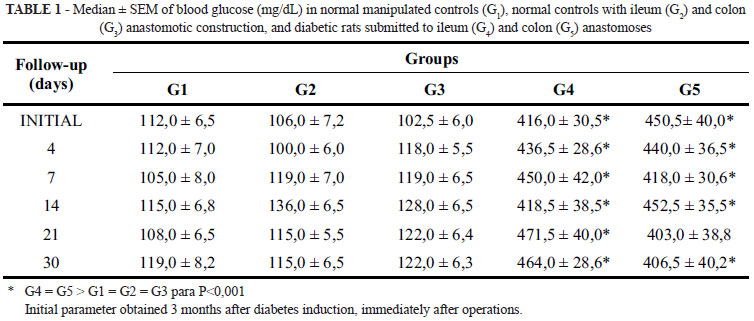
Normal control and normal ileum or colon anastomotic rats showed no clinical or laboratory changes throughout the study. In contrast, anastomotic diabetic rats presented significantly increased food and water intake and urine output, with sustained hyperglycemia at all evaluated moments (Table 1).
Adhesions between the epiploon and intestines were observed in 12 normal control operated rats (5 ileum and 7 colon rats). This was also seen in 14 diabetic anastomosized rats (6 ileum and 8 colon rats).
Dehiscences, anastomotic leakages, and peritoneal infections such as abscesses were only observed in diabetic rats. Anastomotic leakages were observed in two ileum diabetic animals and in 6 colon anastomosis rats. They were more frequently observed in both groups on the first seven days after surgery. Peritoneal abscesses were more evident in colon anastomosis than ileum animals (11 and 5, respectively). Intestinal anastomoses breakdown was not observed in non-diabetic control rats.
Breaking strength was significantly lower in ileum and colon anastomoses of normal and diabetic rats than normal control manipulated rats, without anastomoses. However, breaking strength in ileum and colon anastomoses of diabetic rats was significantly lower than normal operated controls (P<0.05) up to 7 and 14 days after surgery respectively (Figures 1 and 2). No changes in breaking strength were observed between the normal and diabetic groups after day 14.
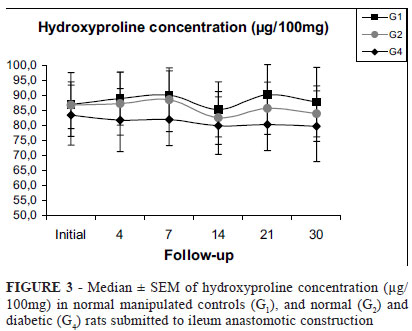
Despite lower breaking strength in diabetic rats up to 14th postoperative day, hydroxyproline (HP) and total tissue protein (TP) levels in ileum (Figures 3 and 4) and colon (Figures 5 and 6) anastomotic fragments from these animals were not significantly different to normal control rats at all evaluated moments (Figures 3 and 4). This was also true for HP/TP relationship.
Discussion
Metabolic imbalance between collagen synthesis and lysis is thought to be the underlying cause of intestinal anastomosis breakdown10. Other alterations may affect both factors; these include tissue blood supply defficiency, advanced age, malnutrition, anemia, irradiation, infection, and other technical, local and systemic factors, including diabetes mellitus.
Diabetes is considered as one of the most important factors in the healing process of surgical wounds because it can impair biomechanical properties and collagen metabolism in anastomotic sutures.
However, most of the evidence that diabetes mellitus adversely affects healing is derived from data on skin, with intestinal healing being poorly studied4,11. Moreover, collagen metabolism in the skin seems to be affected differently by the diabetic state2.
In this study we demonstrated that surgical intestine trauma significantly reduces breaking strength in both the ileum and colon of normal and diabetic rats during the first and second week after anastomosis construction. However, while breaking strength is less affected in the ileum and colon of normal control rats up to 7 days after surgery, it was significantly lower in diabetic rats. Unlike improved breaking strength after 7 days in ileums of normal controls, low values were still observed up to 14 days in colons of diabetic animals.
These findings explain why dehiscences, anastomotic leakages, and infection complications were more frequent in the colon than ileum of diabetic rats.
The use of breaking strength to measure early anastomotic healing in the intestines is discussed. Mansson et al.12 reported that this method is not sufficiently sensitive to for the examination of early anastomotic healing because no changes in breaking strength were observed up to 3 days postoperatively despite colonic anastomoses having leaked immediately after surgery. These authors suggested that bursting pressure and contrast enemas appear to be more suitable for evaluating early anastomotic healing in the rat colon than breaking strength.
However, bursting pressure may also produce inconsistent values for measuring anastomotic integrity in the later postoperative period due to surgical wound contraction which occurs 7 days or more after surgery. In this period, the intestinal segment containing the anastomosis will progressively have a smaller diameter than the adjacent uninjured tissue. As a consequence, according to Laplace's law, this lower diameter segment will also suffer lower inflation tension leading to rupture of adjacent intestinal tissue instead of the anastomotic zone.
In spite of inconveniences, both methods have been widely used in experimental studies on surgical wound tensile strength, although they are rare in the diabetic intestine.
Verhofstad and Hendricks4 while studying streptozotocin-diabetic rats which underwent resection and anastomosis of both ileum and colon, observed a significant decrease in bursting pressure for these animals compared to controls up to 3 and 7 days after ileal and colonic anastomosis, respectively. In contrast to our findings these authors had more anastomotic abscesses, especially in the ileum.
Onodera et al.11 also reported a significant decrease of anastomotic strength in the colon of streptozotocin-diabetic rats on day 7 after surgery, that gradually recovered up to day 14.
Why uncontrolled diabetes impairs anastomotic strength in the early postoperative period of ileum and colon constructions is a good question. Primary changes in total collagen and hydroxyproline quantities in ileum and colon fragments from rats before surgical injury was also first thought to be a good answer. However a previous study in our laboratory7 showed that diabetes on its own is not able to change healing parameters in the colonic wall of uninjuried alloxan-induced diabetic rats. Based on studies in skin, this suggests that diminished anastomotic strength in diabetic intestines would be accompanied by reduced collagen content in anastomoses.
However, unlike impaired wound healing in skin, this study showed that a significant decrease in breaking strength following ileal and colonic anastomoses is not accompanied by a decrease in hydroxyproline and total tissue protein, which remained unchanged compared to controls. This has also been reported by other researchers13,14, including microscopically15.
Verhofstad et al.13, suggested that increased local matrix degradation of collagen may significantly contribute to impaired anastomotic strength in the intestines of streptozotocin-diabetic rats. Their hypothesis was confirmed through a sustained elevated gelatinase activity in intestinal anastomoses of these animals.
Hendricks et al.14 suggested that the integrity of the intestinal wall may not only depend on a loss of collagen content, but also on an alteration in its solubility. These authors while studying unwounded intestine and anastomosis construction in rabbits, observed a significant decrease in acid-soluble hydroxyproline and a significant increase in the acid-solid fraction in the ileum and colon of these animals, 3 and 7 days after surgery, showing that salt and acid solubility of collagen may reflect its degree of crosslinking, which is considered of the utmost importance to suture stability.
Other mechanisms, however, have been postulated to explain variations in collagen content and concentration in intestinal anastomoses of diabetic animals; these include the presence of suture materials16, intraluminal bulk and bacteria17, and qualitative changes in ultrastructural deposition of the different types of collagen18, indicating that further investigations are still necessary.
Conclusion
Experimental diabetes leads to decreased tensile strength of anastomotic healing in the ileum and colon of rats which may explain the higher incidence of dehiscences, leakages, and infectious complications in these animals. Changes observed in breaking strength, however, are not accompanied by decreased hydroxyproline and total tissue protein in the anastomotic site, suggesting that instead of quantitative, a qualitative change in the remodelling process of intestinal repair may be occurring.
Acknowledgements
We are grateful to Luiz Carlos Edevalter Bardella for skilled laboratorial assistance during breaking strength measurements.
Received: August 05, 2008
Review: October 08, 2008
Accepted: November 12, 2008
Conflict of interest: none
Financial source: FAPESP
How to cite this article
Machado JLM, Ortolan EVP, Spadella CT. Anastomotic healing in ileum and colon of alloxan-induced diabetic rats. Acta Cir Bras. [serial on the Internet] 2009 Jan-Feb;24(1). Available from URL: http://www.scielo.br/acb
- 1. Pearl SH, Kanat IO. Diabetes and healing: a review of the literature. J Foot Surg. 1988;27(3):268-70.
- 2. Oxlund H, Christensen H, Seyer-Hansen M, Andreassen TT. Collagen deposition and mechanical strength of colon anastomoses and skin incisional wounds of rats. J Surg Res. 1996;66(1):25-30.
- 3. Spanheimer RG, Umpierrez GE, Stumpf V. Decreased collagen production in diabetic rats. Diabetes. 1988;37(4):371-6.
- 4. Verhofstadt MHJ, Hendriks TH. Diabetes impairs the development of early strength, but not the accumulation of collagen, during intestinal anastomotic healing in the rat. Br J Surg. 1994;82(3):423-4.
- 5. Thornton FJ, Barbul A. Healing in the gastrointestinal tract. Surg Clin North Am. 1997;77(3):549-86.
- 6. Ekmektzoglou KA, Zografos GC. A concomitant review of the effects of diabetes mellitus and hypothyroidism in wound healing. Wld J Gastroenterol. 2006;12(17):2721-9.
- 7. Ortolan EVP, Spadella CT, Machado JLM, Kobayasi S. The evaluation of healing parameters in the colon of diabetic rats without surgical injury. Acta Cir Bras. 2004;19(3):286-95.
- 8. Bergman I, Loxley R. The determination of hydroxyproline in urine hydrolysates. Clin Chim Acta. 1970;27(2):347-9.
- 9. Lowry OH, Rosebrough NJ, Fau AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193(1):265-75.
- 10. Hendriks T, Vereecken TH, Hesp WL, Shillings PH, de Boer HH. Loss of collagen from experimental intestinal anastomoses: early events. Exp Mol Pathol. 1985;42(3):411-8.
- 11. Onodera H, Ikeuchi D, Nagayama S, Imamura M. Weakness of anastomotic site in diabetic rats caused by changes in the integrity of newly formed collagen. Dig Surg. 2004;21(2):146-51.
- 12. Månsson P, Zhang XW, Jeppsson B, Thoriacius H. Anastomotic healing in the rat colon: comparison between a radiological method, breaking strength and bursting pressure. Int J Colorectal Dis. 2002;17(6):420-5.
- 13. Verhofstad MH, Lomme RM, de Man BM, Hendricks T. Intestinal anastomoses from diabetic rats contain supranormal levels of gelatinase activity. Dis Colon Rectum. 2002;45(4):554-61.
- 14. Hendriks T, Hesp WL, Klompmakers AA, Lubbers EJ, de Boer HH. Solubility of tissue hydroxyproline in experimental anastomoses. Exp Mol Pathol. 1985;43(2):253-9.
- 15. Verhofstad MH, Lange WP, van der Laak JA, Verhofstad AA, Hendricks T. Microscopic analysis of anastomotic healing in the intestine of normal and diabetic rats. Dis Colon Rectum. 2001;44(3):423-31.
- 16. Mastboom WJ, Hendricks T, de Boer HH. Intestinal anastomotic healing in the absence of suture material: an experimental study in rats. Int J Colorectal Dis. 1991;1:33-7.
- 17. Jönsson K, Jiborn H, Zederfeldt B. Healing of ileocolic anastomoses. Breaking strength and collagen content in the intestinal wall after ileocolic resection and anastomosis in the rat. Acta Chir Scand. 1985;151(7):629-33.
- 18. Braiman-Wiksman L, Solomonik I, Spira R, Tennenbaum T. Novel insights into wound healing sequence events. Toxicol Pathol. 2007;35(6):767-79.
Publication Dates
-
Publication in this collection
14 Jan 2009 -
Date of issue
Feb 2009
History
-
Accepted
12 Nov 2008 -
Reviewed
08 Oct 2008 -
Received
05 Aug 2008