Abstracts
PURPOSE: To evaluate the placental glycogen storage and fetal development in the pregnancy of neonatally streptozocin-induced diabetic rats and to establish relation with glycemia and insulin levels. METHODS: At the birth day, 147 female rats were randomly distributed in two experimental groups: 1) Non-diabetic Group (Control, n=45) - received the vehicle; 2) Diabetic Group (STZ, n=102) - received 100 mg streptozocin/kg in neonatal period. At day 0 of pregnancy, adult female rats were included in the control group when presented glycemia below 120 mg/dL and, in the group STZ with glycemia between 120 and 300 mg/dL. At day 21 of pregnancy, blood samples were collected for glycemia and insulin determination, and placentas withdrawn for placental glycogen determination. The newborns (NB) were classified in small (SGA), appropriate (AGA) and large (LGA) for gestational age. RESULTS: Rats STZ presented higher glycemia at days 0 and 14 of pregnancy. At end of pregnancy, rats STZ showed higher proportion of NB SGA and LGA; reduced rate of NB AGA and unaltered glycemia, insulin and placental glycogen determinations. CONCLUSION: Mild diabetes altered the maternal glycemia in the early pregnancy, impairing future fetal development, but it caused no alteration on insulin and placental glycogen determination, confirming that this glycemic intensity was insufficient to change glycogen metabolism.
Diabetes Mellitus; Insulin; Pregnancy, Animal; Glycogen; Streptozocin; Glycemia; Rats
OBJETIVO: Avaliar os estoques de glicogênio placentário e o desenvolvimento fetal na prenhez de ratas com diabete moderado induzido no período neonatal e relacionar com glicemia e níveis de insulina. MÉTODOS: No dia de nascimento, foram distribuídas aleatoriamente 147 ratas em dois grupos experimentais: 1) Grupo Não-diabético (Controle, n=45) - recebeu o veículo; 2) Grupo Diabético (STZ, n=102) - recebeu 100 mg streptozocin/kg peso corpóreo. No dia 0 de prenhez, foram incluídas ratas controle que apresentassem glicemia baixo de 120 mg/dL e, no grupo STZ, com glicemia entre 120 e 300 mg/dL. No 21º dia de prenhez, amostras de sangue foram coletadas para glicemia e determinação de insulina e as placentas foram retiradas para determinação de glicogênio placentário. Os recém-nascidos (RN) foram classificados em pequeno (PIP), adequado (AIP) e grande (GIP) para idade de prenhez. RESULTADOS: As ratas STZ apresentaram glicemias maiores nos dias 0 e 14 de prenhez. No final da prenhez, as ratas STZ mostraram maior proporção de RN PIP e GIP, taxa reduzida de RN AIP e inalteração em glicemia, insulina e na determinação de glicogênio placentário. CONCLUSÃO: O diabete moderado alterou a glicemia materna no início da prenhez, prejudicando o futuro desenvolvimento placentário e fetal, mas não causou nenhuma alteração na determinação de insulina e de glicogênio placentário, confirmando que esta intensidade de glicêmica foi insuficiente para modificar o metabolismo de glicogênio.
Diabetes Mellitus; Insulina; Prenhez; Glicogênio; Streptozocin; Glicemia; Ratos
2 - ORIGINAL ARTICLE
MODELS, BIOLOGICAL
Evaluation of placental glycogen storage in mild diabetic rats1 1 Research performed at the Laboratory of Experimental Research in Gynecology and Obstetrics, Botucatú Medical School, State University of São Paulo (UNESP), Brazil.
Avaliação dos estoques de glicogênio placentário em ratas com diabete moderado
Aline BuenoI; Isabela Lovizutto IessiI; Iracema de Mattos Paranhos CalderonII; Marilza Vieira Cunha RudgeIII; Carlos Eduardo Meirelles dos SantosIII; Débora Cristina DamascenoIV
IGraduate student, Laboratory of Experimental Research of Gynecology and Obstetrics, Botucatú Medical School, UNESP, São Paulo, Brazil
IIPhD, Full Professor, Department of Gynecology and Obstetrics, Botucatú Medical School, UNESP, São Paulo, Brazil
IIIMD, Department of Surgery, School of Veterinary Medicine of Botucatú, UNESP and Bauru Veterinary Center (CVB), São Paulo, Brazil
IVPhD, Researcher of Laboratory of Experimental Research of Gynecology and Obstetrics, Botucatú Medical School, UNESP, São Paulo, Brazil
Correspondence Correspondence: Profa. Dra. Débora Cristina Damasceno Gynecology and Obstetrics Department Botucatú Medical School, UNESP Distrito de Rubião Júnior, s/n 18618-000 Botucatú SP Brazil Phone/Fax: (55 14)3811-6181 damasceno@fmb.unesp.br
ABSTRACT
PURPOSE: To evaluate the placental glycogen storage and fetal development in the pregnancy of neonatally streptozocin-induced diabetic rats and to establish relation with glycemia and insulin levels.
METHODS: At the birth day, 147 female rats were randomly distributed in two experimental groups: 1) Non-diabetic Group (Control, n=45) - received the vehicle; 2) Diabetic Group (STZ, n=102) - received 100 mg streptozocin/kg in neonatal period. At day 0 of pregnancy, adult female rats were included in the control group when presented glycemia below 120 mg/dL and, in the group STZ with glycemia between 120 and 300 mg/dL. At day 21 of pregnancy, blood samples were collected for glycemia and insulin determination, and placentas withdrawn for placental glycogen determination. The newborns (NB) were classified in small (SGA), appropriate (AGA) and large (LGA) for gestational age.
RESULTS: Rats STZ presented higher glycemia at days 0 and 14 of pregnancy. At end of pregnancy, rats STZ showed higher proportion of NB SGA and LGA; reduced rate of NB AGA and unaltered glycemia, insulin and placental glycogen determinations.
CONCLUSION: Mild diabetes altered the maternal glycemia in the early pregnancy, impairing future fetal development, but it caused no alteration on insulin and placental glycogen determination, confirming that this glycemic intensity was insufficient to change glycogen metabolism.
Key words: Diabetes Mellitus. Insulin. Pregnancy, Animal. Glycogen. Streptozocin. Glycemia. Rats.
RESUMO
OBJETIVO: Avaliar os estoques de glicogênio placentário e o desenvolvimento fetal na prenhez de ratas com diabete moderado induzido no período neonatal e relacionar com glicemia e níveis de insulina.
MÉTODOS: No dia de nascimento, foram distribuídas aleatoriamente 147 ratas em dois grupos experimentais: 1) Grupo Não-diabético (Controle, n=45) - recebeu o veículo; 2) Grupo Diabético (STZ, n=102) - recebeu 100 mg streptozocin/kg peso corpóreo. No dia 0 de prenhez, foram incluídas ratas controle que apresentassem glicemia baixo de 120 mg/dL e, no grupo STZ, com glicemia entre 120 e 300 mg/dL. No 21º dia de prenhez, amostras de sangue foram coletadas para glicemia e determinação de insulina e as placentas foram retiradas para determinação de glicogênio placentário. Os recém-nascidos (RN) foram classificados em pequeno (PIP), adequado (AIP) e grande (GIP) para idade de prenhez.
RESULTADOS: As ratas STZ apresentaram glicemias maiores nos dias 0 e 14 de prenhez. No final da prenhez, as ratas STZ mostraram maior proporção de RN PIP e GIP, taxa reduzida de RN AIP e inalteração em glicemia, insulina e na determinação de glicogênio placentário.
CONCLUSÃO: O diabete moderado alterou a glicemia materna no início da prenhez, prejudicando o futuro desenvolvimento placentário e fetal, mas não causou nenhuma alteração na determinação de insulina e de glicogênio placentário, confirmando que esta intensidade de glicêmica foi insuficiente para modificar o metabolismo de glicogênio.
Descritores: Diabetes Mellitus. Insulina. Prenhez. Glicogênio. Streptozocin. Glicemia. Ratos.
Introduction
The maternal glucose is considered important energy substrate for the development fetal1. It almost all the energy source of the fetal-placental unit, making the placenta and therefore the fetus totally dependent on maternal source of glucose and its metabolic reserve for the supply of multiple functions of the placenta2.
After the meal, the blood glucose is removed by the liver through the portal vein and the systemic circulation, and temporarily stored as glycogen3. The glycogen storage polysaccharide is the most important existing in animal cells. Each branch of glycogen ends with a non-reducing sugar, so it is not reducing how many terminals ramifications, but with a single terminal reducer. When it is used as an energy source, its units of glucose are removed one by one from terminals not reducing. The enzymes can act in many terminals, making this polysaccharide is reduced to a monosaccharide4. The synthesis of glycogen is the process by which glucose is polymerized the glycogen, which is accumulated in the cells in amounts varying according to the cell type, working there as a deposit of energy accessible to the cell. The metabolism of glycogen is controlled predominantly by the coordinated action of two enzymes, glycogen synthase (promotes the glycogen synthesis) and glycogen phosphorylase (promotes the glycogen lysis)5.
Insulin and glucose may inhibit the glycogenolysis network, stimulating the enzymatic flow of glycogen synthase and/or inhibiting the enzymatic flow of the enzyme glycogen phosphorylase4. Insulin is the hormone responsible for stimulating the glycogen synthesis. It was observed in at term human placentas that the activity of glycogen phosphorylase increases while the activity of glycogen synthase decreases5.
In a normal pregnancy the placental glycogen level is increased, decreasing gradually as pregnancy following the term6. But when there is any endocrine change the glycogen storage may change. Among the changes that occur during pregnancy can include diabetes. Diabetes mellitus is a chronic disorder caused by the lack of insulin production by pancreatic beta (β) cells or the defect in the receptor for insulin in the cells target, resulting in metabolic hyperglycemic disease. This syndrome alters lipid, carbohydrate and protein metabolism7.
Many experimental models using laboratory animals are made to enhance the understanding of the pathophysiological mechanisms involved in diabetic syndrome, such as the use of β -cytotoxic chemical agents (streptozocin and alloxan). The hyperglycemia induced by streptozocin (STZ) is dose-dependent, when low doses of STZ are administered at the beginning of the pregnancy, there is appearance of the gestational diabetes (mild diabetes) occurs with presence of fetal macrosomia, while high doses administered in adulthood of the animal induce insulin deficient diabetes associated with intrauterine growth restriction of uterine8.
The glycogen placental increases both in women and in diabetic animals since diabetes results in the glycogen lysis. The enzymatic mechanism that can result in the glycogen accumulation in the placental diabetes, favoring the glycogen synthesis or decreasing its degradation has been studied in rats presenting STZ- induced diabetes9.
The placenta is glucose-dependent related with of energy. Maternal glucose transfer by maternal placenta is determined by the glucose concentration and glucose amount stored as glycogen10. In late pregnancy the insulin concentration in the fetal circulation is high11, particularly in the presence of maternal hyperglycaemia12. As the placenta depends on its insulin with regard to the glycogen metabolism, hyperglycemia is a major factor in the change of its storage. Then, according to previous studies and interest to investigate the relationship between mild diabetes and placental glycogen in rats, the aim of this study was to evaluate the placental glycogen storage and fetal development in the pregnancy of neonatally streptozocin-induced diabetic rats and to establish relation with glycemia and insulin levels.
Methods
Nine-week-old male and female Wistar rats (n=10), weighting approximately 180g and 220g were kept in collective cages in controlled conditions of temperature of (22 ± 3º C), light (12h light/dark cycle) and relative humidity (60 ± 5%). The animals were fed with laboratory chow and tap water ad libitum and cared for in accordance with the principles of the Guide for Care and Use of Experimental Animals. The local Committee of Ethics in Animal Experimentation approved all experimental procedures of this study. Parental female rats were mated to obtain offspring. The female newborns (NB) received streptozocin in dose of 100 mg/kg body weight, subcutaneous route dissolved in citrate buffer (0.1M, pH 4.5) at day 0 of birth, according to modified procedures13,14. Control NB received citrate buffer only. NB rats remained with their mothers until the end of the weaning period (day 21 of post-natal life) and were maintained under similar conditions to those of their mothers. In the month 3 of life, rats were mated overnight with non-diabetic male Wistar rats. The morning when sperm was found in the vaginal smear was designated Gestational Day 0. At days 0, 7, 14 and 21 of pregnancy, maternal body weights were determined and blood samples were collected from the tail vein in order to evaluate glucose levels using glucose oxidase reagents strips by glucose oxidase method15. Criteria inclusion for this study was rats presenting glycemic level between 120-300 mg/dL classified as mild diabetics rats. Rats with glycemia below 120 mg/dL were considered control. All female rats were mated overnight to non-diabetic male. The morning when sperm was found in the vaginal smear was designated gestational day 0. At day 21 of pregnancy, fed rats were anesthetized with sodium thiopental. Following trichotomy of the abdominal region, the animal was placed in dorsal decubitus, and its libs were fixed to the surgery table. The laparoscopy procedure was carried out by an incision in the medium line beginning in the xiphoid cartilage and ending in the pubis. The intestinal loops were moved cranially for uterus exposure. The hysterectomy was carried out by ligament, artery and ovarian vein section and incision of the body uterine above the cervix. Afterward, the uterus and his content were weighed using analytical scale. Incisions were performed in the whole extension of the uterine horns, on their free margin and on the most avascular area. The fetus, amniotic sac and placenta were removed by slight traction. The umbilical cord was clamped by using Halstead-type hemostatic clamps. Next, each cord was sectioned and each placenta was isolated weighed on an analytical scale to obtain 250mg of tissue. These placental samples were stored in potassium hydroxide (KOH - 30%) at -80ºC. The tissue sample was digested in hot concentrated KOH, precipitated with ethanol, hydrolyzed with phenol and sulphuric acid and so performed by a spectrophotometer with a wavelength of 490 nm according to modified procedure from Nomura et al.16.
The results were reported as mean ± standard error of mean (SEM). All data were statistically analyzed using Two-way analysis of variance (ANOVA), followed by the Student-Newman-Keuls test. p< 0.05 was taken to be statistically significant.
Results
Maternal weight gain during pregnancy
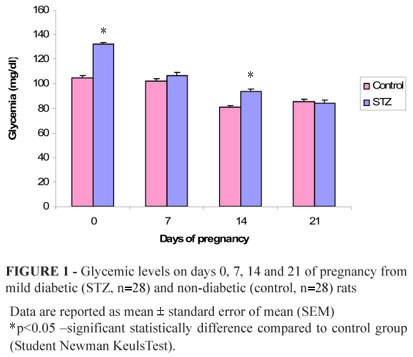
During whole pregnancy, it was observed increased body weight in mild diabetic rats (STZ group). At day 0, 7, 14 and 21 of pregnancy, these animals presented lower (p<0.05) body weight and maternal weight gain (day 21 0) compared to non-diabetic rats (Table 1).
Glycemia during pregnancy
In control rats, normoglycemia was confirmed with mean glucose values bellow 100 mg/dL (day 0, 7, 14 and 21 of pregnancy); while in rats STZ the higher (p<0.05) glycemia was observed at day 0 and 14 of pregnancy compared to control rats (Figure 1).
Insulin levels
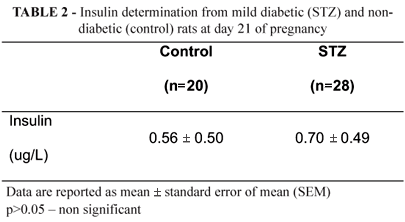
At end of pregnancy, rats STZ presented no significant difference (p>0.05) on the insulin levels compared to control group (Table 2).
Placental glycogen levels
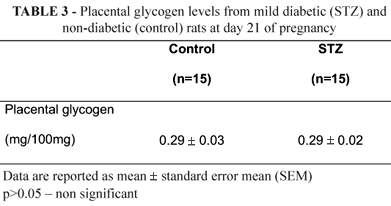
There was no difference on the levels of placental glycogen in rats STZ in the at term pregnancy compared to control rats (p>0.05) (Table 3).
Discussion
In this study, the rats with streptozocin-induced mild diabetes showed weight gain during pregnancy. However, such animals began their pregnancy with lower weights than those in the control group, and these weights remained lower until the end of pregnancy. The smaller body-weight gain of STZ rats during pregnancy might be related to alterations in the growth hormone.
STZ rats began pregnancy with a glycemia which was compatible with that of mild diabetes, that is, between 120 and 300 mg/dL. These results are in accordance with Portha et al.13, who first described the experimental model for mild-intensity diabetes (100 < glicemia < 300mg/dL) in adulthood. Similarly, Bonner-Weir et al.17 reported that neonatal rats which received STZ on the second day of life showed transitory hyperglycemia followed by normal glycemia until the sixth week of life. After this period, the animals developed diabetes with the absence of ketonic bodies and without the need for insulin treatment, which suggest that these hyperglycemic rats presented a selective defect in insulin secretion when it was stimulated by glucose
Movassat et al.18 showed that regeneration of pancreatic β-cells between the 4th and the 7th postnatal days of rats with diabetes induced in the neonatal period contributed to the development of normoglycemic as well as hyperglycemic rats. Nevertheless, various studies in the literature report that STZ administration during the neonatal period causes the onset of mild-intensity diabetes in these animals adulthood13,19, thus confirming our findings. In this study, the glycemic means were higher at days 0 and 14 of pregnancy of rats with mild diabetes. Additionally, they showed glucose intolerance and insulin resistance (data not shown) in the period similar to that presented by women with gestational diabetes, which suggest that both in gestation and in pregnancy pancreatic β-cells develop a sensitive mechanism to respond to glycemic signs. These alterations may be mediated by hormones, such as placental lactogen, estrogen and progesterone.
Our results did not show alterations in the placental glycogen rates of diabetic rats in at-term pregnancy, thus differing from Shafrir and Barash20, leading to the unalteration of glycemic levels at the end of pregnancy, which could be related to the lack of modification of placental glycogen levels. Glycogen accumulation in the placenta, in face of maternal insulin deficiency and the fact thatbhyperinsulinemia on the fetal side is not more accentuated in diabetic than in control rats, indicates that insulin has no direct influence on glycogen metabolism in this tissue10. Glucose flow across the placental is substantially reduced in diabetic hyperglycemia, and there is no convincing demonstration, as yet, of an insulin-dependent, rate-limiting locus in the placenta20.
Conclusion
Mild diabetes altered the maternal glycemia in the early pregnancy, impairing future fetal development, but it caused no alteration on insulin and placental glycogen determination, confirming that this glycemic intensity was insufficient to change glycogen metabolism.
Acknowledgements
The authors are grateful to Fernanda Pereira Lima and Yuri Karen Sinzato from Laboratory of Experimental Research of Gynecology and Obstetrics, Botucatu Medical School, UNESP, Sao Paulo, Brazil) for technical assistance, and Research Support Center (RSC) of the Botucatu Medical School, São Paulo State University (UNESP), for their valuable contribution in study design and statistical analysis.
Received: September 16, 2009
Review: November 12, 2009
Accepted: December 10, 2009
Conflict of interest: none
Financial source: none
- 1. Calderon IMP, Rudge MVC, Ramos MD, Peraçoli JC. Estudo longitudinal, bioquímico e histoquímico de placentas de ratas diabética - relação com a macrossomia e o retardo de crescimento intra-uterino. Rev Bras Ginecol Obstet. 1999;21:91-8.
- 2. Beyth Y, Neuman S, Gutman A, Shafir E. Effect of prolonged gestation on placental and maternal liver enzyme activities in the rat. Diabete Metab. 1977;3:91-6.
- 3. Bischof MG, Krssak M, Krebs M, Bernroider E, Stingl H, Waldhäusl W, Roden M. Effects of short-term improvement of insulin treatment and glycemia on hepatic glycogen metabolism in type 1 diabetes. Diabetes. 2001,50:392-8.
- 4. Lehninger AL, Nelson DL, Cox MM. Carboidratos. In: Lehninger AL, Nelson DL, Cox MM. Princípios de bioquímica. 2ed. New York: Sarvier; 1995. p.222-40.
- 5. Kasuga M, Ogawa W, Ohara T. Tissue glycogen content and glucose intolerance. J Clin Invest. 2003;111:1282-4.
- 6. Sambaugh GE, Mrozak SC, Freinkel N. Fetal fuels. I. Utilization of ketons by isotaled tissues at various stages of maturation and maternal nutrition during late gestation. Metabolism. 1977;26:623-5.
-
7AMERICAN DIABETES ASSOCIATION - ADA, 2009. http://www.diabetes.org (acesso em 05 de fevereiro de 2009).
» link - 8. Holemans K, Aerts L, Van Assche FA. Fetal growth restriction and consequences for the offspring in animals models. Society for gynecologic Investigation. 2003;10:392-9.
- 9. Diamant YZ, Shafrir E. Placental enzymes of glycolysis, gluconeogenesis and lipogenesis in the diabetic rat and in starvation. Comparison with maternal and fetal liver. Diabetologia. 1978;15:481-5.
- 10. Barash V, Gutman A, Shafrir E. Mechanism of placental glycogen deposition in diabetes in the rat. Diabetologia. 1983;24:63-8.
- 11. Girard JR, Kervran A, Soufflet E, Assan R. Factors affecting the secretion of insulin and glucagon by the rat fetus. Diabetes. 1974;23:310-7.
- 12. De Gasparo M, Milner RD. The timing of fetal B cell hyperplasia in diabetic rat pregnancy. Diabetologia. 1980;19:54-7.
- 13. Portha B, Levacher C, Picon L, Rosselin G. Diabetogenic effect of streptozocin in the rat during the perinatal period. Diabetes. 1974;23:889-95.
- 14. Tsuji K, Taminato T, Usami M, Ishida H, Kitano N, Fukumoto H, Koh G, Kurose T, Yamada Y, Yano Y, Seino H, Imura H. Characteristic features of insulin secretion in the streptozotocin-induced NIDDM rat model. Metabolism. 1988;37(11):1040-4.
- 15. Tietz C, Jelezko F, Gerken U, Schuler S, Schubert A, Rogl H, Wrachtrup J. Single molecule spectroscopy on the light-harvesting complex II of higher plants. Biophys J. 2001;81:556-62.
- 16. Nomura Y, Okamoto S, Sakamoto M, Feng Z, Nakamura T. Effect of cobalto in the liver glycogen content in the streptozotocin-induced diabetic rats. Mol Cell Biochem. 2005;277:127-30.
- 17. Bonner-Weir S, Trent DF, Honey RN, Weir GC. Responses of neonatal rat islets to streptozotocin: limited B-cell regeneration and hyperglycemia. Diabetes. 1981;30:64-9.
- 18. Movassat J, Saulnier C, Portha B. Insulin administration enhances growth of the beta-cell mass in streptozotocin-treated newborn rats. Diabetes. 1997;46:1445-52.
- 19. Capobianco E, Jawerbaum A, White V, Pustovrh C, Sinner D, Gonzalez ET. Elevated levels of endothelin-1 and prostaglandin E2 and their effect on nitric oxide generation in placental tissue from neonatal streptozotocin-induced diabetic rats. Prostaglandins Leukot Essent Fatty Acids. 2003;68:225-31.
- 20. Shafrir E, Barash V. Placental glycogen metabolism in diabetic pregnancy. Isr J Med Sci. 1991;27:449-61.
Publication Dates
-
Publication in this collection
18 Oct 2010 -
Date of issue
Apr 2010
History
-
Reviewed
12 Nov 2009 -
Received
16 Sept 2009 -
Accepted
10 Dec 2009