Abstracts
PURPOSE: To qualify the FT-Raman spectral data of primary and metastatic cutaneous melanoma in order to obtain a differential diagnosis. METHODS: Ten normal human skin samples without any clinical or histopathological alterations, ten cutaneous melanoma fragments, and nine lymph node metastasis samples were used; 105, 140 and 126 spectra were obtained respectively. Each sample was divided into 2 or 3 fragments of approximately 2 mm³ and positioned in the Raman spectrometer sample holder in order to obtain the spectra; a monochrome laser light Nd:YAG at 1064 nm was used to excite the inelastic effect. RESULTS: To differentiate the three histopathological groups according to their characteristics extracted from the spectra, data discriminative analysis was undertaken. Phenylalanine, DNA, and Amide-I spectral variables stood out in the differentiation of the three groups. The percentages of correctly classified groups based on Phenylalanine, DNA, and Amide-I spectral features was 93.1%. CONCLUSION: FT-Raman spectroscopy is capable of differentiating melanoma from its metastasis, as well as from normal skin.
Melanoma; Spectrum Analysis, Raman; Diagnosis; Biopsy
OBJETIVO: Qualificar os dados espectrais FT-Raman do melanoma cutâneo primário e metastático e assim realizar o diagnóstico diferencial. MÉTODOS: Foram utilizadas amostras de 10 fragmentos de pele sem alterações clínicas ou histopatológicas, 10 de melanomas cutâneos e 9 de metástases linfonodais; 105, 140 and 126 espectros foram obtidos respectivamente. Cada amostra foi dividida em 2 ou 3 frações de 2 mm³ e posicionada no porta amostras do espectrômetro Raman para obtenção dos espectros, por meio da excitação do espalhamento inelástico pelo laser de Nd:YAG em 1064 nm incididos na amostra. RESULTADOS: Para diferenciar os três grupos formados de acordo com as características fornecidas pelos espectros, realizamos a análise discriminante dos dados. As variáveis espectrais Fenilalanina, DNA e Amida-I se destacaram na capacidade de diferenciação dos três grupos histológicos. A porcentagem de classificação correta utilizando estes critérios foi de 93,1%; o que mostra a eficiência da análise realizada. CONCLUSÃO: A espectroscopia FT-Raman é capaz de diferenciar o melanoma de sua metástase, assim como da pele normal.
Melanoma; Análise Espectral Raman; Diagnóstico; Biópsia
9 - ORIGINAL ARTICLE
PLASTIC SURGERY
Differential diagnosis in primary and metastatic cutaneous melanoma by FT-Raman spectroscopy1 1 Research performed at the Plastic Surgery Experimental Laboratory, Post-graduate Program in Plastic Surgery, Division of Plastic Surgery, Department of Surgery, Federal University of Sao Paulo (UNIFESP), Brazil.
Diagnóstico diferencial no melanoma primário e metastático por espectroscopia FT-Raman
Andrea Fernandes de OliveiraI; Ivan Dunshee de Abranches Oliveira SantosII; Sidney Bandeira CartaxoI; Renata Andrade BitarIII; Mílvia Maria Simões e Silva EnokiharaIV; Herculano da Silva MartinhoV; Airton Abrahão MartinVI; Lydia Masako FerreiraVII
IFellow Master degree, Post-graduate Program in Plastic Surgery, UNIFESP, Sao Paulo, Brazil
IIPhD, Head of Division of Plastic Surgery, Department of Surgery, UNIFESP, Sao Paulo, Brazil
IIIFellow PhD degree, Post-graduate Program in Plastic Surgery, UNIFESP, Sao Paulo, Brazil
IVMD, PhD, Pathology Department, UNIFESP, Sao Paulo, Brazil
VPhD, Head of Center for Human and Natural Sciences (CCNH), Federal University of ABC, Santo Andre-SP, Brazil
VIPhD, Head of Biomedical Vibrational Spectroscopy Laboratory, Institute of Research and Development - IP&D, UNIVAP, Sao Jose dos Campos-SP, Brazil
VIIPhD, Full Professor, Head of Division of Plastic Surgery, Department of Surgery, UNIFESP, Sao Paulo, Brazil
Correspondence Correspondence: Andrea Fernandes de Oliveira Division of Plastic Surgery, Department of Surgery Federal University of Sao Paulo Rua Napoleão de Barros, 715/4º andar 04024-002 São Paulo SP Brasil Phone: (55 11)5576-4065/5576-4118 dra.afo@gmail.com
ABSTRACT
PURPOSE: To qualify the FT-Raman spectral data of primary and metastatic cutaneous melanoma in order to obtain a differential diagnosis.
METHODS: Ten normal human skin samples without any clinical or histopathological alterations, ten cutaneous melanoma fragments, and nine lymph node metastasis samples were used; 105, 140 and 126 spectra were obtained respectively. Each sample was divided into 2 or 3 fragments of approximately 2 mm3 and positioned in the Raman spectrometer sample holder in order to obtain the spectra; a monochrome laser light Nd:YAG at 1064 nm was used to excite the inelastic effect.
RESULTS: To differentiate the three histopathological groups according to their characteristics extracted from the spectra, data discriminative analysis was undertaken. Phenylalanine, DNA, and Amide-I spectral variables stood out in the differentiation of the three groups. The percentages of correctly classified groups based on Phenylalanine, DNA, and Amide-I spectral features was 93.1%.
CONCLUSION: FT-Raman spectroscopy is capable of differentiating melanoma from its metastasis, as well as from normal skin.
Key words: Melanoma. Spectrum Analysis, Raman. Diagnosis. Biopsy.
RESUMO
OBJETIVO: Qualificar os dados espectrais FT-Raman do melanoma cutâneo primário e metastático e assim realizar o diagnóstico diferencial.
MÉTODOS: Foram utilizadas amostras de 10 fragmentos de pele sem alterações clínicas ou histopatológicas, 10 de melanomas cutâneos e 9 de metástases linfonodais; 105, 140 and 126 espectros foram obtidos respectivamente. Cada amostra foi dividida em 2 ou 3 frações de 2 mm3 e posicionada no porta amostras do espectrômetro Raman para obtenção dos espectros, por meio da excitação do espalhamento inelástico pelo laser de Nd:YAG em 1064 nm incididos na amostra.
RESULTADOS: Para diferenciar os três grupos formados de acordo com as características fornecidas pelos espectros, realizamos a análise discriminante dos dados. As variáveis espectrais Fenilalanina, DNA e Amida-I se destacaram na capacidade de diferenciação dos três grupos histológicos. A porcentagem de classificação correta utilizando estes critérios foi de 93,1%; o que mostra a eficiência da análise realizada.
CONCLUSÃO: A espectroscopia FT-Raman é capaz de diferenciar o melanoma de sua metástase, assim como da pele normal.
Descritores: Melanoma. Análise Espectral Raman. Diagnóstico. Biópsia.
Introduction
Raman spectroscopy is an optical technique that provides information on the vibrational levels of molecules. It is widely used in analytical studies, both qualitative and quantitative. With Raman spectroscopy the molecular structure and composition of the material under study is coded as a group of spread light frequency changes. Thus, the Raman spectrum can provide a digital print of material from which its molecular composition can be determined1,2.
Raman spectroscopy has lately been used as an analytical tool in the biochemical characterization of biological tissues, including neoplasias, due to advantages such as its sensitivity to detect small structural changes in the molecules, as well as being a non-invasive test that does not require much preparation in data collection1-3. These molecular changes can assist in the early detection of neoplastic lesions, and in the scanning of lesions that are not clinically detected, by determining the safe margins for the excision of tumors, based on the concept that chemical changes precede the morphologic changes4.
The histopathologic test is the gold standard to diagnose cutaneous melanoma. It uses a thickness biopsy of the tumor. In the case of metastatic disease that cannot be clinically detected, it provides better evaluation and information about the patients prognosis5. However, the histopathological test of these injuries is not easy due to a relevant amount of lesion that can imitate melanoma. The use of markers (HMB-45, S100, Melan-A) diminish this diagnostic difficulty, but can fail as these antibodies can also be found in benign lesions5,6. Raman spectroscopy attempts to diminish the subjectivity in the diagnosis of cutaneous pigmented lesions. The aim of this study therefore was to qualify the Raman spectral data in primary and metastatic cutaneous melanoma, aiming towards a differential diagnosis.
Methods
The research was approved by the Ethics Committee of the Federal University of Sao Paulo-UNIFESP (CEP 1642/05). For the experimental procedure, 10 fragments of normal skin with no clinical or pathologic alterations, 10 fragments of cutaneous melanoma, and 9 fragments with lymph nodal metastasis were used. The samples originated from excisions of primary tumours or therapeutical lymphectomies. Patients were selected consecutively rather than randomly. They were informed of the research and asked to sign a consent form, authorizing the collection of their tissue samples.
After the surgical procedure, all samples were identified, snap-frozen, and stored in liquid nitrogen at minus 77 K in cryogenic Nalgene® vials, before FT-Raman spectra recording. For FT-Raman data collection, samples were brought to room temperature and kept moist in 0.9 % physiological solution to preserve their structural characteristics. They were then placed in a windowless aluminum holder for the Raman spectra collection. Soon after, samples were fixed in 10 % formaldehyde solution for further histopathological analysis. A FT-Raman Bruker RFS 100/S spectrometer was used with Nd:YAG laser at 1064 nm as the excitation light source. The laser power on the sample was kept at 300 mW while the spectrometer resolution was set to 4 cm-1. There were five collection points per sample. Spectra of normal skin and primary melanoma plus metastatic melanoma tissues were recorded with 100 and 150 scans, respectively. OPUS software (Bruker) was used to evaluate spectral characteristics in the region from 800 cm-1 to 1800 cm-1. Before the visual evaluation, all spectra were baseline corrected and vector normalized. For analysis, the Raman spectra were first pre-processed by correcting the base line and performing vector normalization using Minitab software (Minitab® 15.1.1.0).
Once the entire databases were established, the variables obtained from specific samples were investigated, and their capacity to differentiate the groups from a statistical point of view. At first, analysis of variance with a constant factor was used. This demonstrated that all variables showed an association with their own group. The next phase of the statistical analysis was the employment of a discriminating analysis, the results of which showed a significant differentiation between the three groups.
After measurement, the samples were fixed in a 10 % formalin solution and thereafter sent to the Pathological Anatomy Department of UNIFESP/EPM, for diagnostic confirmation. At this phase, the samples were regularly processed for the confection of histological slides by being stained with Hematoxilin and Eosin. Thus, each spectrum obtained could be related to a confirmed histological finding, enabling the formation of spectral groups to allow for univariate and multivariate analysis.
Results
Ten samples without clinical and histopathological alterations of different patients were evaluated; 105 spectra were obtained from this group, and used as the control group. All samples were histologically diagnosed as normal skin. Ten samples of the primary melanoma group and nine of the metastasis group were studied, of which, respectively, 140 and 126 spectra were obtained. All the spectra were grouped to proceed with the visual analysis, showing well-defined standards on the three groups, which indicated a differentiation among them.
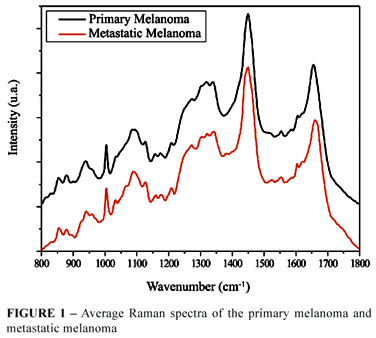
Some variables were investigated among the possible spectra from a statistical point of view. These variables were based on information regarding the biochemical compounds present in biological (skin) tissues analysed through the identification of the Raman spectra (Figure 1). This identification was based on Gniadecka et al.7 proposal.
With visual analysis, compared with normal skin, the melanoma spectra presented a decrease on the Amide I band, with spectral area distortion between 1640 and 1670 cm-1, which suggests change in the proteins molecular composition. On the 1475 to 1640 cm-1 region, an increase was observed in the intensity for the melanoma group, corresponding to proteins and lipids. In the Raman shift region of 1200 to 1300 cm-1, corresponding to Amide III and Lipids biomolecules, there was an increase in the intensity, and on the region representing the melanin molecule there was also an intensity increase (1300 to 1400 cm-1). In the region corresponding to the protein band between 920 to 980 cm-1, there was a decrease in the intensity. With the metastasis and normal skin group, the alterations on the bands and peaks were quite similar, although relevant.
The metastasis spectral group presented differences in relation to the primary melanoma group on the Amide I (1640 to 1670 cm-1) band, where the metastasis groups presented a decrease in the intensity in relation to the primary melanoma group, an increase in the Amide III and lipids (1222 to 1300 cm-1), as well as in the protein area between 900 and 940 cm-1. On the melanin, corresponding band there was an increase in the intensity of the metastasis group (1300 to 1380 cm-1). Figure 1 shows the spectra median of the melanoma and metastasis groups. Visually, the differences on the bands and peaks are discreet.
In order to proceed with the statistical analysis, the spectra were divided according to the histologic group to which they belonged. Inside each group, the spectra were united in sampling units. These units were constructed through the average calculation of the original spectra of each patient in order to summarize the data and organize their analysis. Ten sampling units for normal skin, ten sampling units for primary melanoma and nine sampling units for the metastasis were obtained.
Following the identification of the biochemical bands relevant to statistical analysis, the first step was to employ analysis of variance with a constant factor, revealing that all variables, or Raman vibrational modes, presented a significant association with each diagnostic group.
In order to better understand these results, Box plots were constructed for each vibrational mode analyzed (Bands 1 to 10, described in Table 1). These plots clearly showed the distribution of biomolecules within each group studied.
This set of figures, where the qualitative description of all vibrational modes was resumed, showed that the spectra of the Metastatic Melanoma group obtained the greatest intra-group difference for all analyzed items, followed by the Primary Melanoma and then Normal Skin groups. It is likely that this difference refers to the significant fluorescence generated by the tissue due to its high degree of pigmentation.
In the discriminating analysis, the results presented significant differentiations between the three groups studied; the following skin components (variables) deserve attention: Phenylalanine, DNA and Amide-I (Figure 2).
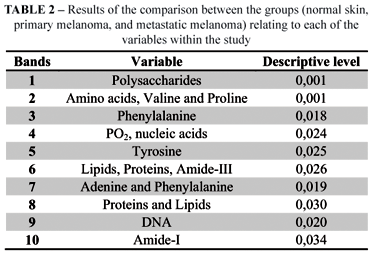
Finally, for each vibrational mode, multivariate analysis of the spectra was calculated. The multivariate analysis included the descriptive level calculation. These results are shown in Table 2.
The variables Phenylalanine, DNA and Amide I stood out at the differentiation of the three groups, in agreement with the classification functions detailed in Tables 3 and 4.
Focusing on the differentiation of the three sampling groups formed according to the characteristics obtained from the spectra, a discriminant analysis of the data was undertaken. The percentage of individuals correctly classified with this criterion was 93.1%, showing the efficiency of the analysis (Figure 3).
The spectra collected from the skin with no alterations (control group) were similar to those collected previously7,8. The Amide I band (1640-1700 cm-1) corresponds mainly to the stretch vibrational mode of the ligations C=O, whereas Amide III (1222-1362 cm-1), is nominated by the vibration in the ligations N-H plan and the stretch of C-N. Both regions thus reflect the secondary structure of the proteins7. The lipid structure is represented by the vibration of CH2 in the region between 1420 to 1500 cm-1. The bands between 855 and 937 cm-1 are typical of the collagen spectrum, are due to proline vibration, and stretch vibration of the C-C in the protein9.
Discussion
This technique did not require wide sample preparation or pretreatment. Using the FT-Raman technique employing light sources in the infrared region, the detection of weak Raman signals became easier due to fluorescence suppression. Moreover, the excitation in the near infrared, at 1064 nm, also minimizes sample photo degradation, allowing the employment of larger powered densities to compensate weak Raman signals generated by longer wavelengths.
In previously published data, authors compared the spectra of malignant and benign pigmented cutaneous: seborrheic keratosis, pigmented nevi, pigmented basal cell carcinoma and melanoma, and through visual analysis of the spectra, they could differ the lesions. Using the neural system, the sensitivity of melanoma diagnosis was 85 % and the specificity 99 %. The authors discussed, based on these observations, that the neoplastic transformation in the tissues, can start cellular changes no matter the kind of tissue involved and the alterations on the amide bands in the Raman spectra are attributed to the protein conformation10. In the melanoma group of this study, the alterations visually examined were similar, diminishing on the Amide I band, with spectral area distortion between 1640 and 1670 cm-1. However, in the metastasis groups, this reduction was higher when compared to the melanoma group. On the Amide III band, there was an increase in the intensity, and in the metastasis group, the increase was more relevant. It can be concluded that for a cell to become metastatic, a higher number of protein mutations need to occur.
In the present study, in the regions of Raman shifts 1540 to 1620 cm-1 and 1040 to 1100 cm-1, corresponding to DNAs vibrational mode, an increase of spectral intensity was observed metastatic melanoma and primary melanoma. In the first group, the intensity gain was stronger. Maybe, this intensity gain of the metastatic melanoma spectrum in these bands could be related to an increase in nucleic acids and protein synthesis in malignant tissues due to an elevated quantity of mitoses and, consequently, increased duplication of genetic material responsible for the proliferation of malignant cells compared with primary melanoma. Similar effect was observed by Mahadevan-Jansen et al.12, who conducted studies on molecular biology and energy transformation in the DNA of cancerous and pre-cancerous tissues using Raman spectroscopy.
The statistical analysis helped to decrease the subjectivity of the data observation and demonstrated that there is a higher heterogeny in the metastasis group, indicative that cell clones can differ themselves through mutations to the point that they become more invasive, and so, different from the primary melanoma cells. In the band corresponding to the melanin, the melanoma group presented higher intensity, showing a higher production of this pigment in the neoplasia studied; in the metastasis group, this increase was more intense at 1360 to 1400 cm-1, showing that metastatic cells are capable of producing pigment.
The cutaneous melanoma presents a high quantity of melanin due to characteristics of the neoplasia, making it difficult to use optical biopsy with FT-Raman spectroscopy, and therefore, further studies to enable the diagnosis of pigmented lesions are necessary.
Certainly, this study will serve, as the basis to use FT-Raman spectroscopy in the diagnosis of cutaneous melanoma in vivo, however further research is necessary.
Conclusion
FT-Raman spectroscopy is capable of differentiating melanoma from its metastasis, as well as from normal skin.
Received: February 25, 2010
Review: April 22, 2010
Accepted: May 26, 2010
Conflict of interest: none
Financial source: FAPESP and CNPq
How to cite this article
Oliveira AF, Santos IDAO, Cartaxo SB, Bitar RA, Enokihara MMSS, Martinho HS, Martin AA, Ferreira LM. Differential diagnosis in primary and metastatic cutaneous melanoma by FT-Raman spectroscopy. Acta Cir Bras. [serial on the Internet] 2010 Sept-Oct;25(5). Available from URL: http://www.scielo.br/acb
- 1. Bitar RA, Martinho HS, Tierra-Crioulo CJ, Ramalho NZ, Netto MM, Martin AA. Biochemical analysis of human breast tissues using Fourier-transform Raman spectroscopy. J Biomed Opt. 2006;11:1-8.
- 2. Hanlon EB, Manoharan R, Koo TW, Shafer KE, Motz JT, Fitzmaurice M, Kramer JR, Itzkan I, Dasari RR, Feld MS. Prospects for in vivo Raman spectroscopy. Phys Med Biol. 2000;45(2):R1-59.
- 3. Stone N, Kendall C, Shepherd N, Crow P, Barr, H. Near-infrared Raman spectroscopy for the classification of epithelial pre-cancers and cancers. J Raman Spectrosc. 2002;33:564-73.
- 4. Schut TCB, Wolthuis R, Caspers GJ. Real-time tissue characterization on the basis of in vivo Raman spectra. J Raman Spectrosc. 2002;33:580-5.
- 5. Riker AI, Glass F, Perez I, Cruse CW, Messina J, Sondak VK. Cutaneous melanoma: methods of biopsy and definitive surgical excision. Dermatol Ther. 2005;18:387-93.
- 6. Slater DN. Doubt and uncertainty in the diagnosis of melanoma. Histopathology. 2000;37:469-72.
- 7. Gniadecka M, Wulf HC, Mortensen NN, Nielsen OF, Christensen DH. Diagnosis of basal cell carcinoma by Raman spectroscopy. J Raman Spectrosc. 1997;28:125-9.
- 8. Williams AC, Barry BW, Edwards HG, Farwell DW. A critical comparison of some Raman spectroscopic techniques for studies of human stratum corneum. Pharm Res. 1993;10:1642-7.
- 9. Nijssen A, Schut TCB, Heule F, Caspers PJ, Hayes DP, Neumann MHA, Pupples GJ. Discriminating basal cell carcinoma from its surrounding tissue by Raman spectroscopy. J Invest Dermatol. 2002;119:64-9.
- 10. Gniadecka M, Philipsen PA, Sigurdsson S, Wessel S, Nielsen OF, Christensen DH, Hercogova J, Rossen K, Thomsen HK, Gniadecki R, Hansen LK, Wulf HC. Melanoma diagnosis by Raman spectroscopy and neural networks: structure alterations in proteins and lipids in intact cancer tissue. J Invest Dermatol. 2004;122:443-9.
- 11. Huang Z, Lui H, Chen XK, Alajlan A, McLean DI, Zeng H. Raman spectroscopy of in vivo cutaneous melanin. J Biomed Opt. 2004;9(6):1198-205.
- 12. Mahadevan-Jansen A, Mitchell MF, Ramanujam N, Malpica A, Thomsen S, Utzinger U, Richards-Kortum R. Near-infrared Raman spectroscopy for in vitro detection of cervical precancers. Photochem Photobiol. 1998;68(1):123-32.
Publication Dates
-
Publication in this collection
20 Sept 2010 -
Date of issue
Oct 2010
History
-
Received
25 Feb 2010 -
Reviewed
22 Apr 2010 -
Accepted
26 May 2010