Abstracts
PURPOSE: To compare increment of bone mineral density (BMD) with pamidronate, zoledronate and the isolated effect of proteinous diet in undernourished oophorectomized and non-oophorectomized female rats, besides validating BMD's indexes. METHODS: 60 young female Lewis rats were divided into five experimental groups and a control group, oophorectomized and non-oophorectomized. The administration of drugs were submitted to proteinous and aproteinous diets. The variables analyzed were weight, bone densitometry, histomorphometry and biochemical evolution. RESULTS: In weight evaluation, the first interval showed a statistically meaningful increase in oophorectomized sample. In densitometry evaluation, the first interval showed statistically meaningful decrease in four medicated groups and third showed a statistically meaningful increase in 2 non-oophorectomized groups. In laboratory evaluation, there were an increase of total proteins and globulin, decrease of alkaline phosphatase, phosphorus and calcium (except for the oophorectomized) in four medicated groups and increase of phosphorus and calcium in 2 not medicated groups. In histomorphometric evaluation, the oophorectomized groups had smaller increment of BMD. CONCLUSIONS: The pamidronate and zoledronate have shown effectives in the increment of BMD. The proteinous diet itself possesses therapeutic effect in BMD though not significant compared with medicated animals. The results of histomorphometry allow validating BMD's indexes in this experimental model.
Osteoporosis; Bone Density; Densitometry; Ovariectomy, Diphosphonates; Rats
OBJETIVO: Comparar o incremento de densidade mineral óssea (DMA) com pamidronato, zoledronato e o efeito isolado de dieta protéica em ratas desnutridas ooforectomizadas e não ooforectomizadas, além de validar índices de DMA. MÉTODOS: 60 ratas jovens Lewis foram divididas em cinco grupos experimentais e um grupo controle, com e sem ooforectomia. A administração das drogas foi submetida às dietas protéica e aprotéica. As variáveis analisadas foram peso, densitometria óssea, histomorfometria e evolução bioquímica. RESULTADOS: Na avaliação ponderal, o primeiro intervalo mostrou aumento estatisticamente significante nos grupos ooforectomizados. Na avaliação densitométrica, o primeiro intervalo mostrou diminuição estatisticamente significante nos quatro grupos medicados e o terceiro mostrou aumento estatisticamente significante nos dois não ooforectomizados. Na avaliação laboratorial, houve aumento de proteínas totais e globulina, diminuição de fosfatase alcalina, fósforo e cálcio (exceto nos ooforectomizados) nos quatro grupos medicados e aumento de fósforo e cálcio nos dois não medicados. Na avaliação histomorfométrica, os grupos ooforectomizados tiveram incremento menor de DMA. CONCLUSÕES: O pamidronato e o zoledronato se mostraram efetivos no incremento de DMA. A dieta protéica em si possui efeito terapêutico na DMA, porém menos significativa, comparada aos animais medicados. Os resultados da histomorfometria permitem validar os índices de DMA neste modelo experimental.
Osteoporose; Densidade Óssea; Densitometria; Ovariectomia; Difosfonatos; Ratos
7 - ORIGINAL ARTICLE
EFFECT OF DRUGS
Pamidronate and zoledronate effects in the increment of bone mineral density and histomorphometry in rats1 1 Research performed at the Central Animal Colony, Parana University Hospital, Londrina State University (UEL), Brazil. Part of Doctorate thesis. Tutor: Antonio Barbieri.
Efeitos do pamidronato e zoledronato no incremento da densitometria óssea e histomorfometria em ratos
Antônio Fiel Cruz JúniorI; Carlos BuchpiguelII; Roberto GuarnieroIII; Antonio BarbieriIV
IAssociate Professor, Department of Clinical Medicine, Faculty of Medicine of Uninga, Maringa-PR, Brazil. Acquisition and interpretation of data and technical procedures
IIFull Professor, Department of Radiology, Faculty of Medicine, University of Sao Paulo (USP), Brazil. Provided guidelines, collection of study informations, manuscript preparation
IIIFull Professor, Department of Orthopaedics and Traumatology, Faculty of Medicine, USP, Sao Paulo-SP, Brazil. Conception and design of the study, designed the protocol
IVAssociate Professor, Department of Diagnostic Imaging, Federal University of Sao Paulo, Brazil. Acquisition and interpretation of data, manuscript writing
Correspondence Correspondence: Antônio Barbieri Rua Higino Pellegrini, 37 05003-060 São Paulo - SP Brasil afiel@bs2.com.br
ABSTRACT
PURPOSE: To compare increment of bone mineral density (BMD) with pamidronate, zoledronate and the isolated effect of proteinous diet in undernourished oophorectomized and non-oophorectomized female rats, besides validating BMD's indexes.
METHODS: 60 young female Lewis rats were divided into five experimental groups and a control group, oophorectomized and non-oophorectomized. The administration of drugs were submitted to proteinous and aproteinous diets. The variables analyzed were weight, bone densitometry, histomorphometry and biochemical evolution.
RESULTS: In weight evaluation, the first interval showed a statistically meaningful increase in oophorectomized sample. In densitometry evaluation, the first interval showed statistically meaningful decrease in four medicated groups and third showed a statistically meaningful increase in 2 non-oophorectomized groups. In laboratory evaluation, there were an increase of total proteins and globulin, decrease of alkaline phosphatase, phosphorus and calcium (except for the oophorectomized) in four medicated groups and increase of phosphorus and calcium in 2 not medicated groups. In histomorphometric evaluation, the oophorectomized groups had smaller increment of BMD.
CONCLUSIONS: The pamidronate and zoledronate have shown effectives in the increment of BMD. The proteinous diet itself possesses therapeutic effect in BMD though not significant compared with medicated animals. The results of histomorphometry allow validating BMD's indexes in this experimental model.
Keywords: Osteoporosis. Bone Density. Densitometry. Ovariectomy, Diphosphonates. Rats.
RESUMO
OBJETIVO: Comparar o incremento de densidade mineral óssea (DMA) com pamidronato, zoledronato e o efeito isolado de dieta protéica em ratas desnutridas ooforectomizadas e não ooforectomizadas, além de validar índices de DMA.
MÉTODOS: 60 ratas jovens Lewis foram divididas em cinco grupos experimentais e um grupo controle, com e sem ooforectomia. A administração das drogas foi submetida às dietas protéica e aprotéica. As variáveis analisadas foram peso, densitometria óssea, histomorfometria e evolução bioquímica.
RESULTADOS: Na avaliação ponderal, o primeiro intervalo mostrou aumento estatisticamente significante nos grupos ooforectomizados. Na avaliação densitométrica, o primeiro intervalo mostrou diminuição estatisticamente significante nos quatro grupos medicados e o terceiro mostrou aumento estatisticamente significante nos dois não ooforectomizados. Na avaliação laboratorial, houve aumento de proteínas totais e globulina, diminuição de fosfatase alcalina, fósforo e cálcio (exceto nos ooforectomizados) nos quatro grupos medicados e aumento de fósforo e cálcio nos dois não medicados. Na avaliação histomorfométrica, os grupos ooforectomizados tiveram incremento menor de DMA.
CONCLUSÕES: O pamidronato e o zoledronato se mostraram efetivos no incremento de DMA. A dieta protéica em si possui efeito terapêutico na DMA, porém menos significativa, comparada aos animais medicados. Os resultados da histomorfometria permitem validar os índices de DMA neste modelo experimental.
Descritores: Osteoporose. Densidade Óssea. Densitometria. Ovariectomia. Difosfonatos. Ratos.
Introduction
As number and gravity of spine and femoral fractures increase, mainly caused by osteoporosis, it has been noted the need for an efficient and short-term treatment. These fractures cause huge social and economical damages to individuals who have less work capability, the elderly, stricken by this disease. Another important aspect is that these individuals usually need longer recovery time.
Due to the seriousness of the issue, the SBOT - Brazilian Society of Orthopedics and Traumatology has been broadcast a national campaign for preventing osteoporosis since 1998.
New drugs for osteoporosis treatment are being tested, many of them developed in various research centers worldwide1,2. The progress in the treatment of osteoporosis is supported by an improvement in medicine treatment that takes place with the findings of drugs that promote bone resorption decrease.
Sodium fluoride is one of the available agents for osteoporosis treatment that stimulates bone tissue formation. Other medicines, such as pamidronate, zoledronate, residronate and alendronate act by reducing bone resorption.
The pamidronate and zoledronate effects in reducing bone resorption level are an issue one should focus on intravenous pamidronate and zoledronate capacity to act by decreasing bone resorption with and without the aggravation of proteinous malnourishment is the issue that will be evaluated and measured by experiment through criteria of weight, densitometry, biochemistry and histomorphometry3,4.
The objectives of this study are to evaluate pamidronate and zoledronate effects in the increase of bone mineral density in malnourished oophorectomized and non-oophorectomized female rats. Beyond this, to evaluate the isolated effect of proteinous diet on bone mineral density's increment in malnourished female rats, and validate bone mineral density indexes obtained by bone densitometry at the end of the treatment with pamidronate, zoledronate and proteinous diet by analysis of bone histomorphometry in different groups of animals studied.
Methods
Female Lewis rats, albines, in a total of sixty young (ages under 120 days) healthy, initially weighting between 140-260g, were raised at the Central Animal colony in Parana University Hospital, belonging Londrina State University.
These animals were initially divided into six groups (control group and A, B, C, D and E experimental groups), each one with ten animals, divided into 12 cages with five animals each. Cages were made of opaque polyethylene and closed with stainless steel lid, as a grid, covered with wood shavings, changed twice a week. The light period was twelve hours with stable temperature, humidity and noise levels. Ten animals (from control group) were given standardized food (balanced diet for rats) and ad libitium distilled water. The other fifty animals (experimental groups) underwent aproteinous diet and ad libitum distilled water. The animals were numbered from 1 to 60 on its ears, according to the laboratory animal's patterning.
Cages were identified into 2 groups: the ones of the experimental groups A, B, C, D and E which received aproteinous diet for 3 weeks (components described below); and the ones of the control group which received balanced diet for rats and include pamidronate and zoledronate. Components of aproteinous diet1: MgSO4(94,5g); NaCl (240g); KCl (120g); FeSO4(NH4)2SO4, 6H2O (21,6g); CaSO4 ou CaCl2(1,2g); MnSO4(1,2g); ZnSC4 (1,5g); K (0,063g); CaHSO4 (720g); benzoic acid (30g) and corn starch q. s. p. (30.000g).
Study's exclusion criteria were animals with any type of disease, besides restriction of food other than established diets; it was mainly rejected the administration of other drugs and liquids different from those of the experiment. This total experimental period was 89 days.
The animals were randomly divided into groups, where weight, and densitometric, biochemical and histomorphometric criteria were evaluated, measured and compared:
- Control group: comprised 10 animals that received a normal lab diet along the whole study, and were after evaluated according to this study parameters;
- Experimental group A: comprised 10 oophorectomized animals that received aproteinous diet along three weeks, and by the end they received pamidronate medication, in dose of 1 mg/kg (weight) and were after evaluated according to this study parameters.
- Experimental group B: comprised 10 oophorectomized animals that received aproteinous diet along three weeks and by the end, they were given zoledronate in dose of 0.04 mg/kg and were later evaluated by these study parameters.
- Experimental group C: comprised 10 animals that received aproteinous diet along three weeks, and by the end were given zoledronate, in dose of 0.04 mg / kg and were later evaluated by these study parameters.
- Experimental group D: comprised 10 animals, that received aproteinous diet during three weeks, and after were given pamidronate, in dose of 1 mg/ kg, later assessed by this study parameters;
- Experimental group E: comprised 10 animals, that received aproteinous diet during three weeks, without medication administration, and were later evaluated by these study parameters.
Oophorectomy and drugs administration criteria
The animals were anesthetized for oophorectomy. Anesthesia was intraperitoneally injected, after inhalatory sulfuric ether sedation. It was used a mixture of ketamine, an anesthetic drug, and myorelaxant xilazine in proportion of 1:1.
Bone densitometry was carried out only after sedation with inhalatory sulfuric acid during 1 minute. Oophorectomy was performed bilaterally, with the animal on decubitus and by central incision. Only one rat from the pamidronate group died of inflammatory complications.
Pamidronate and zoledronate were provided by a pharmaceutical laboratory. After this, it was prepared for administration on animals at the Bioterium of the Post Graduation Laboratory at College of Medicine in Londrina State University. Doses dilutions were 1 mg/kg of pamidronate and 0.04 mg/ kg of zoledronate. The administration of the first dose was given shortly after the end of aproteinous diet (21 days), and the second dose occurred after each one's biological half-life (pamidronate at 38 days and zoledronate at 55 days).
Evaluation criteria and measurement methods
Study's animals were evaluated according to weight evolution, densitometry, histomorphometry, albumin's blood dosage, total proteins and globulin, and calcium, phosphorus and blood alkaline phosphatase of dosages. All animals were weighted three times: at the first day of the study, after pamidronate's drug effect (eighth week) and after zoledronate's drug effect (thirteenth week). Animals' body weight's evolution was checked through Helmac HM 5000® electronic balance, by grams.
All animals femoral bone mineral density measured by a LUNAR densitometer (DPX ALFA), set at a private clinic, the Clinilab Imagem. It was used a software in a high resolution mode for small animals.
Bone mineral densities of right femoral shafts were measured in grams per cm2. Demarcation was performed by delimited areas (ROIs) and quantified by specific software. After processing, the graphics were analyzed in order to compare bone mineral density's values between various medicated groups and control group.
Histomorphometrical evaluations were performed using an image analysis system (Kontron Electronic 300). The workstation consisted of a Zeiss ifferent light microscope, a colored video camera (Sony CCD - Iris), images scanner, a microcomputer with Pentium processor 133MHz, IBM-PC compatible, operating in Windows 95-32 bits.
Images were digitalized through a specific software for image analysis (Kontron Electronic 300), allowing data recording with text processor (Microsoft Word) and in an electronic worksheet (Microsoft Excel). By using these softwares, it was possible to set the analysis treatment, interpretation, measurement values of the structures, with all the variables and data automatic distribution, generated by the images workstation analyzer for electronic worksheets and text processors, automatically.
Images calibration was performed amplified four times. This quantification was set at the smallest special unit, the pixel. Calibration factor (CF) was automatically calculated in pixels and as such it was used by the calculation program in micrometers, according with calibration. Images were assessed by Kontron 300 (Zeiss), program which allowed area determination. This tool allowed to identify the structure mark and automatize its identification for future readings, like filters usage and appliance of multiple macros for various tasks' automation.
Histological cuts were processed through image analyzing system, by using a 4x objective and 10x ocular. Images were captured by a video camera and digitalized by the computer's special digitalization plaque, used for images optimization. After image delivery, it was used a graphical resource for making the structures to be quantified. By using the mouse, one has delimited bone area to be measured by square micrometer. All these measures were saved at computer's specific program, for future statistical analysis.
Albumin, total proteins and globulin's blood dosage was carried out at the Post Graduation Laboratory of the University Hospital at Londrina State University. It was proceeded in a SELECTRA automation machine. Albumin was dosed by bromocresol green method (BCG), and total proteins by a biuret method. Results were measured in grams by deciliter of blood and normal results are respectively: 3.40 - 5.00 g/dl and 6.40 - 8.20 g/dl.
Globulin is the result of albumin's total protein subtraction and the relationship between albumin and globulin's division. Results were measured in grams by deciliter of blood and normal results are, respectively: 2.00-3.50 g/dl and 1.50-2.50 g/dl. Among lab investigations of bone metabolism, it was performed the measurement of calcium seric concentrations, phosphorus and alkaline phosphatase.
The process occurred in a SELECTRA automation machine. Calcium was dosed by ortho-cresolphtalein (o-CPC) method and measured in milligram by deciliter; and phosphorus was dosed by ultraviolet molybdate method and measured in milligram / deciliter. For your turn, alkaline phosphatase by Bowers and McComb kinetic method and measured by international units per liter. Normal results are respectively: 8.50 - 10.1 mg/dl, 2.50 - 4.90 mg/dl and 50 - 136 iu/l.
Statistical analysis
Weight, densitometric, laboratory and histomorphometric variables were analyzed in order to establish the following comparisons: 1) Group A x control group; 2) Group B x control group; 3) Group C x control group; 4) Group D x control group and 5) Group E x control group. For variable's description, one used arithmetic mean and patter deviation shown in tables according to study's medications. The hypothesis formulated at study's planning was checked by repeated measures analysis of variance technique with a classification factor.
Tests were performed in a 5% significance level which means that tests were considered meaningful when p < 0.05, and processed on software SAS (Statistical Analysis System).
Results
The three weight variables were achieved on the first day of study (weight 1), on the 55th day, after the effect of medications first doses (weight 2), and on the 89th day, after second doses of medications effect (weight 3), as shows Figure 1.
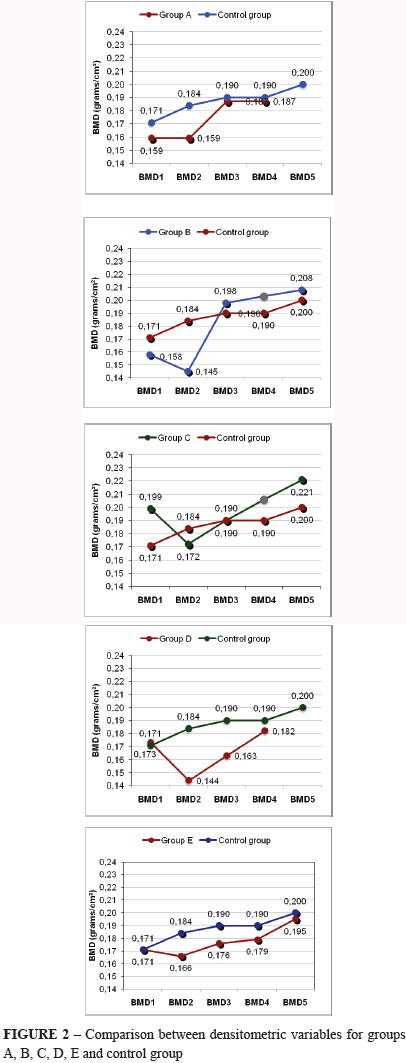
The five densitometric variables were achieved on the first day of study (BMD 1); on the 21st day, after ending aproteinous diet (BMD 2); on the 38th day after 1st doses effect of pamidronate (BMD 3). In order of succession, on the 55th day, after 2nd doses effect of pamidronate and 1st zoledronate doses (BMD 4) and on the 89th day, after effect of zoledronate 2nd doses effect (BMD 5), as shows Figure 2.
Results of experimental groups in comparison with control group for weight and densitometric variables are shown on Table 1.
The two laboratorial variables were carried out at the beginning of medication use (21st day) and at the end of the response to medications used (89th day). Table 2 shows the results of albumin dosages, total proteins, globulins, relation albumin/globulin, alkaline phosphatase, phosphorus and calcium of experimental groups in comparison to the control group.
Histomorphometric variable was performed at the end of the whole study (89th day). The areas were compared by using the Student's T test, because the Test by Bartlett detected homogeneity of variances. Results showed no significant differences in all the comparisons made.
Discussion
In this experiment, the species under study was female rats under four months old, considered young adults, although they were still growing, it was considered a slow growth. Moreover, other researchers reported having used animals with a similar age in previous studies5. It is worth to highlight that the uniform general characteristics of all animals at the beginning of the study, which, in fact allowed a longitudinal and vertical groups analysis. Baron et al.6 showed that animals at this age present a degree of bone remodeling / transformation similar to an adult human. The studies of animals, which have a more stable bone development (from six months to one year old) are not so practical, due to the longer maintenance period of these animals. Moreover, bone transformations are slower.
The time necessary to observe bone alterations after performing oophorectomy was around three weeks. Some authors did not need a period of more than four weeks7, whereas other researchers were able to observe that only after six months8. However, in the great majority of studies, the time needed was just a few weeks9. It is an interesting model, since it is known that bone transformations in human beings are slower and bone loss occurs throughout many years, which hinders or limits some clinical studies.
The weight of the animals increased in all groups, as expected, since they were undergoing a growing phase. There was no significant difference among the groups, a fact that contrasts with the literature data that demonstrated higher weight gains in the oophorectomized animals10. This is a fact that minimizes bone loss but does not prevent it11.
BMD analysis was carried out by densitometry, a technique has already been used in human beings for more than one decade. Such method is considered more practical and direct than other methods previously used, and it is broadly used for osteoporosis diagnosis. Several studies have shown that such technique is highly accurate and also precise in animals12,13. The first densitometers, such as the single photon-absorptiometry and the dual photon-absorptiometry (dual-energy x-ray absorptiometry), whose sources of energy were the gadolinium, have already shown superiority in relation to conventional x-rays14. However, the development of densitometers, whose source is ray-X (DEXA), has supplanted the original equipments15.
In principle, one could establish a more relevant analogy between femur's proximal area and osteoporosis. However, it was shown a conflicting result regarding the characteristics of bone loss. Bagi et al.16 observed a cortical thinning in this area, as it presents smaller periosteal formation but larger subendosteal resorption, whereas Shinoda et al.9 have not obtained the same results. Another criticism made, is the fact that this is a rather narrow region, thus hindering its interpretation. It was chosen not to carry out the analysis of isolated vertebras, because they are very small areas.
The variation coefficient (VC), index used to measure the precision of the densitometric technique, revealed results similar to those obtained by other researchers. However, there is a great variation of this index (0.8% to 6.4%), which the authors attribute to factors such as type of decubitus used to study the animal, whether the analysis of the bone segment is made in vivo or in vitro. Besides, there are other differences regarding the use of one or more observers17. Studies with dissected bone in vitro show lower values compared to the ones obtained in vivo. Such fact is, probably, due to the interference of the soft tissues, difficulty in delimitating the areas of interest, mainly in the femur region, where there is the interference of interlinked bones, such as tibia and hip. Such facts do not exist when the bones are analyzed in vitro. However, the analysis in vitro does not allow longitudinal studies, impairing, for instance, the evaluation of the drug response. Nevertheless, the study in vivo makes its analysis more significant, thus making the study more dynamic.
In the control group one observed in all studied phases a bone mineral density increase, probably related to the fact that animals were still growing and to weight increase with proteinous diet. Whether in the group of oophorectomized animals as in the ones submitted to aproteinous diet, bone mineral density decrease on the area of cortical bone (medial femur) was seen, thus achieving one of our purposes since oophorectomy and aproteinous diet take to bone loss.
Various studies were performed showing bone loss; on metaphysary regions in some important works; in others on lumbar spine what makes evident a specific differen of each bone segment18. In the oophorectomized rats group, medicated with zoledronate, it was observed meaningful increase of bone mineral density in all studied phases. At the end of the study, zoledronate caused a bone mass increase when compared with pamidronate. At the end of the study histomorphometric data analyzed did not differ among groups, demonstrating that the medicated groups results were the same of the control group.
We could obtain, thus, significant correlations among densitometric, biochemical and histomorphometric parameters. This fact is important for its practical implications.
Conclusions
The pamidronate and zoledronate have shown effectives in the increment of BMD. The proteinous diet itself possesses therapeutic effect in BMD though not significant compared with medicated animals. The results of histomorphometry allow validating BMD's indexes in this experimental model.
Received: September 11, 2010
Review: November 12, 2010
Accepted: December 14, 2010
Conflict of interest: none
Financial source: none
- 1. Bartl R, Frisch B. Osteoporosis: an epidemic overwhelming proportions. In: Bartl R, Frisch B. Osteoporosis: diagnosis, prevention, therapy. 1ed. Berlin: Springer Verlag; 2004. p.1-4.
- 2. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7-29, 2000: highlights of the conference. South Med J. 2001;94(6):569-73.
- 3. Lindsay R. Hormones and bone health in postmenopausal women. Endocrine. 2004;24:223-30.
- 4. Orimo H, Schacht E. The D-hormone analog alfacalcidol: the pioneer beyond the horizon of osteoporosis treatment. J Rheumatol Suppl. 2005;76:4-10.
- 5. Kalu DN, Liu CC, Salermo, Hollis B, Echon R, Ray M. Skeletal response of ovariectomized ratos to low and high doses of 17 beta-estradiol. Bone Miner. 1991;14(3):175-87.
- 6. Baron R, Tress R, Vignery A. Evidence of sequencial remodeling in rat trabecular bone: morphology, dynamic histomorphometry and changes during skeletal maturation. Anat Rec. 1984;208:137-45.
- 7. Pastoriau P, Chomel A, Bonnet J. Specific evaluation of localized bone mass and bone loss in the rat using dual-energy absortometry subregional analysis. Osteoporosis Int. 1995;5:143-9.
- 8. García-Moreno C, Calvo OM, Herrero S, Martín E, Suquía B, San Román JI, Martín M, García-Talavera JR, Calvo JJ, del Pino J. Heterogenous decrease of bone mineral density in vertebral column of ovariectomized rats. Bone. 1995;16(4 Suppl):295S-300S.
- 9. Shinoda H, Adamek G, Felix R, Fleisch H, Schenk R, Hagan P. Structure-activity relationship of various bisphosphonates. Calcif Tissue Int. 1997;35:87-99.
- 10. Wronski TJ, Lowry PL, Walsh CC, Ignaszewski A. Skeletal alterations in ovariectomized rats. Calcif Tissue Int. 1985;37:324-8.
- 11. Roudebush RE, Magee DE, Benslay DN, Bendele AM, Bryant HU. Effect of weight manipulation on bone loss due to ovariectomy and the proctective effects of estrogen in the rat. Calcif Tissue Int. 1993;53:61-4.
- 12. Ammann P, Rizzolo R, Slosman D, Bonjour JP. Sequencial and precise in vivo measurement of bone mineral density in rats using dual energy X-ray absorptiometry. J Bone Miner Res. 1992;7:311-6.
- 13. Griffin MG, Kimble R, Hopper W, Pacifici R. Dual-energy x-ray absortptiometry of the rat: ifferen, precision and measurement of bone loss. J Bone Miner Res. 1993;8:795-800.
- 14. Safadi M, Shapira D, Leichter I, Reznick A, Silbermann M. Ability of different techniques of measuring bone loss in aging female rats. Calcif Tissue Int. 1988;42:375-82.
- 15. Yamaguchi H, Kushida K, Yamazaki K, Inove T. Assessment of spine bone mineral density in ovariectomized rats using DXA. J Bone Miner Res. 1995;10:1033-9.
- 16. Bagi CM, DeLeon E, Ammann P, Rizzoli R, Miller SC. Histoanatomy of the proximal femur in rats: impact of ovariectomy on bone mass, structure, and stiffness. Anat Rec. 1996;245(4):633-44.
- 17. Rozenberg S, Vandromme J, Neve J, Aguilera A, Muregancuro A, Peretz A, Kinthaert J, Ham H. Precision and accuracy of in vivo bone mineral measurement in rats using dual-energy X-ray absorptiometry. Osteoporos Int. 1995;5(1):47-53.
- 18. Sato M, McClintock C, Kim J, Turner CH, Bryant HU, Magee D, Slemenda CW. Dual-energy X-ray absortiometry of raloxifene effects on lumbar vertebrae and femora of ovariectomized rats. J Bone Miner Res. 1994;9:715-24.
Publication Dates
-
Publication in this collection
22 Mar 2011 -
Date of issue
Apr 2011
History
-
Received
11 Sept 2010 -
Accepted
14 Dec 2010 -
Reviewed
12 Nov 2010