Abstracts
PURPOSE: To study the antitumor action of Tabebuia avellanedae in experimentally induced colon carcinogenesis by azoxymethane in mice. METHODS: Fifty (n=50) mice were divided into five groups: in group I azoxymethane (AOM) was administered, in Group II - β-lapachone, in group III - vehicle (diluent) and in group IV - vehicle + AOM and finally in group V - β-lapachone + AOM. RESULTS: It was observed the presence of aberrant crypt foci in all animals of groups I and IV, 50% in group II and 90% in group V. CONCLUSION: The β-lapachone extracted from the Tabebuia avellanedae showed no protective effect of lesions induced by azoxymethane in colon of mice.
Carcinogens; Colorectal Neoplasms; Azoxymethane; Tabebuia; Naphthoquinones; Mice
OBJETIVO: Estudar a ação antitumoral da Tabebuia avellanedae (Ipê-Roxo) na carcinogênese colônica induzida experimentalmente pelo azoximetano em camundongos. MÉTODOS: Foram utilizados 50 camundongos divididos em 5 grupos: grupo I administrado Azoximetano (AOM); grupo II - β-lapachona; grupo III - veículo (diluente); grupo IV - veículo + AOM; e grupo V - β-lapachona + AOM. RESULTADOS: Observou-se presença de focos de criptas aberrantes em todos os animais dos grupos I e IV, 50% no grupo II e 90% no grupo V. CONCLUSÃO: A β-lapachona extraída da Tabebuia avellanedae não apresentou efeito protetor das lesões induzidas pelo azoximetano em cólon de camundongos.
Carcinógenos; Neoplasias Colorretais; Azoximetano; Ipê-Roxo; Naftoquinonas; Camundongos
9 - ORIGINAL ARTICLE
EXPERIMENTAL ONCOLOGY
Study of the antineoplastic action of Tabebuia avellanedae in carcinogenesis induced by azoxymethane in mice1 1 Research performed at the Health and Development Postgraduate Program, Federal University of Mato Grosso do Sul (UFMS), Campo Grande, Brazil. Part of Master degree thesis. Tutor: Ricardo Dutra Aydos.
Estudo da ação antineoplásica da Tabebuia avellanedae (Ipê-Roxo) na carcinogênese induzida pelo azoximetano em camundongos
Roberta Alves HigaI; Ricardo Dutra AydosII; Iandara Schettert SilvaIII; Rondon Tosta RamalhoIV; Albert Schiaveto de SouzaV
IMD, Master in Health and Development Postgraduate Program, UFMS, Campo Grande-MS, Brazil. Experimental study and histopathological examinations
IIPhD, Associate Professor, Department of Surgery, UFMS, Campo Grande-MS, Brazil. Guidance and experimental design
IIIPhD, Associate Professor, Department of Surgery, UFMS, Campo Grande-MS, Brazil. Veterinary assistance in the experimental study
IVFellow PhD degree, Health and Development Postgraduate Program, UFMS, Campo Grande-MS. Master in Biotechnology, UCDB, Brazil. Experimental study and histopathological examinations
VPhD, Associate Professor, Department of Morphophysiology, UFMS, Campo Grande-MS, Brazil. Statistical analysis
Correspondence Correspondence: Ricardo Dutra Aydos Av. Senador Felinto Muller, s/n 79080-190 Campo Grande - MS Brasil ricadoaydos@gmail.com
ABSTRACT
PURPOSE: To study the antitumor action of Tabebuia avellanedae in experimentally induced colon carcinogenesis by azoxymethane in mice.
METHODS: Fifty (n=50) mice were divided into five groups: in group I azoxymethane (AOM) was administered, in Group II - β-lapachone, in group III - vehicle (diluent) and in group IV - vehicle + AOM and finally in group V - β-lapachone + AOM.
RESULTS: It was observed the presence of aberrant crypt foci in all animals of groups I and IV, 50% in group II and 90% in group V.
CONCLUSION: The β-lapachone extracted from the Tabebuia avellanedae showed no protective effect of lesions induced by azoxymethane in colon of mice.
Keywords: Carcinogens. Colorectal Neoplasms. Azoxymethane. Tabebuia. Naphthoquinones. Mice.
RESUMO
OBJETIVO: Estudar a ação antitumoral da Tabebuia avellanedae (Ipê-Roxo) na carcinogênese colônica induzida experimentalmente pelo azoximetano em camundongos.
MÉTODOS: Foram utilizados 50 camundongos divididos em 5 grupos: grupo I administrado Azoximetano (AOM); grupo II - β-lapachona; grupo III - veículo (diluente); grupo IV - veículo + AOM; e grupo V - β-lapachona + AOM.
RESULTADOS: Observou-se presença de focos de criptas aberrantes em todos os animais dos grupos I e IV, 50% no grupo II e 90% no grupo V.
CONCLUSÃO: A β-lapachona extraída da Tabebuia avellanedae não apresentou efeito protetor das lesões induzidas pelo azoximetano em cólon de camundongos.
Descritores: Carcinógenos. Neoplasias Colorretais. Azoximetano. Ipê-Roxo. Naftoquinonas. Camundongos.
Introduction
The Tabebuia avellanedae a typical tree of the Brazilian savannah, has been used for years in folk medicine as treatment for various diseases1. In the '60s and '70s, there was significant investment in studies of substances extracted from this plant, especially lapachol and β-lapachone, which started to be commercialized for use in adjuvant chemotherapy for treatment of leukemia1,2.
Lapachol is an important naphthoquinone obtained from the bark of Tabebuia avellanedae, and its derivative, a quinone β-lapachone, exists mainly in this type of ipe. Several pharmacological activities have been attributed to lapachol and its semi-synthetic derivatives, such as antimicrobial and antifungal action, cercaricide, molluscicide, leishmanicidal, trypanocidal, antimalarial, antiinflammatory, anticancer, anti-ulcerating, contraceptive and againstenteroviruses1,3-5.
Lapachol and its derivatives have very important properties in the induction of apoptosis, by acting on topoisomerases enzymes I and II, and any change in the balance between these enzymes is sufficient to induce apoptosis - which is the programmed cell death. The biochemistry of induction is not totally clear, however, the information available in the literature can point to an action of inducing apoptosis through inhibition of topoisomerase-DNA1 complex.
The inhibitory action on repairing systems seems to be the only mode of action of β-lapachone, as fungi, which do not express the topoisomerases, are also inhibited by this quinone1. Apparently, the β-lapachone has a critical role as xenobiotic on more than one intracellular target, and not only topoisomerase I. Another hypothesis was the attack by β-lapachone to specific points in the catalytic cycle that expresses the action of topoisomerase I, for example, the checkpoints G1 and S, by action other than a simple merge. It was recently reported that β-lapachone induces apoptosis of malignant epithelial cells, human glioma, and human retinal pigments1.
There are few scientific studies that used lapachol and its derivatives as antineoplastic agents, especially in the study of colon cancer6. The main objective of this study was to evaluate the antitumor action of lapachol in experimentally induced colonic carcinogenesis by azoxymethane in mice.
Methods
The procedures were approved by the Ethics Committee in the use of animals, protocol No. 71/2007.
Fifty 8-week-old male mice were use, average weight 20 grams, from UFMS Central Animal Colony. They were held at the Laboratory of Carcinogenesis, Department of Surgical Clinic of UFMS in boxes with polypropylene with galvanized cap, standard size for six animals and adjustment period of 7 days.
The 50 animals were randomly divided into five groups according to protocol:
Group I: 10 (n) mice were submitted to two doses of azoxymethane, one in the first week and another in the second;
Group II: 10 (n) mice were submitted to β-lapachone in daily doses for 6 weeks;
Group III: 10 (n) mice were submitted to vehicle (diluent) of β-lapachone in daily doses for 6 weeks;
Group IV: 10 (n) mice were submitted to vehicle (diluent) of β-lapachone in daily doses for 6 weeks and two doses of azoxymethane, one in the first week and another in the second week;
Group V: 10 (n) mice were submitted to β-lapachone in daily doses for six weeks and two doses of azoxymethane, one in the first week and another on Monday.
Administration of substances
The beta-lapachone was diluted in solution (vehicle) of 20% ethanol, and was administered daily by oral gavage at 25mg/kg for 6 weeks and in the control group it was administered vehicle with the same volume and frequency. Azoxymethane at a dose of 15mg/Kg weight / dose diluted in 0.9% saline solution subcutaneously in two doses during the experiment - 1st and 2nd weeks.
Euthanasia
Mice were identified, weighed and euthanasia was performed with thiopental infusion at a dose of 150mg/kg, intraperitoneally. Then, in a supine position, it was made a median incision of the skin, extending from the xiphoid process to pubis; dieresis of abdominal wall plans, identification of the ileocaecal region and repair with forceps; total colectomy after section of the entire length of mesocolon, excluding the cecum and rectum, which were not used in the experiment.
Histopathology
The preparation of the piece to histopathology was made by opening the colon in the anti-mesenteric border, washing the mucosa with a ringer solution; colon fixation on cardboard and placed in formol lampooned in 10%, for 24 hours, then, inclusion in paraffin, diereses with microtome in proximal edge, at 3 cm level using the entire length of the width of the rectum in longitudinal section, and placed on glass slides for histological processing with hematoxylin and eosin (HE).
We performed histological and morphological study, categorizing them into: hyperplastic aberrant crypt focus: presents a moderate hypercellularity, with slight enlargement of light and abnormal form, dysplastic aberrant crypt focus: besides the enlargement of light, there is enough tortuosity of the crypt that is markedly hypercellular and pseudostratification; microadenoma: severely hypercellular, presence of cell differentiation, hyperchromasia and loss of cellular polarity7.
Statistical analysis
Statistical analysis was performed using the chi square test and the z test, considering a significance level of 5%.
Results
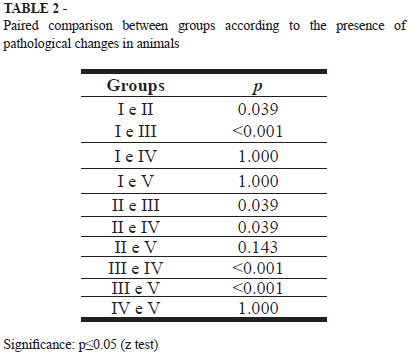
After histopathological analysis, we found the result shown in Table 1. It was considered positive the presence of hyperplastic or dysplastic aberrant crypt focus. It was not found the presence of microadenomas.
According to the presence or absence of histopathological criteria, we observed a significant difference (p<0.05) between groups I and II, and III I, II and III, II and IV, III and IV and III and V. Among groups I and IV, and V I, II and V and IV and V no significant differences were observed.
Discussion
The focus of aberrant crypt has been used for early detection of factors influencing colorectal carcinogenesis8,9, found in rodents treated with carcinogens, and in humans10,11,12. Experimental evidence supports the hypothesis that this type of lesion is pre-neoplastic11-16, consisting of crypts that have expanded lumen and abnormal form10,16 probably evolved in time with proliferative, hyperplastic, dysplastic phenomena, and finally, small adenomas9,10.
There was need to hold two control groups of β-lapachone vehicle, since the diluent is alcohol (ethanol 20%)2, from which group III, treated only with vehicle, showed no histopathological changes, as seen in literature17. Group IV showed similar changes to group I due to the use of tumor promoter (AOM).
Lapachol and its derivative β-lapachone are naphthoquinones with therapeutic potential against some types of cancer2. The β-lapachone exhibits in vitro activity against many types of different lineages of cells, especially malignant cancers of lung, breast, colorectal, prostate, melanoma and leukemia. Despite the broad spectrum of bioactivities, the mechanisms of action of β-lapachone in experimental models are not well delineated. And yet, there is a strong interest focused on the commercial use of the substance, which can be demonstrated by several patents granted in recent years involving this quinone, probably as a guarantee for commercial use in the future1.
In vitro study it is known that β-lapachone has activity against approximately 60 human tumor cell lines, among them colon cancer18. It also induces apoptosis of breast cancer cells; it has considerable anticancer activity in multiple myeloma cells, inhibition of NO synthase, which may lead to new findings on the development of anti-inflammatory; at low concentrations it can induce cell death of prostate cancer; it is cytotoxic against various tumor cells, including activity against cells resistant to other chemotherapics1.
In group II we found hyperplastic aberrant crypt foci in 50% of cuts. Studies show that Lapachol require increased levels to achieve a cytotoxic effect, but in larger doses the major toxic effects of Lapachol arise, such as nausea, vomiting, reversible increase of prothrombin time. The clinical study of Lapachol has not been determined yet as it hasn't achieved a high plasma level which apparently produce a toxic effect. In the study by Dinnen et al.21, it is demonstrated that the concentration of Lapachol, required to induce cell differentiation is lower than necessary to promote a cytotoxic effect17-21.
This study only found aberrant crypt foci of hyperplastic and dysplastic types; the first type was found in all animals with histopathological changes, and the second was uncommon in all groups. Most of the work makes only descriptive evaluation of histopathology slides stained by using hematoxylin and eosin, and present evaluation results with immunohistochemistry, genetics (DNA) or biochemicals1,10,11,13,15,16,22.
Only one animal in group V showed no pathological changes, which brought no significant changes in reducing aberrant crypt foci in mice that were induced with azoxymethane colon tumor and treated with β-lapachone. Thus, there was evidence that β-lapachone extracted from the Tabebuia avellanedae inhibits the appearance of lesions in the colon of mice when administered in AOM-induced model.
Conclusions
β-lapachone extracted from Tabebuia avellanedae, at the concentration used, showed no protective effect of lesions induced by azoxymethane in the colon of mice. The β-lapachone was considered capable of inducing lesions in the colon of mice, as compared to control. And the azoxymethane is an excellent model to induce aberrant crypt foci in hyperplastic and dysplastic colon of mice.
Acknowledgments
Prof. Dr. Vitor Francisco Ferreira for having given the extract of Tabebuia avellanedae for this work, Prof. Gilberto Gonçalves Facco for helping in histological evaluation and Profa. Isabella Saliba Pereira for revising the English language.
Received: September 20, 2010
Review: November 16, 2010
Accepted: December 14, 2010
Conflict of interest: none
Financial source: none
- 1. Silva MN, Ferreira VF, Souza MCBV. Um panorama atual da química e da farmacologia de naftoquinonas, com ênfase na β-lapachona e derivados. Quim Nova. 2003;26(3):407-16.
- 2. Guerra MO, Mazoni ASB, Brandão MAF, Peters VM. Toxicology of lapachol in rats: embryolethality. Rev Bras Biol. 2001;61(1):171-4.
- 3. Oliveira MF, Lemos TLG, Mattos TAS, Santiago GMP, Braz-filho R. New enamine derivatives of lapachol and biological activity. An Acad Bras Cienc. 2002;74(2):211-21.
- 4. Goel RK, Pathak NK, Biswas M, Pandey VB, Sanyal AK. Effect of lapachol, a naphthaquinone isolated from Tectona grandis, on experimental peptic ulcer and gastric secretion. J Pharm Pharmacol. 1987;39(2):138-40.
- 5. Carmo Lagrota MH, Wigg MD, Aguiar AN, Pinto AV, Pinto Mdo C. Antiviral activity of naphthoquinones. I. Lapachol derivatives against enteroviruses. Rev Latinoam Microbiol. 1986;28(3):221-5.
- 6. Choi BT, Cheong J, Choi YH. beta-Lapachone-induced apoptosis is associated with activation of caspase-3 and inactivation of NF-kappaB in human colon cancer HCT-116 cells. Anticancer Drugs. 2003;14(10):845-50.
- 7. Nambiar PR, Nakanishi M, Gupta R, Cheung E, Firouzi A, Ma XJ, Flyn C, Dong M, Guda K, Levine J, Raja R, Achenie L, Rosenberg DW. Genetic signatures of high-and-low-risk aberrant crypt foci in a mouse model of sporadic colon cancer. Cancer Res. 2004;64(18):6394-6401.
- 8. Demarzo MM, Garcia SB. Exhaustive physical exercise increases the number of colonic preneoplastic lesions in untrained rats treated with a chemical carcinogen. Cancer Lett. 2004;216(1):31-4.
- 9. Murillo G, Choi JK, Pan O, Contantinou AI, Mehta RG. Efficancy of garbanzo and soybean flour in suppression of aberrant crypt foci in the cólons of CF-1 mice. Anticancer Res. 2004;24(5A):3049-55.
- 10. Montenegro MA, Negrette MS, Lertora WJ, Catuogno MS. Focos de criptas displásicas inducidas com 1,2-dimetilhidrazina em intestino gruesso de ratas tratadas com molibdeno y tungsteno. Rev Vet. 2003;14(1):14-9.
- 11. Di Gregorio C, Losi L, Fante R, Modica S, Ghidoni M, Pedroni M, Tamassia MG, Gafa L, Ponz de Leon M, Roncucci L. Histology of aberrant crypt foci in the human colon. Histhopatology. 1997;30(4):328-34.
- 12. Shimpo K, Chihara T, Beppu H, Ida C, Kaneko T, Nagatsu T, Kuzuya H. Inhibition of azoxymethane-induced aberrant crypt foci formation in rat colorectum by whole leaf Aloe arborescens Miller var. Nataliensis Berger. Phytother Res. 2001; 15(8):705-11.
- 13. Yoshimi N, Morioka T, Kinjo T, Inamine M, Kaneshiro T, Shimizu T, Suzui M, Yamada Y, Mori H. Histological and immunohistochemical observations of mucin-depleted foci (MDF) stained with Alcian blue, in rat colon carcinogenesis induced with 1,2-dimethylhydrazine dihydrochloride. Cancer Sci. 2004;95(10):792-7.
- 14. Nobuoka A, Takayama T, Miyanishi K, Sato T, Takanashi K, Hayashi T; Kukitsu T, Sato Y, Takahashi M, Okamoto T, Matsunaga T, Kato J, Oda M, Azuma T, Niitsu Y. Glutathione-S-transferase P1-1 protects aberrant crypt foci from apoptosis induced by deoxycholic acid. Gastroenterology. 2004;127(2):428-43.
- 15. Fujita K, Matsuda E, Sekine K, Iigo M, Tsuda H. Lactoferrin modifies apoptosis-related gene expression in the colon of the azoxymethane-treated rat. Cancer Lett. 2004;213(1):21-9.
- 16. Takeda J, Kitajima K, Fujii S, Horiuchi H, Hori H, Chibana Y, OkuyamaT Tominaga K; Ichikawa K, Ono Y, Teramoto T, OhkuraY, Imura J, Shinoda M, Chiba T, Sakamoto C, Kawamata H, Fujimori T. Inhibitory effects of etodolac, a selective COX-2 inhibitor, on the occurrence of tumors in colitis-induced tumorigenesis model in rats. Oncol Rep. 2004;11(5):981-5.
- 17. de Sandoval NA, Rodríguez CP, de Martínez NR. Tumors caused by methylcholanthrene and lapachol. Follow-up of development with cytology. Acta Physiol Pharmacol Ther Latinoam. 1996;46(4):257-64.
- 18. Block JB, Serpick AA, Miller W, Wiernik PH. Early clinical studies with Lapachol (NSC-11905), Cancer Chemother Rep 2. 1974;4(4):27-8.
- 19. Santana CF, Silva AAF. Primeiras observações com o emprego de lapachol em pacientes humanos portadores de neoplasias malignas. Rev Inst Antibiot (Recife). 1981;8(1/2):89-94.
- 20. Sieber SM, Mead JAR, Adamson RH. Pharmacology of antitumor agents from higher plants. Cancer Treat Rep. 1976;60(8):1127-39.
- 21. Dinnen RD, Ebisuzaki K. The search for novel anticancer agents: a differentiation-based assay and analysis of a folklore product. Anticancer Res. 1997;17(2A):1027-33.
- 22. Renou SG, Asis SE, Abasolo MI, Bekerman DG, Bruno AM. Monoarylhydrazones of alpha-lapachone: syntheis, chemical properties and antineoplasric activity. Pharmazie. 2003;58(10):690-5.
Publication Dates
-
Publication in this collection
21 Mar 2011 -
Date of issue
Apr 2011
History
-
Received
20 Sept 2010 -
Accepted
14 Dec 2010 -
Reviewed
16 Nov 2010