Abstracts
PURPOSE: To evaluate different protocols to isolate stem cells from ovine umbilical cord blood and adipose tissue. METHODS: There were used 5 samples of umbilical blood and 5 samples of perirenal adipose tissue from 10 female sheep. All the samples were obtained through surgery, to harvest aseptic samples. There were used 3 protocols for obtainment and culture of umbilical cord blood stem cells and 4 protocols for ovine adipose tissue stem cells. RESULTS: It was possible to observe only one successful protocol for the obtainment of umbilical cord blood stem cells. When analyzing the techniques used to obtain adipose tissue stem cells, only one of the methods was effective as well. Through colony forming unit assay, there were obtained 58 colonies of cells after seven days in culture. Flow citometry tests revealed the cells were positive to CD44 and exhibited negative reaction to CD38, CD45, CD41/61. These cells showed a growth curve with very well defined phases LOG, LAG and PLATEAU. This phases are typically seem in mesenchymal stem cells growth curves. CONCLUSIONS: The isolation and culture of mesenchymal stem cells from ovine umbilical cord blood are complex and request more detailed assays. Stem cells from fat tissue sheep showed mesenchymal characteristics, according to their cell growth curve, ability to origin colonies of fibroblastoid cells and positive reactivity with the antibody CD44 by flow citometry.
Stem Cells; Umbilical Cord; Adipose Tissue; Sheep
OBJETIVO: Testar diferentes protocolos para o isolamento de células tronco a partir de sangue de cordão umbilical e tecido adiposo de ovinos. MÉTODOS: Foram utilizadas cinco amostras de sangue de cordão umbilical e cinco amostras de tecido adiposo perirrenal de 10 fêmeas de ovelha. A coleta das amostras foi realizada através de procedimento cirúrgico para coleta do material de forma mais asséptica possível. Foram realizados três protocolos de isolamento e cultivo das células-tronco do cordão umbilical e quatro protocolos para o isolamento e cultivo das células-tronco de gordura de ovinos RESULTADOS: Somente um dos protocolos utilizados para o isolamento das células-tronco de cordão umbilical foi efetivo. Dos quatro protocolos utilizados para isolamento das células-tronco de gordura, da mesma forma, apenas um obteve sucesso. Foi realizado o ensaio de unidades formadoras de colônias destas células, sendo contadas 58 colônias ao final de sete dias. Na citometria de fluxo essas células mostraram-se positivas para CD44 e negativas para CD38, CD45, CD41/61. Estas células apresentaram curva de crescimento com fases de LOG, LAG e PLATEAU bem definidas, características das curvas de crescimento das células-tronco de origem mesenquimal. CONCLUSÕES: O isolamento e cultivo das células-tronco mesenquimais do cordão umbilical de ovinos é de difícil realização, exigindo maiores ensaios e estudos profundos. Células tronco do tecido adiposo de ovelhas demonstraram características mesenquimais, de acordo com a curva de crescimento, habilidade de formação de colônias, células com morfologia fibroblastóide e reação positiva ao anticorpo CD44.
Células-Tronco; Cordão Umbilical; Tecido Adiposo; Ovinos
4 - ORIGINAL ARTICLE
MODELS, BIOLOGICAL
Protocols for obtainment and isolation of two mesenchymal stem cell sources in sheep1 1 Research performed at Postgraduate Program of Anatomy in Domestic and Wild Animals, Department of Veterinary Surgery, Faculty of Veterinary Medicine and Animal Science, Sao Paulo University (USP), Brazil.
Protocolos para obtenção e isolamento de células tronco mesenquimais de duas fontes em ovinos
Leandro FadelI; Brunno Rosa VianaII; Matheus Levi Tajra FeitosaIII; Anna Caroline Mazeto ErcolinII; Kelly Cristine Santos RoballoII; Juliana Barbosa CasalsIV; Naira Caroline Godoy PieriIV; Flávio Vieira MeirellesV; Daniele dos Santos MartinsV; Maria Angélica MiglinoVI; Carlos Eduardo AmbrósioV
IMaster, Surgery Department, Faculty of Veterinary Medicine and Animal Science, USP, Sao Paulo, Brazil. Main author, surgery and cell culture
IIGraduate student, Basic Sciences Department, Faculty of Animal Sciences and Food Engineering, USP, Pirassununga-SP, Brazil. Cell culture and flow citometry
IIIFellow PhD degree, Department of Surgery, Faculty of Veterinary Medicine and Animal Science, USP, Sao Paulo, Brazil. Anesthesia and surgery
IVFellow Master degree, Surgery Department, Faculty of Veterinary Medicine and Animal Science, USP, Sao Paulo, Brazil. Cell culture and postoperative care
VPhD, Associate Professor, Faculty of Animal Sciences and Food Engineering, USP, Pirassununga-SP, Brazil. Cell culture protocols, manuscript writing and English review
VIPhD, Chairwoman, Department of Surgery, Anatomy Unit, Faculty of Veterinary Medicine and Animal Science, USP, Sao Paulo, Brazil. Manuscript writing and English review
Correspondence Correspondence: Matheus Levi Tajra Feitosa Cid. Univ. Armando de Salles Oliveira Setor de Anatomia Av.Prof. Dr. Orlando Marques de Paiva, 87 05508-000 São Paulo - SP Brasil Tel: (55 11)3091-7805 mtajra@gmail.com
ABSTRACT
PURPOSE: To evaluate different protocols to isolate stem cells from ovine umbilical cord blood and adipose tissue.
METHODS: There were used 5 samples of umbilical blood and 5 samples of perirenal adipose tissue from 10 female sheep. All the samples were obtained through surgery, to harvest aseptic samples. There were used 3 protocols for obtainment and culture of umbilical cord blood stem cells and 4 protocols for ovine adipose tissue stem cells.
RESULTS: It was possible to observe only one successful protocol for the obtainment of umbilical cord blood stem cells. When analyzing the techniques used to obtain adipose tissue stem cells, only one of the methods was effective as well. Through colony forming unit assay, there were obtained 58 colonies of cells after seven days in culture. Flow citometry tests revealed the cells were positive to CD44 and exhibited negative reaction to CD38, CD45, CD41/61. These cells showed a growth curve with very well defined phases LOG, LAG and PLATEAU. This phases are typically seem in mesenchymal stem cells growth curves.
CONCLUSIONS: The isolation and culture of mesenchymal stem cells from ovine umbilical cord blood are complex and request more detailed assays. Stem cells from fat tissue sheep showed mesenchymal characteristics, according to their cell growth curve, ability to origin colonies of fibroblastoid cells and positive reactivity with the antibody CD44 by flow citometry.
Key words: Stem Cells. Umbilical Cord. Adipose Tissue. Sheep.
RESUMO
OBJETIVO: Testar diferentes protocolos para o isolamento de células tronco a partir de sangue de cordão umbilical e tecido adiposo de ovinos.
MÉTODOS: Foram utilizadas cinco amostras de sangue de cordão umbilical e cinco amostras de tecido adiposo perirrenal de 10 fêmeas de ovelha. A coleta das amostras foi realizada através de procedimento cirúrgico para coleta do material de forma mais asséptica possível. Foram realizados três protocolos de isolamento e cultivo das células-tronco do cordão umbilical e quatro protocolos para o isolamento e cultivo das células-tronco de gordura de ovinos
RESULTADOS: Somente um dos protocolos utilizados para o isolamento das células-tronco de cordão umbilical foi efetivo. Dos quatro protocolos utilizados para isolamento das células-tronco de gordura, da mesma forma, apenas um obteve sucesso. Foi realizado o ensaio de unidades formadoras de colônias destas células, sendo contadas 58 colônias ao final de sete dias. Na citometria de fluxo essas células mostraram-se positivas para CD44 e negativas para CD38, CD45, CD41/61. Estas células apresentaram curva de crescimento com fases de LOG, LAG e PLATEAU bem definidas, características das curvas de crescimento das células-tronco de origem mesenquimal.
CONCLUSÕES: O isolamento e cultivo das células-tronco mesenquimais do cordão umbilical de ovinos é de difícil realização, exigindo maiores ensaios e estudos profundos. Células tronco do tecido adiposo de ovelhas demonstraram características mesenquimais, de acordo com a curva de crescimento, habilidade de formação de colônias, células com morfologia fibroblastóide e reação positiva ao anticorpo CD44.
Descritores: Células-Tronco. Cordão Umbilical. Tecido Adiposo. Ovinos.
Introduction
Mesenchymal stem cells, first obtained from bone marrow aspirate, have a great plasticity and are able to differentiate into bone, cartilage, fat tissue and even muscle1. Recent studies have shown the isolation of such cells from fetal tissue and blood2. Mesenchymal stem cells from umbilical cord can be used for autologous or heterologous transplant3 due to their high proliferative potential3,4. Advantages of their use include transplantation with no risk to the donor, easy and safety during the collection and a reduction in the rejection potential5-7. Researches with cord blood stem cells still face some difficulties, mainly during the isolation process8,9. However, studies have reported the possibility of cord blood stem cells represent a new source of multipotent cells from non-hematopoietic tissues 7.
Chang et al.10 isolated human umbilical cord blood mesenchymal cells, which showed two different phenotypic characteristics but a similar osteogenic, adipogenic and chondrogenic capacity5. Differentiation into hepatocytes11 and neurons12 have also been carried out. Fuchs et al.13 and Jager et al.14 described the feasibility of adipogenic and osteogenic differentiation of cartilage and cord blood stem cells from sheep.
The first report with the isolation of fat cells were performed by Rodbell15 and Rodbell and Jones16 from samples of fat mice. Later, this isolation procedure was modified for use in human models17. The collection of samples of adipose tissue, which was occasionally performed during other surgical procedures, has become more feasible since the advent of plastic surgery and liposuctioned. These cosmetic procedures are currently an inexhaustible source of adipose stem cells. Studies by Zuk et al.18,19 revealed that adipose tissue is a source of multipotent stem cells with characteristics of cell marking and expression of genes with unique features that differ from mesenchymal stem cells in some respects. This research was performed by flow cytometry and tests of differentiation and biochemical and molecular tests taken from human fat cells.
Due easy protocol for sample collection, cell isolation and the great potential for tissue differentiation, adipose tissue could be a good source for application in biotechnology20.These cells can be used in the field of regenerative medicine. The ovine model for research with the isolation and culture of mesenchymal stem cells is important for pre-clinical orthopedic21, reactions to xenotransplantation15 and uterine surgery22.
In order to use this animal model in preclinical tests applied to regenerative medicine, this study aimed to purpose an adequate protocol for collect and isolation of stem cells from umbilical (Cord blood and fat) of sheep, in order to obtain a suitable cell line with characteristics of proliferative and differentiation potential for use in cell therapy.
Methods
Ten female sheep Santa Ines were used acquired in Sao Paulo and kept on the premises of the Faculty of Veterinary Medicine, University of Sao Paulo-FMVZ / USP. All animals underwent the same handling conditions, fed twice daily (massive) and 200g of concentrated food (food for sheep) once a day.
The animals were fasted for 24 hours (solid) and 12 hours (liquid). After this period, 0.3 mg/kg hydocloride of meperidine associated to 2 mg/kg midazolam maleate intravenously in the same syringe were administered as premedication (MPA). Five minutes later, the animals were induced to anesthesia with propofol 3mg/kg associated with 5 mg/kg of fentanyl intravenously, intubated with endotracheal tube size 10G and maintained with inhalational anesthetic isoflurane. All anesthetic procedures were performed with cardiac monitoring and pulse oximetry. The animals submitted to the trial received 0.2 mg/kg of meloxicam and 25 mg/kg of sodium dipyrone for immediate analgesia (thereafter maintained with 0.1 m / kg meloxicam once a day for about five days). Prophylactic antibiotic based on penicillin at a dose of 20,000 IU / kg therapy was performed in all animals for 5 days.
To collect the cord blood there was adopted a surgical procedure for intrauterine fetal blood sampling (Figure 1).
We collected five samples of material from fetuses and sheep, and 20 mL of blood was aspirated from each animal. The blood obtained by puncturing the umbilical artery, was transported in heparinized tubes until the Stem Cell Laboratory, Department of Surgery, Sector of Anatomy of Domestic and Wild Animals FMVZ/USP. The procedure of cordocentesis included puncture of the umbilical artery from the externalization of the term pregnant uterus and exposure of the umbilical cord. Related to animals which underwent a cesarean section, we adopted the same procedure of cordocentesis. However, after collecting the blood, the placenta was ruptured the umbilical cord was clamped and incised suture attached to the uterus and abdominal muscles. For the isolation of mesenchymal stem cells from umbilical cord were performed three different tests. The first protocol involved the dilution of the fraction of blood for the cultivation solution with DMEM-H (1:1), addition of Ficoll HypaqueTM Plus and subsequent centrifugation to obtain a pellet of mononuclear cells. After this process it was observed the formation of a whitish buffy coat, which was isolated, washed with saline and centrifuged again. This procedure was repeated three times to ensure the elimination of Ficoll HypaqueTM Plus - toxic to the cells. The pellet obtained after the spin was resuspended and then counts were performed in cells with the aid of a Neubauer chamber. The cells were plated in 25cm2 culture bottles and incubated in a temperature of 37ºC and 5% CO2. The second set protocol included the dilution of the fraction of blood for the cultivation in solution with DMEM-H (3:1) and subsequent filtration with a 100um mesh, followed by centrifugation. The pellet formed was resuspended in 10mL of PSB and received Ficoll HypaqueTM Plus. This procedure was followed by a further centrifugation, from which was possible to observe the formation of a discrete whitish halo. This buffy coat, after being isolated was twice washed with PSB and centrifuged. The pellet obtained after the spin was resuspended and then counts were performed in cells with the aid of a Neubauer chamber. There were plated 2.0 x 106 mononuclear cells in a 25cm2 culture bottle. The material was incubated under the same conditions described for the first protocol. For the third protocol, there was made a dilution of the fraction of blood for the cultivation solution with DMEM-H (3:1) and centrifuged. The pellet was resuspended in 10mL composed of DMEM-H and centrifuged again (procedure was repeated three times). After the pellet had been resuspended and the number of cells had already been estimated, there were plated 2.5 x 104 mononuclear cells in a 25cm2 culture bottle. The incubation was performed under the same conditions described for the first protocol. After 72 hours, the cells that had not adhered to the bottom of the bottle were discarded. To study the ability of in vitro expansion of cord blood cells there were used three different culture media. The material isolated by each of the protocols described above was distributed equally into three bottles of cultivation. The culture medium used were: Alpha-MEM, DMEM and DMEM-High-Low, all supplemented with 10% fetal calf serum (GIBCO) and 1% penicillin and streptomycin. Each bottle, previously identified and filled with a kind of medium, received the cells isolated from one of three protocols established and was incubated under conditions described below.
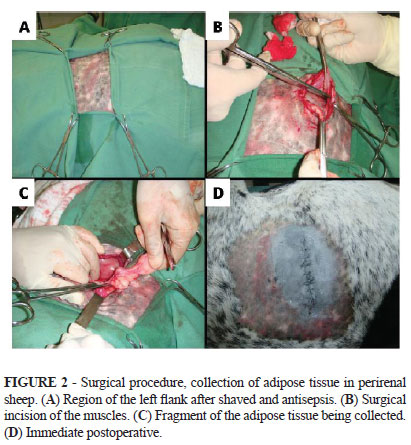
The adipose tissue was collected from the left perirenal region. Access to this region was made through a paracostal incision (Figure 2).
Five samples were placed in sterile Falcon tubes and sent to the same laboratory mentioned above. There were used four different protocols for isolation of mesenchymal stem cells from adipose tissue fragments. In the first protocol, the samples were washed with PBS and subsequently held the dissociation chemistry of tissue samples. In these fragments there was added a solution of PBS containing 0.05% collagenase and this step was followed by incubation for 90 minutes at 37ºC. After this period, the cell suspension was filtered through a 100um mesh and subsequently centrifuged. The pellet formed was resuspended in culture medium and washed three times. The medium used was MEM ALPHA supplemented with 10% fetal bovine serum, and 0,5uL/mL of amikacin and 0,5uL/mL of nystatin. The cells were then plated on culture bottles and incubated in a temperature of 37ºC and 5% CO2. The second protocol differs from the first in just one point: the incubation time of the fragments, after its dissociation, was reduced to half (45 minutes). The third protocol differs from the second by the fact that the enzyme trypsin was used instead of collagenase. The fourth set protocol is similar to the first, except for the fact that there were also plated small fragments of adipose tissue.
There were used around 2x105 cells to begin the study of growth curve. The amount of cells was estimated by the following formula: NC/NS x 104 (NC= number os counted cells; NS= number of squares in Neubauer chamber). The number of cells was measured during 18 days (from 48 to 48 hours). In order to verify the ability of colony forming units of fibroblastoid cells, 1 x 103 mesenchymal stem cells from fat tissue of sheep were plated in 60cm2 dish with culture medium ALPHA-MEM (supplemented with 15% fetal bovine serum Hyclone®, 1% penicillin-streptomycin , 1% MEM-NEAA, 1% L-glutamine) in the heater with medium replacement of 48-48h for 10 days. In order to determine the Colony Forming Unit-fiblobastic (CFU-F) assay, we adopted the following procedures: removing the medium and washing the culture dish with PBS free of Ca2+ and Mg2+, incubating the material with paraformol 4% for 30 minutes for fixation of the cells and finally staining the material with crystal violet. After an incubation time of 10 minutes, the culture dish was washed with distilled water and it was possible to analyze the colonies. The temperature of incubation was the normal room temperature.
For flow cytometry (FACScalibur, BD) there were used around 105 cells in the passage one, per tube. The cells were distributed equally in the tubes and ressuspended in 200µL of PBS for subsequent addition of markers. We used the following markers: CD38, CD41/CD61 associated, CD44 and CD45. Propidium iodide was used to test the viability of cells in the cytometer. After 15 minutes of incubation in a recipient without light contact, each tube was centrifuged at 1800 RPM for three minutes and after that, washed with 1 mL of PBS. For the following analysis in the cytometer, the resulting pellet was resuspended in 200µL of PBS with 1% formaldehyde to each tube.
Results
The procedure adopted did not cause problems for the fetus and female as the pregnancy went to term without the need for veterinary intervention. There were neither postpartum, nor transoperative or postoperative (in cases where there was a caesarean) complications.
The cultivation of cells from the umbilical cord was performed by using different protocols. Protocols 1 and 2, which contained Ficoll HypaqueTM Plus for cell separation by density gradient, showed no satisfactory results. Blood cells derived from protocol 3 demonstrated good results regarding the cultivation and expansion of mesenchymal stem cells. These cells remained in culture during 15 days, incubated (37.5ºC and 5% CO2) with three different culture media indicated. The cells were plated at concentrations of 2.5 x 105, 5 x 105 and 1 x 106 and the culture medium were replaced after 24, 72, 96 and 120 hours of plating.
Monitoring the process of growth and adhesion of cord blood stem cells revealed that the cultures were composed of numerous small circular non-adherent cells, and a large part was composed of red blood cells. It was not possible to observe the adhesion and hence expansion of mesenchymal stem cells.
Further tests, using a protocol with the culture medium alpha-MEM, allowed a rapid cell growth and fibroblastoid features (Figure 3), but after the first passage the culture has not progressed.
The collection of adipose tissue from the perirenal region was performed by surgical procedure. Due to the inherent characteristics of selected ovine specie a little amount of cells could be collected because of the storage in the subcutaneous fat.
The cell culture of adipose tissue analyzed with the four established protocols showed the following finds: The first protocol used in isolation achieved satisfactory outcomes. It was observed that the cells adhered to plastic after 24 hours in primary culture, were refractile and also showed a variety of formats ranging from the triangular, dendritic and rounded. From the study of growth curve of mesenchymal stem cells from fat sheep it was possible to assemble the following graph (Figure 4), which shows the stages of LOG, which runs from the day 26-02 until 06-03; PLATEAU, which runs from 06-03 to 14-03, and the LAG phase which runs from 14-03 to 16-03.
The study of colony forming units (CFU) showed that mesenchymal stem cells were able to form colonies of fibroblastic cells (CFU-F). After the material was treated with crystal violet there was possible to count over 58 colonies of cells (Figure 5). The protocol used to this assay was modified due to the high proliferative behavior of the cells. There were plated 102 instead of 103 cells on a 100cm2 dish. There were no medium renewal during the seven (instead of fourteen) days of harvest.
In flow cytometry there was positive staining for CD44 antibody with a positive reactivity for mesenchymal cells. And negativity for other markers: CD38, CD45, CD41/61 (Figure 6).
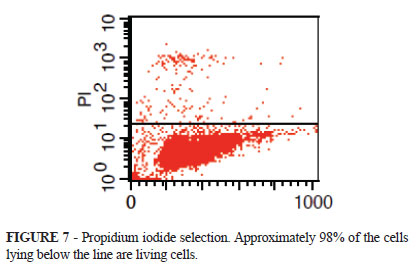
The viability through propidium iodide selection in this experiment indicated 97.83% of lively cells (Figure 7).
According to the second protocol could be verified degree of adhesion of cells isolated. However, after 240 hours the cells, which showed characteristic spherical and refractile, died or showed differentiation into unwanted cell types. The use of trypsin to the detriment of collagenase (protocol 3) did not enable cell adherence and these had to be viable after 10 days of cultivation.
Satisfactory results could be observed in the cultivation of the cells with tissue fragments. There was cellular adhesion and after 360 hours of cultivation, the presence of cells with fibroblastoid morphology and rapid growth. After appropriate degree of confluence was held the replating of cells, however, the material was lost by fungal contamination.
Discussion
The first protocol, drawn up according to Feitosa23 (bone marrow of sheep), was ineffective since it did not enable cell adherence and viability. The second protocol, based on methods used by Fuchs et al.14 showed formation of a halo of white and red blood cell supernatants that persisted even after washing the material. A second sample underwent the same protocol provided adherent cells, but without the typical fibroblastoid morphology. These results corroborate the studies of Secco et al.5. These authors obtained only 10% of success in the isolation of mesenchymal cells from human umbilical cord from 10 samples.
The third protocol was established according to studies by Zucconi et al.24, who isolated mesenchymal cells from cord blood of dogs. On the first attempt, adhered cells with fibroblastoid morphology became unviable after 30 days of cultivation. The successful isolation of cells with morphology of mesenchymal stem cells obtained from the second sample was subjected to protocol 3. The cells in culture showed expansion and confluence, but became unworkable after the first pass. These results corroborate data Secco et al.5.
The procedures for collection and isolation of cells from adipose tissue were made from research by Knippenber et al.25. These authors have proposed techniques to harvest and isolation of fat cells in the goat model. For the present study there were used similar protocols in the ovine model, due to anatomical and physiological similarities found in sheep and goat. Whereas the accumulation of adipose tissue in sheep is located in intra-abdominal viscera, unlike other species that have fat concentrated on special sub-cutaneous.
The procedure of collecting cells from the perirenal region was reported by Knippenberg et al.25. His execution did not represent very complexity and there were no post-operative complications.
The success of isolation in first protocol allowed to obtaining a new cell concentration for the establishment of colony forming units of fibroblastoid cells. It was crucial to the successful formation of these colonies. These modifications argued with that published by Yoshimura et al.26.
The positive staining for CD44 antibody in flow cytometry argued with that published by Barry et al.27 and Martínez et al.28. These studies showed that CD44 is an antibody that characterizes mesenchymal cells for human and sheep fat tissue derived cells. In case of rabbits, CD44 is negative, according to researches published by Martínez et al.28.
The second protocol was established from a reduced time for enzymatic digestion by collagenase. It was possible to observe some adhesion of cells to the surface of the bottle, though the cells were not very refractile and therefore unviable.
In the third protocol there was used the enzyme trypsin to chemical cleavage fragments of adipose tissue. Cells that adhered to the surface of the bottle were more refractile, but unviable due to some degree of differentiation into an osteogenic lineage of cells.
The fourth protocol, different from the others by the presence of adipose tissue explants, resulted in a population of cells adherent to the surface of the cylinder, showing high levels of refringence, fibroblastoid morphology and growth characteristic of mesenchymal cells. However, the cells did not survive after the first pass.
Conclusions
The method used to umbilical blood collection and isolation was innocuous to fetus and pregnant sheep. The surgical technique used to gather adipose tissue samples from kidney area was very well succeeded, without any complication.
The efficiency found while isolating Cord blood stem cells was higher when compare with dog models as previous report by our studies. The isolation and explants of fat cells was more efficient if compared with other assays, all evaluated under aseptic standards. Stem cells from fat sheep showed up mesenchymal characteristics, according to their growth curve, ability to form colonies of fibroblastoid cells and positive reactivity with the antibody CD44.
Acknowledgments
We would like to thank FAPESP/CNPq for providing the necessary funds and the fellowships for this study; the Postgraduate Program of the Anatomy in Domestic and Wild Animals from Faculty of Veterinary Medicine and Animal Science, Sao Paulo University (FMVZ-USP) and National Institute of Science and Technology in Stem Cells and Cell Therapy (INCTC). We also would like to thank Profa. Dra. Aparecida Maria Fontes, Profa. Patricia Bonini Palma and Alícia Greyce Turatti Pessolato from the Regional Center of Hemotherapy of the Faculty of Medicine of Ribeirao Preto, Sao Paulo University (FMRP-USP) by the advisor with flow citometry assays.
Received: December 15, 2010
Review: February 17, 2011
Accepted: March 18, 2011
Conflict of interest: none
Financial source: CAPES/CNPq
- 1. Pountos I, Giannoudis PV. Biology of mesenchymal stem cells. Injury. 2005;36 Suppl 3:S8-S12.
- 2. Romanov YA, Svintsitkaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cell: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21(1):105-10.
- 3. Cohen Y, Nagler A. Umbilical cord blood transplantation - how, when and for whom? Blood Rev. 2004;18:167-9.
- 4. Dimitriou H, Matsouka C, Perdikoyanni C, Stiakaki E, Bolonaki I, Lydaki E, Koumantakis E, Kalmanti M. Phenotypic characteristics of blood cord hemopoietic cell. Leuk Res. 1998;22(8):755-8.
- 5. Secco M, Zucconi E, Vieira NM, Fogaça LLQ, Cerqueira A, Carvalho MDF, Jazedje T, Okamoto OK, Muotri AR, Zatz M. Multipotent stem cells from umbilical cord: cord is richer than blood. Stem Cells. 2008;26:146-50.
- 6. Waldlow RC, Porter DL. Umbilical cord transplantation: where do we stand? Biol Blood Marrow Transplant. 2002;8:637-47.
- 7. Barker JN, Wagner JE. Umbilical cord transplantation: current practice and future innovations. Crit Rev Oncol Hematol. 2003;48:35-43.
- 8. Mareschi K, Biasin E, Piacibello W, Aglietta M, Madon E, Fagioli F. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001;86:1099-100.
- 9. Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal 'stem'cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368-74.
- 10. Chang Y, Tseng C, Hsu L, Hsieh T, Hwang S. Characterization of two populations of mesenchymal progenitor cells in umbilical cord blood. Cell Biol Int. 2006;30:495-9.
- 11. Kang X, Zang W, Bao L, Li D, Xu X, Yu X. Differentiating characterization of human umbilical cord blood-derived mesenchymal stem cells in vitro. Cell Biol Int. 2006;30:569-75.
- 12. Park K, Lee Y, Kang K. In vitro neuronal and osteogenic differentiation of mesenchymal stem cells from human umbilical cord blood. J Vet Sci. 2006;7(4):343-8.
- 13. Fuchs JR, Hannouche D, Terada S, Zand S, Vacanti JP, Fauza DO. Cartilage engineering from ovine umbilical cord blood mesenchymal progenitor cells. Stem Cells. 2005;23:958-64.
- 14. Jager M, Bachmann R, Scharfstadt A, Krauspe R. Ovine cord blood accommodates multipotent mesenchymal progenitor cells. In Vivo. 2006;20(2):205-14.
- 15. Rodbell M. Metabolism of isolated fat cells. II. The similar effects of phospholipase c (clostridium perfringens alpha toxin) and of insulin on glucose and amino acid metabolism. J Biol Chem. 1966;241:130-9.
- 16. Rodbell M, Jones AB. Metabolism of isolated fat cells. 3. The similar inhibitory action of phospholipase c (clostridium perfringens alpha toxin) and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. J Biol Chem. 1966;241:140-2.
- 17. Van RL, Bayliss CE, Roncari DA. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J Clin Invest. 1976;58:699-704.
- 18. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211-28.
- 19. Zuk PA, Zhu Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-95.
- 20. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24(4):150-5.
- 21. Manggold J, Sergi C, Becker K, Lukoschek M, Simank HG. A new animal model of femoral head necrosis induced by intraosseous injection of ethanol. Lab Anim. 2002;36:173-80.
- 22. Duncombe GJ, Barker AP, Moss TJM, Gurrin LC, Charles AK, Smith NM, Newnham JP. The effects of overcoming experimental bladder outflow obstruction in fetal sheep. J Matern Fetal Neonatal Med. 2002;11(2):130-7.
- 23. Feitosa MLT, Fadel L, Beltrão-Braga PCB, Wenceslau CV, Kerkis I, Kerkis A, Birgel-Júnior EH, Martins JFP, Martins DS, Miglino MA, Ambrósio CE. Successful transplant of mesenchymal stem cells in induced osteonecrosis of the ovine femoral head. Preliminary results. Acta Cir Bras. 2010;25(5):416-22.
- 24. Zucconi E, Vieira NM, Bueno DF, Secco M, Jazedje T, Ambrosio CE, Passos-Bueno MR, Miglino MA, Zatz M. Mesenchymal stem cells derived from canine umbilical cord vein. A novel source for cell therapy studies. Stem Cells Dev. 2010;19(3):395-402.
- 25. Knippenberg M, Helder MN, Doulabi BZ, Wuisman PIJM, Klein-Nulend J. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem Biophys Res Commun. 2006;342:902-8.
- 26. Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2006;327:449-62.
- 27. Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochem Biophys Res Commun. 1999;265:134-9.
- 28. Martínez-Lorenzo M, Royo-Canãs M, Alegre-Aguarón E, Desportes P, Castiella T, Garcia-Alvarez F, Larrad L. Phenotype and chondrogenic differentiation of mesenchymal cells from adipose tissue of different species. J Orthop Res. 2009;27:1499-507.
Publication Dates
-
Publication in this collection
25 July 2011 -
Date of issue
Aug 2011
History
-
Received
15 Dec 2010 -
Reviewed
17 Feb 2011 -
Accepted
18 Mar 2011