Abstracts
PURPOSE: To investigate and compare the efficacy of oral midazolam with two different dosages in orange juice on perioperative hemodynamics and behavioral changes in children who underwent skin laser treatment in an academic educational Hospital. METHODS: Ninety children, candidates for skin laser treatment were randomly assigned to 1 of 3 groups of 30 each: the placebo group received 0.1 ml/kg orange flavored juice, group 2 and 3 receiving 0.5 and 1 mg/kg of injectable midazolam mixed with an equal volume of orange juice, respectively. The main outcome measures included the mask acceptance, patients' behavioral scales and postoperative events. RESULTS: There were no significant differences in heart rate, respiratory rate, and systolic blood pressure among the three groups. However, arterial oxygen saturation was significantly reduced in those given 1 mg.kg-1 midazolam. The median scores of anxiety, separation from parent, preparing an intravenous line, acceptance of the oxygen mask, good sedation, crying reduction and consciousness level were better in midazolam group. Postoperative agitation and re-crying were also more frequent in placebo receivers. Those given 1 mg.kg-1 midazolam were significantly more optimal for sedation, crying, consciousness, preparing an intravenous line, and postoperative re-crying compared with 0.5 mg.kg-1 midazolam receivers. CONCLUSION: As a preanaesthetic medication, the 1 mg.kg-1 dose of orally given midazolam especially in a volume of orange juice and can optimize the children's behavior during skin laser treatment with no serious adverse effects, enhancing their parents' satisfactions about the sedative protocol.
Midazolam; Premedication; Anesthesia; Laser Therapy; Skin
OBJETIVO:Investigar e comparar a eficácia do uso oral de midazolam com duas diferentes doses de suco de laranja na hemodinâmica peropeatória e mudanças de desempenho em crianças submetidas tratamento de pele por laser em Hospital educacional e acadêmico. MÉTODOS:Noventa crianças candidatas a tratamento de pele por laser foram distribuídas aleatóriamente em três grupos de 30 cada: o grupo placebo recebeu 0.1mg/kg de suco de laranja, grupos dois e três receberam 0.5 e 1mg/kg de midazolam injetável misturado em igual volume de suco de laranja respectivamente. Os principais registros incluíam a aceitação da máscara, escalas de comportamento e eventos pós-operatórios. RESULTADOS:Não houve diferenças significantes cardíacas, respiratórias e pressão sanguinea sistólica nos três grupos. Contudo, o nível de saturação de oxigênio foi reduzido significantemente nos que receberam 1mg.kg-1 de midazolam. Os níveis médios de ansiedade, separação dos pais, preparo intravenoso, aceitação da máscara de oxigênio, boa sedação, redução do choro e nível de consciência, foram melhores no grupo midazolam. Agitação pós-operatória e retorno do chora foi mais freqüente nos que receberam placebo. Observou-se que o grupo que recebeu 1mg.kg-1 foi melhor comparado ao que recebeu 0.5mg.kg-1. CONCLUSÃO:Como medicação pré-anestésica na dose de 1mg.kg-1 de midazolam, fornecida em igual volume de suco de laranja, é satisfatória no comportamento de crianças durante tratamento de pele por laser, proporcionando satisfação dos pais.
Midazolam; Pré-Medicação; Anestesia; Terapia a Laser; Pele
10 - ORIGINAL ARTICLE
ANESTHESIA
Perioperative effects of oral midazolam premedication in children undergoing skin laser treatment. A double-blinded randomized placebo-controlled trial1 1 Research performed at Department of Anaesthesia, Razi Hospital, Tehran University of Medical Sciences, Tehran, Iran.
Efeitos peroperatórios da premedicação oral de midazolam em crianças submetidas a tratamento de pele por laser. Estudo duplo-cego randomizado e controlado
Mehrdad ShoroghiI; Shahriyar ArbabiII; Farshid FarahbakhshIII; Mehrdad SheikhvatanIV; Ali AbbasiV
IMD, Assistant Professor, Department of Anaesthesia, Razi Hospital, Tehran University of Medical Sciences, Tehran, Iran. Conception, data acquisition and design. Drafting and revising the article. Responsible for anaesthesia procedure and for the integrity of the work as a whole
IIMD, Assistant Professor, Department of Anaesthesia, Razi Hospital, Tehran University of Medical Sciences, Tehran, Iran. Conception, design and data acquisition
IIIMD, Assistant Professor, Department of Anaesthesia, Ali-Ebn Abitaleb Hospital, Rafsanjan University, Rafsanjan, Iran. Critical revision
IVMD, Research Fellow, Department of Cardiology, Tehran Heart Center, Tehran University of Medical Sciences, Tehran, Iran. Analysis, interpretation of data, drafting and revising the article
VMD, Fellow PhD degree, Department of Epidemiology, University Medical Center Groningen, Groningen, Netherlands. Drafting the article, data acquisition, critical revision, analysis and interpretation of data
Correspondence Correspondence: Ali Abbasi Department of Epidemiology University Medical Center Groningen Hanzeplein 1, P.O. Box 30.001 9700 RB Groningen, the Netherlands Tel: 0031 50 361 8068 Fax: 0031 50 361 4493 aliiabbasi@yahoo.com; a.abbasi@epi.umcg.nl
ABSTRACT
PURPOSE: To investigate and compare the efficacy of oral midazolam with two different dosages in orange juice on perioperative hemodynamics and behavioral changes in children who underwent skin laser treatment in an academic educational Hospital.
METHODS: Ninety children, candidates for skin laser treatment were randomly assigned to 1 of 3 groups of 30 each: the placebo group received 0.1 ml/kg orange flavored juice, group 2 and 3 receiving 0.5 and 1 mg/kg of injectable midazolam mixed with an equal volume of orange juice, respectively. The main outcome measures included the mask acceptance, patients' behavioral scales and postoperative events.
RESULTS: There were no significant differences in heart rate, respiratory rate, and systolic blood pressure among the three groups. However, arterial oxygen saturation was significantly reduced in those given 1 mg.kg-1 midazolam. The median scores of anxiety, separation from parent, preparing an intravenous line, acceptance of the oxygen mask, good sedation, crying reduction and consciousness level were better in midazolam group. Postoperative agitation and re-crying were also more frequent in placebo receivers. Those given 1 mg.kg-1 midazolam were significantly more optimal for sedation, crying, consciousness, preparing an intravenous line, and postoperative re-crying compared with 0.5 mg.kg-1 midazolam receivers.
CONCLUSION: As a preanaesthetic medication, the 1 mg.kg-1 dose of orally given midazolam especially in a volume of orange juice and can optimize the children's behavior during skin laser treatment with no serious adverse effects, enhancing their parents' satisfactions about the sedative protocol.
Key words: Midazolam. Premedication. Anesthesia. Laser Therapy. Skin.
RESUMO
OBJETIVO:Investigar e comparar a eficácia do uso oral de midazolam com duas diferentes doses de suco de laranja na hemodinâmica peropeatória e mudanças de desempenho em crianças submetidas tratamento de pele por laser em Hospital educacional e acadêmico.
MÉTODOS:Noventa crianças candidatas a tratamento de pele por laser foram distribuídas aleatóriamente em três grupos de 30 cada: o grupo placebo recebeu 0.1mg/kg de suco de laranja, grupos dois e três receberam 0.5 e 1mg/kg de midazolam injetável misturado em igual volume de suco de laranja respectivamente. Os principais registros incluíam a aceitação da máscara, escalas de comportamento e eventos pós-operatórios.
RESULTADOS:Não houve diferenças significantes cardíacas, respiratórias e pressão sanguinea sistólica nos três grupos. Contudo, o nível de saturação de oxigênio foi reduzido significantemente nos que receberam 1mg.kg-1 de midazolam. Os níveis médios de ansiedade, separação dos pais, preparo intravenoso, aceitação da máscara de oxigênio, boa sedação, redução do choro e nível de consciência, foram melhores no grupo midazolam. Agitação pós-operatória e retorno do chora foi mais freqüente nos que receberam placebo. Observou-se que o grupo que recebeu 1mg.kg-1 foi melhor comparado ao que recebeu 0.5mg.kg-1.
CONCLUSÃO:Como medicação pré-anestésica na dose de 1mg.kg-1 de midazolam, fornecida em igual volume de suco de laranja, é satisfatória no comportamento de crianças durante tratamento de pele por laser, proporcionando satisfação dos pais.
Descritores: Midazolam. Pré-Medicação. Anestesia. Terapia a Laser. Pele.
Introduction
Preoperative adequate preparation of children can lead to improved surgical experiences and faster recovery after surgery. This protocol helps the children feel less anxious about the anesthesia induction and surgery and can reduce the post-surgical complications and maladaptive behavioral changes1,2 .Thus, in order to minimize the crying and struggling prior to surgery and during induction of anesthesia, pre-anesthetic medication is recommended for children who are candidates for surgery3.
Despite a number of pre-medications being advocated to facilitate the separation of children from their parents and to reduce the anxiety associated with the operation, no choice pre-medication has universal acceptance. In spite of the rare side effects such as respiratory depression, midazolam has become a commonly used agent for conscious sedation of children before diagnostic or therapeutic procedures or before induction of anesthesia. Until recently, only the intravenous form of the drug was available. However, it has been clear that the oral midazolam is very bitter even with added flavoring. The liquid form of drug has been shown to be an extremely safe premedicant for children with a dose range of 0.25 to1.0 mg/kg4,5.
Some studies showed that the premedication with midazolam could reduce pre-operative distress and facilitate patient management6-8. However, the effects of oral midazolam especially mixed with orange juice on hemodynamic status, anxiety level and behavioral changes of children after operation has not been clearly determined. We tried to investigate and compare the efficacy of oral midazolam with the different dosages and mixed with orange juice on both postoperative behavioral changes and hemodynamics in children who underwent skin laser treatment.
Methods
In a randomized, prospective double-blind placebo controlled study, 90 children between the ages of two and eight years with ASA I-II status presenting for under-anesthesia skin laser therapy were included the study. The study protocol was explained to the parents and informed consent was obtained from them before beginning the study. The study was approved by the Ethics Committee in Tehran University of medical sciences. Additionally, it conforms to the principles in the Helsinki Declaration.
The subjects with the hypersensitivity to benzodiazepines and who were treated with drugs that affect the nervous system were excluded. Study patients were randomly assigned to one of the three groups: 1) the placebo group (as control group) received 0.1 ml/kg orange flavored juice; 2 and 3) the midazolam groups, received injectable midazolam 0.5 mg/kg and 1 mg/kg in an equal volume of the orange juice, respectively. The placebo group was received the similar preparation. The research physician prepared the placebo or midazolam glasses labeled multi-care A, B or C to prescribe for the children. The children, their parents and the research anesthetist who assessed hemodynamics and behavioral changes preoperatively, were blinded to drug and placebo assignment. Random allocation was performed by using a randomized six-block order of A, B and C. All children were separated from their parents entered the operating room 30 min after the oral administration of placebo or midazolam containing orange juice.
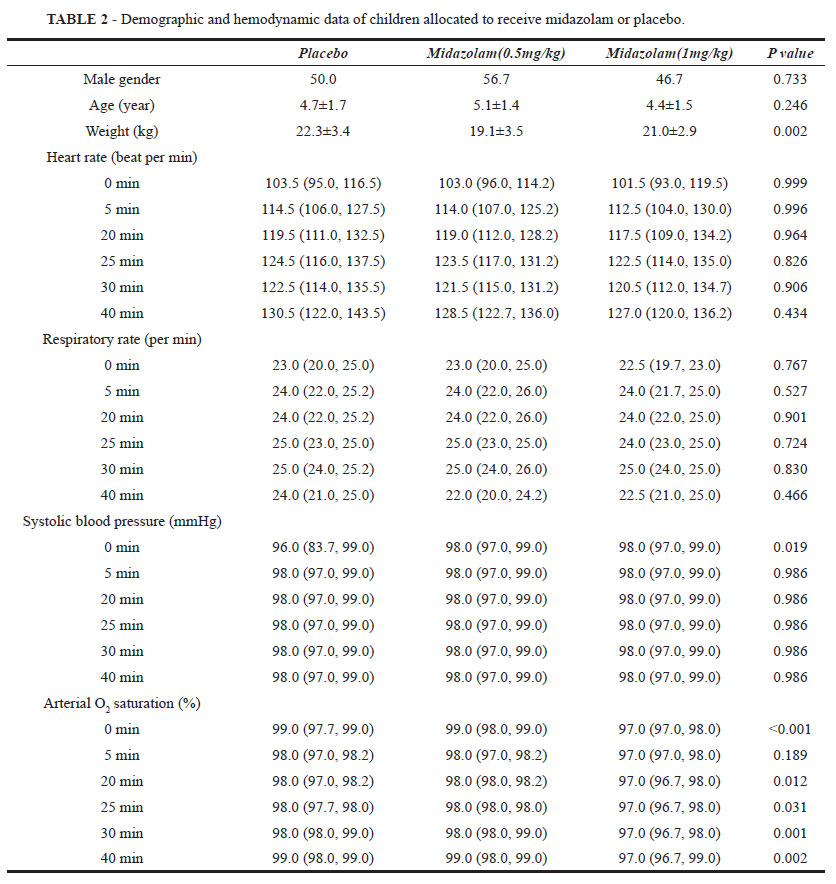
Hemodynamic parameters (heart rate, respiratory rate, systolic blood pressure, and arterial oxygen saturation) were recorded immediately before the entry to operation room and also 5, 20, 25, 30, and 40 minutes after the beginning of laser therapy. A blinded observer (same observer for all patients) scored the patient's behavior during the perioperative period by using the scales assessing the level of anxiety, separation from parent, preparing an intravenous line, acceptance of the drug administration, acceptance of the oxygen mask, sedation, crying, and consciousness. A higher score on these scales indicates a better situation (Table 1).
Furthermore, postoperative events were recorded for each patient. They were a need to airway, nausea and vomiting, re-crying, abnormal movement, and restless. At the time of discharge from the recovery, the parents were asked to rate their satisfaction with the pre-medication on a visual analogue scale from 0 to 10 (0= extremely dissatisfied; 10= extremely satisfied).
Results were reported as mean±S.D. for the quantitative variables and percentages for the categorical variables. Non-parametric and ordinal variables were presented by median (1st, 3rd quartiles). For the difference of distribution of the scales, the groups were compared using the Kruskal- Wallis' test and differences between two treatments groups were analysed with Mann-Whitney's U-test. P values of 0.05 or less were considered statistically significant. For multiple comparisons, P values of 0.01 or less were considered statistically significant after the Bonferroni's correction. All the statistical analyses were performed using Statistical Package for Social Sciences version 16 (SPSS Inc., Chicago, IL, USA).
Results
The patients in the three treatment groups were not significantly different with regard to age and sex, but they had a different distribution of weight. There were no significant differences in heart rate, respiratory rate, and systolic blood pressure at any time before and during the operation (0, 5, 20, 25, 30, and 40 min). However, arterial oxygen saturation was significantly lower in those premedicated with 1 mg.kg-1 oral midazolam (Table 2).
The willing acceptance of the drug administration was similar between the three groups. The median scores of anxiety, separation from parent, preparing an intravenous line, acceptance of the oxygen mask, good sedation, crying reduction, consciousness, postoperative agitation and re-crying level were better in midazolam groups compared with placebo group (P<0.001). The children premedicated with 1 mg.kg-1 midazolam were sedated better than those with 0.5 mg.kg-1 midazolam (P<0.01). Also, the 1 mg.kg-1 midazolam group had more optimal level for crying, consciousness, preparing an intravenous line compared with other premedicated group (P<0.001) (Table 3).
Adverse events after the operation in the three groups are shown in Table 4.
Postoperative assessment showed that an increased incidence of agitation and re-crying in placebo group compared with midazolam premedicated groups (P<0.001). In 1 mg.kg-1 midazolam group the incidence of postoperative re-crying was comparable with 0.5 mg.kg-1 midazolam receivers (P=0.002).
Discussion
Several medications are used to relax and calm patients specially children before certain procedures or before anesthesia for surgery that can help decrease memory of the events. However, clinical studies have confirmed some serious complications during the post- anesthetic period and child's discharge time in the use of these medications. Thus, the selection of the most effective preoperative sedation can accompanied with a trend towards better recovery from anesthesia and a higher degree of parental satisfaction.
In the present study, we investigated the effects of the administration of orange juice with and without midazolam on children behavioral changes, their parents' satisfaction and the changes of hemodynamics after juice drinking. We firstly found that the increase of midazolam dosages led to the child's better behaviors and parent's satisfaction. Several studies revealed similar results, however in their studies, different dosages of oral midazolam were applied. Cote et al.9 found that the oral midazolam juice was effective for producing sedation and anxiolysis at a dose of 0.25 mg.kg-1 and this dosage was led to the minimal effects on respiration and oxygen saturation. In a study by Cox et al.10, oral midazolam premedication in children was found to reduce the anxiety associated with separation from parents with midazolam 0.5 mg.kg-1 administered 20 to 30 min preoperatively. Also, in another study by Kuganeswaran et al.11, medicated patients reported less pain and anxiety and physicians observed less pain and anxiety compared with placebo during the procedure. Furthermore, in a study by Liacouras et al.12, a significant difference was noted in the group that was administered oral midazolam for the level of sedation for intravenous placement, pre-procedural sedation, ease of intravenous insertion, ease of separation from parents, and ease of the nursing personnel's ability to monitor the patient during the procedure. These results have been also shown in McErlean et al.13, McGraw et al.14, Pandit et al.15 and Cray et al.16 and studies. Similar to our study, the positive relationship between the dosage of administered midazolam and reduction of abnormal behavioral changes was noted in some studies. In a study by Marshall et al.17, a significant linear relationship between plasma drug concentration and maximal sedation score, but not anxiety score, was observed and concluded that the sedative effects were related to plasma concentrations of midazolam and the primary metabolite, alpha-hydroxymidazolam. They confirmed that oral midazolam with the dose of 1.0 mg.kg-1, administered within 30 min of the expected procedure or anesthetic induction should provide safe and effective sedation for a majority of children. In Masue et al.18 study, infants and children premedicated with oral midazolam 1.5mg.kg-1 were better sedated than those with a standard dose of midazolam. Also, in their study, most of infants and children given 1.5 mg.kg-1 of midazolam achieved satisfactory sedation in 30 min, in comparison with those given 1.0 or 0.5 mg.kg-1. Besides, in some other studies, different effect of oral midazolam in sedation of children who were candidates for surgery was not proven. In a study by Fine et al.19, no differences in resistance, success of delivery, problems with separation and mask acceptance were found. Also, Kain et al.20 indicated that although midazolam was an effective anxiolytic for most children, 14.1% of children still exhibit extreme distress. In addition, in Kapur et al.21 study, there was no significant difference in the acceptability of the test solutions in the children who received 0.5 mg.kg-1 midazolam mixed in strawberry juice via the oral-transmucosal route and those in control group were given the same juice diluted with normal saline. It seems that the bioavailability of midazolam in the commercial preparation was surprisingly appropriate and the results were consistently acceptable. However this favorable result can be dependant to the different dosages of drug so that the recommended dose for children is a single dose of 0.25 to 0.5 mg.kg-1 to a maximum dose of 20 mg. Also, good outcome of oral midazolam administration in children can be related to the children age, obesity, level of basal anxiety, and medical need22. Thus, more studies with greater sample sizes are needed to determine the best dosages of midazolam to treat the children with variant demographic characteristics.
In our study, except for the reduction of O2 saturation in patients received higher dosage of midazolam, other hemodynamics were not different between the three groups. Similarly, in study by Fine et al.19 arterial oxygen saturation and heart rate were not significant changed after the administration of 0.5 mg.kg-1 oral midazolam. In another study by Masue et al.18 Midazolam 1.5 mg.kg-1 did not cause any statistically significant decrease in blood pressure, arterial oxygen saturation and heart rate. However, in Wan et al.23 study, heart rate and systolic blood pressure in intervention group who received 0.5 mg.kg-1 of midazolam were much lower than that in control group. These results can indicated that the changes of vital signs are not only dependant to the dosage of oral midazolam and other variables such as the type of operation and other patients variables can predict these changes that should be considered in further ingestigations. Midazolam has a bitter taste that is difficult to disguise even when given in a mixture with grape juice24. Whereas, we observed that acceptance rate to swallow was similar in placebo or midazolam group. In other word, children judged the taste of oral midazolam mixed with orange juice not to be different from orange juice alone.
Conclusions
In conclusion, our data suggest that in spite of some complications of midazolam premedication such as reduction of arterial O2 saturation, the administration of 1 mg.kg-1 dosage oral midazolam especially in volume of the orange juice can significantly reduce children anxiety and agitation for operation. As midazolam premedication optimized effectively the children's behavior, it will enhance their parents' satisfactions about this sedative protocol. After midazolam medicatiom, the more oxygen supplement may be applied during operation to resolve the blood oxygen saturation.
Received: December 10, 2010
Review: February 14, 2011
Accepted: March 15, 2011
Conflict of interest: none
Financial source: none
- 1. Elliot JK, Peter JD, Smith RM. Preoperative preparation. In: Motoyoma EK, Davis PJ, eds. Smith's anesthesia for infants and children. 6ed. St Louis: C.V. Mosby; 1996. p.213-29.
- 2. Van der Walt JH, Jacob R, Murrell D, Bentley M. The perioperative effects of oral premedication in children. Anaesth Intensive Care.1990;18:5-10.
- 3. Levine MF, Hartley EJ, Macpherson BA, Burrows FA, Lerman J. Oral midazolam premedication for children with congenital cyanotic heart disease undergoing cardiac surgery: a comparative study. Can J Anaesth. 1993;40:934-8.
- 4. McMillan CO, Spahr-Schopfer IA, Sikich N, Hartley E, Lerman J. Premedication of children with oral midazolam. Can J Anaesth. 1992;39:545-50.
- 5. Feld LH, Negus JB, White PF. Oral midazolam preanesthetic medication in pediatric outpatients. Anesthesiology. 1990;73:831-4.
- 6. Caldas JC, Pais-Ribeiro JL, Carneiro SR. General anesthesia, surgery and hospitalisation in children and their effects upon cognitive, academic, emotional and sociobehavioral development - a review. Paediatr Anaesth. 2004;14(11):910-5.
- 7. Horiuchi T, Kawaguchi M, Kurehara K. Evaluation of relatively low dose of oral transmucosal ketamine premedication in children: a comparison with oral midazolam. Paediatr Anaesth. 2005;15(8):643-7.
- 8. Samarkandi A, Naguib M, Riad W, Thalaj A, Alotibi W, Aldammas F, Albassam A. Melatonin vs. midazolam premedication in children: a double-blind, placebo-controlled study. Eur J Anaesthesiol. 2005;22:189-96.
- 9. Cote' CJ, Cohen IT, Suresh S, Rabb M, Rose JB, Weldon C, Davis PJ, Bikhazi GB, Karl HW, Hummer KA, Hannallah RS, Khoo KC, Collins P. A comparison of three doses of a commercially prepared oral midazolam juice in children. Anesth Analg. 2002;94:37-43.
- 10. Cox RG, Nemish U, Ewen A, Crowe MJ. Evidence-based clinical update: does premedication with oral midazolam lead to improved behavioural outcomes in children? Can J Anaesth. 2006;53(12):1213-9.
- 11. Kuganeswaran E, Clarkston WK, Cuddy PG, Quiason SG, Pandya PK, Dierenfeldt WT, Jonnalagadda SS, Smith OJ, Chen ST. A double-blind placebo controlled trial of oral midazolam as premedication before flexible sigmoidoscopy. Am J Gastroenterol. 1999;94(11):3215-9.
- 12. Liacouras CA, Mascarenhas M, Poon C, Wenner WJ. Placebo-controlled trial assessing the use of oral midazolam as a premedication to conscious sedation for pediatric endoscopy. Gastrointest Endosc. 1998;47(6):455-60.
- 13. McErlean M, Bartfield JM, Karunakar TA, Whitman MC, Turley DM. Midazolam juice as a premedication to reduce the discomfort associated with pediatric intravenous catheter insertion. J Pediatr. 2003;142(4):429-30.
- 14. McGraw T, Kendrick A. Oral midazolam premedication and postoperative behaviour in children. Paediatr Anaesth. 1998;8(2):117-21.
- 15. Pandit UA, Collier PJ, Malviya S, Voepel-Lewis T, Wagner D, Siewert MJ. Oral transmucosal midazolam premedication for preschool children. Can J Anaesth. 2001;48(2):191-5.
- 16. Cray SH, Dixon JL, Heard CM, Selsby DS. Oral midazolam premedication for paediatric day case patients. Paediatr Anaesth. 1996;6(4):265-70.
- 17. Marshall J, Rodarte A, Blumer J, Khoo KC, Akbari B, Kearns G. Pediatric pharmacodynamics of midazolam oral juice. Pediatric Pharmacology Research Unit Network. J Clin Pharmacol. 2000;40(6):578-89.
- 18. Masue T, Shimonaka H, Fukao I, Kasuya S, Kasuya Y, Dohi S. Oral high-dose midazolam premedication for infants and children undergoing cardiovascular surgery. Paediatr Anaesth. 2003;13(8):662-7.
- 19. Fine B, Castillo R, McDonald T, Paisansathan C, Zsigmond E, Hoffman WE. Jet injector compared with oral midazolam for preoperative sedation in children. Paediatr Anaesth. 2004;14(9):739-43.
- 20. Kain ZN, MacLaren J, McClain BC, Saadat H, Wang SM, Mayes LC, Anderson GM. Effects of age and emotionality on the effectiveness of midazolam administered preoperatively to children. Anesthesiology. 2007;107(4):545-52.
- 21. Kapur A, Chawla SH, Goyal A, Gauba K, Bhardwaj N. Efficacy and acceptabilty of oral-transmucosal midazolam as a conscious sedation agent in pre-school children. J Indian Soc Pedod Prev Dent. 2004;22(3):109-13.
- 22. Personal Communication, Anne L. Lear, Medical Center Representative, Indianapolis, IN, Roche Laboratories, February 1, 1999.
- 23. Wan K, Jing Q, Zhao JZ. Evaluation of oral midazolam as conscious sedation for pediatric patients in oral restoration. Chin Med Sci J. 2006;21(3):163-6.
- 24. Peterson M. Making oral midazolam palatable for children. Anesthesiology. 1990;73:1053.
Publication Dates
-
Publication in this collection
25 July 2011 -
Date of issue
Aug 2011
History
-
Reviewed
14 Feb 2011 -
Received
10 Dec 2010 -
Accepted
15 Mar 2011