Abstracts
PURPOSE: To investigate the effects of preventive enteral administration of ornithine alpha-ketoglutarate (OKG) in an ischemia-reperfusion rat model. METHODS: Sixty rats were randomized into five groups (G1-G5, n = 12). Each group was divided into two subgroups (n = 6) and treated with calcium carbonate (CaCa) or OKG by gavage. Thirty minutes later, the animals were anesthetized with xylazine 15mg + ketamine 1mg ip and subjected to laparotomy. G1-G3 rats served as controls. Rats in groups G4 and G5 were subjected to ischemia for 30 minutes. Ischemia was achieved by clamping the small intestine and its mesentery, delimiting a segment of bowel 5 cm long and 5 cm apart from the ileocecal valve. In addition, G5 rats underwent reperfusion for 30 minutes. Blood samples were collected at the end of the laparotomy (G1), after 30 minutes (G2, G4) and 60 minutes (G3, G5) to determine concentrations of metabolites (pyruvate, lactate), creatine phosphokinase (CPK), thiobarbituric acid reactive substances (TBARS) and glutathione (GSH). RESULTS: There was a significant decrease in tissue pyruvate and lactate and plasma CPK levels in OKG-treated rats at the end of reperfusion period. GSH levels did not change significantly in ischemia and reperfusion groups. However, TBARS levels increased significantly (p<0.05) in tissue samples in OKG-treated rats subjected to ischemia for 30 minutes. CONCLUSION: Short-term pretreatment with OKG before induction of I/R decreases tissue damage, increases pyruvate utilization for energy production in the Krebs cycle and does not attenuate the oxidative stress in this animal model.
Intestine; Ischemia; Reperfusion; Oxidative Stress; Ornithine; Rats
OBJETIVO: Investigar os efeitos da administração enteral preventiva de ornitina alfa-cetoglutarato (OKG) em modelo de isquemia-reperfusão no rato. MÉTODOS: Sessenta ratos foram randomizados em cinco grupos (G1-G5, n=12). Cada grupo foi redistribuído em dois subgrupos (n=6) e tratado com carbonato de cálcio (CaCa) ou OKG por gavagem. Trinta minutos mais tarde, os animais foram anestesiados com xilazina 1mg+cetamina 15mg i.p. e submetidos à laparotomia. Os ratos dos grupos G4-G5 foram submetidos à isquemia por 30 minutos. A isquemia foi obtida por pinçamento do intestino delgado, delimitando um segmento com 5 cm de comprimento e distando 5 cm da válvula ileocecal. O grupo G5 foi submetido à reperfusão por 30 minutos. Amostras de sangue foram coletadas no final da laparotomia (G1), após 30 minutos (G2, G4) e 60 minutos (G3, G5) para determinação das concentrações de metabolitos (piruvato, lactato), creatinofosfoquinase (CPK), substâncias reativas ao ácido tiobarbitúrico (TBARS) e glutationa (GSH). RESULTADOS: Observou-se redução significante (p<0,05) das concentrações de piruvato e lactato, teciduais e CPK plasmático em ratos tratados com OKG, no final do período de reperfusão. Não houve alteração significante nos níveis plasmáticos e teciduais de GSH. Entretanto os níveis de TBARS aumentaram significativamente (p<0,05) em amostras de tecido de ratos tratados com OKG submetido à isquemia por 30 minutos. CONCLUSÃO: o pré-tratamento em curto prazo com OKG antes da indução da I/R diminui a lesão tecidual, aumenta a utilização de piruvato para produção de energia no ciclo de Krebs, mas não atenua o estresse oxidativo neste modelo animal.
Intestino Delgado; Isquemia; Reperfusão; Estresse Oxidativo; Ornitina; Ratos
2 - ORIGINAL ARTICLE
ISCHEMIA-REPERFUSION
Effect of short-term ornithine alpha-ketoglutarate pretreatment on intestinal ischemia-reperfusion in rats1 Correspondence: Paulo Roberto Leitão de Vasconcelos Rua Professor Costa Mendes, 1608/3º andar 60430-140 Fortaleza - CE Brasil Tel: (55 85)3366-8083 Fax: (55-85)3366-8064 paulo.vasconcelos@ufc.br
Efeitos do pré-tratamento em curto prazo com ornitina alfa-cetoglutarato na isquemia-reperfusão intestinal em ratos
Eduardo Silvio Gouveia GonçalvesI; Camila Menezes RabeloII; Alberico Ximenes do Prado NetoII; José Huygens Parente GarciaIII; Sérgio Botelho GuimarãesIV; Paulo Roberto Leitão de VasconcelosV
IFellow MS Degree, Department of Surgery, Postgraduate Program, UFC, Ceara, Brazil. Technical procedures, acquisition and interpretation of data. The article is part of a master degree dissertation
IIGraduate student, UFC, Ceara, Brazil. Helped with technical procedures and acquisition of data
IIIPhD, Associate Professor, Head, Department of Surgery, UFC, Ceara, Brazil. Critical revision and analysis of data
IVPhD, Associate Professor, Department of Surgery, Head, Experimental Surgery Laboratory - LABCEX, UFC, Ceara, Brazil. Manuscript writing, statistical analysis and graphics design
VPhD, Associate Professor, Coordinator, Postgraduate Program, Department of Surgery, UFC, Ceara, Brazil. Tutor, responsible for conception, design, intellectual and scientific content of the study, critical analysis, final approval of manuscript
Correspondence Correspondence: Paulo Roberto Leitão de Vasconcelos Rua Professor Costa Mendes, 1608/3º andar 60430-140 Fortaleza - CE Brasil Tel: (55 85)3366-8083 Fax: (55-85)3366-8064 paulo.vasconcelos@ufc.br
ABSTRACT
PURPOSE: To investigate the effects of preventive enteral administration of ornithine alpha-ketoglutarate (OKG) in an ischemia-reperfusion rat model.
METHODS: Sixty rats were randomized into five groups (G1-G5, n = 12). Each group was divided into two subgroups (n = 6) and treated with calcium carbonate (CaCa) or OKG by gavage. Thirty minutes later, the animals were anesthetized with xylazine 15mg + ketamine 1mg ip and subjected to laparotomy. G1-G3 rats served as controls. Rats in groups G4 and G5 were subjected to ischemia for 30 minutes. Ischemia was achieved by clamping the small intestine and its mesentery, delimiting a segment of bowel 5 cm long and 5 cm apart from the ileocecal valve. In addition, G5 rats underwent reperfusion for 30 minutes. Blood samples were collected at the end of the laparotomy (G1), after 30 minutes (G2, G4) and 60 minutes (G3, G5) to determine concentrations of metabolites (pyruvate, lactate), creatine phosphokinase (CPK), thiobarbituric acid reactive substances (TBARS) and glutathione (GSH).
RESULTS: There was a significant decrease in tissue pyruvate and lactate and plasma CPK levels in OKG-treated rats at the end of reperfusion period. GSH levels did not change significantly in ischemia and reperfusion groups. However, TBARS levels increased significantly (p<0.05) in tissue samples in OKG-treated rats subjected to ischemia for 30 minutes.
CONCLUSION: Short-term pretreatment with OKG before induction of I/R decreases tissue damage, increases pyruvate utilization for energy production in the Krebs cycle and does not attenuate the oxidative stress in this animal model.
Keywords: Intestine, Small. Ischemia. Reperfusion. Oxidative Stress. Ornithine. Rats.
RESUMO
OBJETIVO: Investigar os efeitos da administração enteral preventiva de ornitina alfa-cetoglutarato (OKG) em modelo de isquemia-reperfusão no rato.
MÉTODOS: Sessenta ratos foram randomizados em cinco grupos (G1-G5, n=12). Cada grupo foi redistribuído em dois subgrupos (n=6) e tratado com carbonato de cálcio (CaCa) ou OKG por gavagem. Trinta minutos mais tarde, os animais foram anestesiados com xilazina 1mg+cetamina 15mg i.p. e submetidos à laparotomia. Os ratos dos grupos G4-G5 foram submetidos à isquemia por 30 minutos. A isquemia foi obtida por pinçamento do intestino delgado, delimitando um segmento com 5 cm de comprimento e distando 5 cm da válvula ileocecal. O grupo G5 foi submetido à reperfusão por 30 minutos. Amostras de sangue foram coletadas no final da laparotomia (G1), após 30 minutos (G2, G4) e 60 minutos (G3, G5) para determinação das concentrações de metabolitos (piruvato, lactato), creatinofosfoquinase (CPK), substâncias reativas ao ácido tiobarbitúrico (TBARS) e glutationa (GSH).
RESULTADOS: Observou-se redução significante (p<0,05) das concentrações de piruvato e lactato, teciduais e CPK plasmático em ratos tratados com OKG, no final do período de reperfusão. Não houve alteração significante nos níveis plasmáticos e teciduais de GSH. Entretanto os níveis de TBARS aumentaram significativamente (p<0,05) em amostras de tecido de ratos tratados com OKG submetido à isquemia por 30 minutos.
CONCLUSÃO: o pré-tratamento em curto prazo com OKG antes da indução da I/R diminui a lesão tecidual, aumenta a utilização de piruvato para produção de energia no ciclo de Krebs, mas não atenua o estresse oxidativo neste modelo animal.
Descritores: Intestino Delgado. Isquemia. Reperfusão. Estresse Oxidativo. Ornitina. Ratos.
Introduction
Small bowel ischemia induces an early reduction of villi height in jejunum and ileum, causing decreased mucosal thickness in rats1. In humans, only 30 minutes of small bowel ischemia brings about an early destruction of villus tips, with enterocyte shedding into the lumen2.
Intestinal ischemia occurs in the absence or decrease in blood flow due to acute or chronic mesenteric vessels obstruction. During ischemia, the intestinal tissue needs to use anaerobic alternative routes to replace adenosine triphosphate (ATP) required to preserve the minimal metabolism that maintain cell homeostasis and function. Such mechanisms are fragile and generally effective for only a few hours, while producing potentially harmful substances such as lactic acid and precursors of free radicals such as xanthine oxidase. Resulting from the inability to replenish ATP in the ischemic area, energy production ceases. Next, by-products of purine hypoxanthine, xanthine and iosine metabolism,are produced. With the return of blood flow (reperfusion) the oxygen will react with these metabolites generating highly reactive free radicalsm resulting in local and systemic oxidative stress3-5. Several methods can be used to obtain ischemia: arterial obstruction by vascular clamping, shock, hypothermia and surgical tapes for timed release of blood flow. A review of the literature showed that the preference of researchers is by vascular clamping, followed by stenosis or extrinsic vascular compression6.
Alpha-ketoglutarate is a precursor of glutamine and when linked to two molecules of ornithine becomes a new compound, the ornithine alpha-ketoglutarate (OKG). The administration of pharmacological doses of OKG in burned rats controls the reduction of muscle glutamine (GLN) as opposed to direct administration of GLN7. Raul et al.8 demonstrated that after three days of fasting, rats fed with OKG (1g/kgdia) showed hyperplasia in the intestinal villi and increase in brush border hydrolases8. OKG administration is associated with reduced protein turnover, decreased myofibrillar protein catabolism, attenuation in protein synthesis and in muscle glutamine drop9. Oral and parenteral OKG preparations are available. Considering that parenteral OKG is not easily obtainable, the use of oral preparations administered by gavage or in diet is frequently chosen by researchers9-11.
GLN is a conditionally essential nutrient during sepsis or trauma12. Recently, glutamine has been demonstrated to protect against ischemia/reperfusion (I/R) injury of the gut, heart, liver and skeletal muscle13. The mechanism is still incompletely understood and may be partly related to the preservation of GSH content14-15. Considering all the above, this study aims to investigate whether short-term enteral OKG pretreatment has beneficial systemic and/or local effects in rat small bowel ischemia/reperfusion model.
Methods
Approval for experimental use of laboratory animals was obtained on February, 2008 from the local Ethics Committee on the Use of Animals (CEUA) former Ethics Committee on Animal Use (CEPA) (Protocol #127/07) in view of the Federal Law No. 11794 of October 8, 2008, http://www.planalto.gov.br/ccivil_03/_Ato2007-2010/2008/Lei/L11794.htm and Decree No. 6689 of July 15, 2009 that regulated the Law 11794, available at: http://www.planalto.gov.br/ccivil_03/_Ato2007-2010/2009/Decreto/D6899.htm. The study was designed so as to minimize the number of animals required for the experiments. All animals were housed in polypropylene cages at ambient temperature of 24ºC on a 12 h light-dark cycle.
Study design
Sixty rats were randomized into five groups (G1-G5, n = 12). Each group was divided into two subgroups (n = 6) and treated by gavage with calcium carbonate 5.0g/kg (CaCa) or OKG 5.0g/Kg dissolved in 2.0 ml of distilled water. Thirty minutes later, the animals were anesthetized with xylazine 15mg + ketamine 1mg ip and subjected to laparotomy. G1-G3 rats served as controls. Rats in groups G4 and G5 were subjected to ischemia for 30 minutes. Ischemia was achieved by clamping the small intestine and its mesentery, delimiting a segment of bowel 5 cm long and 5 cm apart from the ileocecal valve. In addition, G5 rats underwent reperfusion for 30 minutes after the second operation for removal of hemostatic clips. Blood samples were collected at the end of the laparotomy (G1), after 30 minutes (G2, G4) and 60 minutes (G3, G5) to determine concentrations of metabolites (pyruvate, lactate), creatine phosphokinase (CPK), thiobarbituric acid reactive substances (TBARS) and glutathione (GSH).
Chemicals and drugs
OKG powder for solution (Cétornan®) was purchased from Chiese SA, Courbevoie, France. All other chemicals were purchased from standard commercial sources and were of the highest quality available.
Laboratory parameters
Plasma and tissue metabolites (pyruvate, lactate)16, oxidative stress (malondialdehyde - MDA, reduced glutathione - GSH)17,18 were evaluated by methods described in the literature.CPK was determined using the optimized standard method.
Statistical analysis
Graphpad Prism 5.0 (GraphPad Software, www.graphpad.com) was used for statistical analysis and graphics design. Results are reported as mean ± SD. All data were tested for distribution by Kolmorogov-Smirnov test. Unpaired t-test was used for comparisons between CaCa and OKG groups. ANOVA was used to compare sham groups. P values of less than 0.05 were considered significant.
Results
Surgical trauma induced an apparent gradual increase of CPK levels in rats treated with CaCa (155.32±9.83, 178.82±5.06 and 181.01±6.13, in G1, G2 and G3 groups respectively) but without significant differences. The values were unchanged in rats treated with OKG (Figure 1). There was a significant decrease in plasma CPK (Figure 2) levels in OKG-treated rats at the end of reperfusion period.
There was a significant decrease in tissue pyruvate (Figure 3) and lactate (Figure 4) levels in OKG-treated rats at the end of reperfusion period..
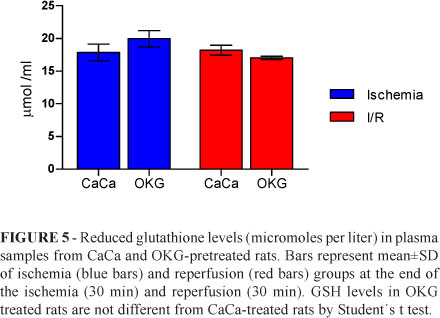
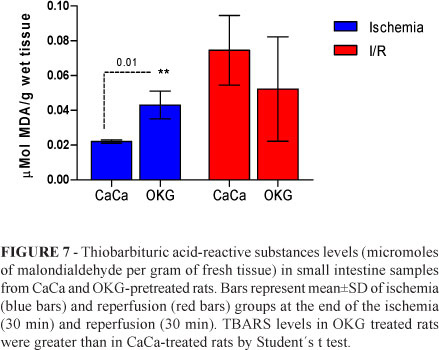
GSH levels did not change significantly in ischemia and reperfusion groups (Figures 5 and 6). However, TBARS levels increased significantly (p<0.05) in tissue samples in OKG-treated rats subjected to ischemia for 30 minutes (Figure 7).
Discussion
In this study, the influence of OKG administration on I/R-related intestine injury was investigated in an ischemic small bowel segment model. Although a possible preventive effect of OKG administration against I/R injury has been evoked, no major effect of this therapy was noted here as hypothesized, considering that OKG is a precursor of arginine and GLN19 which are presumed to potently interfere with I/R-induced lesions.
The fact that CPK levels were not different in sham rats suggests that the surgical trauma was similar in all timepoints (Figure 1). However, when OKG-treated rats were subjected to I/R, CPK levels fell significantly after reperfusion. The decrease in CPK levels (Figure 2) demonstrates a decrease in tissue damage in OKG-treated rats.
The significant decrease in tissue pyruvate (Figure 3) and lactate levels (Figure 4) at the end of reperfusion period in OKG-treated rats suggest an increased use of pyruvate for energy production in the Krebs cycle by reducing its conversion to lactate.besides demonstrating the energy-enhancing action of OKG. Pyruvate is an energy substrate with known cytoprotective properties. Today we know that this is due not only to its antioxidant action, but also because it reduces the intracellular acidosis. However, the precise mechanisms of action of pyruvate in the mitochondria are still largely unknown20.
The increase in TBARS levels (Figure 7) in rats subjected to ischemia for 30 minutes points to a possible peroxidative activity of OKG during ischemia.
Schuster et al.21 studied the effects of dietary OKG supplementation in a rodent model of liver ischemia and found no alterations in lipid peroxidation products. They concluded that OKG seems to act on the inflammatory response rather than on oxidative reactions.
GSH is a tripeptide (glutamyl-cysteinyl-glycine) that has functions as the maintenance of cellular activity, the detoxification of xenobiotic compounds and the action against free radicals22. Plasma GSH is largely derived from the liver and skeletal muscle which releases GSH in proportion to tissue concentrations23,24. Thus, plasma GSH levels may reflect systemic oxidative stress25.
GSH is the source of Intracellular glutamate by the action of glutaminase. Glutamate is transported poorly across cell membranes and glutamine readily crosses this membrane. The depletion of GLN directly causes a reduction in cellular GSH content; an adequate supply of glutamine is essential for GSH synthesis26. The absence of changes in GSH levels during ischemia and reperfusion (Figures 5 and 6) in OKG-treated rats may reflect increased consumption of the tripeptide by damaged tissue. Another possible explanation is that short-term pretreatment with OKG before induction of I/R does not attenuate the oxidative stress, at least in this animal model.
Conclusion
Short-term pretreatment with ornithine alpha-ketoglutarate before induction of ischemia-reperfusion decreases tissue damage, increases pyruvate utilization for energy production in the Krebs cycle but does not attenuate the oxidative stress in this animal model.
Conflict of interest: none
Financial source: none
1 Research performed at Experimental Surgery Laboratory (LABCEX), Faculty of Medicine, Federal University of Ceara (UFC), Fortaleza-CE, Brazil.
- 1. Chang JX, Chen S, Ma LP, Jiang LY,Chen JW, Chang RM, Wen LQ, Wu W,Jiang ZP, Huang ZT. Functional and morphological changes of the gut barrier during the restitution process after hemorrhagic shock. World J Gastroenterol. 2005;11(35):5485-91.
- 2. Derikx JP, Matthijsen RA, de Bruine AP, van Dam RM, Buurman WA, Dejong CH. A new model to study intestinal ischemia-reperfusion damage in Man. J Surg. 2011;166(2):222-6.
- 3. Schoemberg MH, Fredholm BB, Haglund U, Jung H, Sellin D, Younes M, Schildberg FW. Studies on the oxygen radical mechanism involved in the small intestinal reperfusion damage. Acta Physiol Scand. 1985;124(4):581-9.
- 4. Lammers KM, Innocenti G, Venturi A, Rizzello F, Helwig U, Bianchi GP, Pedrini L, Di Nino G, Gionchetti P, Campieri M. The effect of transient intestinal ischemia on inflammatory parameters. Int J Colorectal Dis. 2003;18(1):78-85.
- 5. Szijártó A, Hahn O, Batmunkh E, Stangl R, Kiss A, Lotz G, Schaff Z, Váli L, Blázovics A, Gero D, Szabó C, Kupcsulik P, Harsányi L. Short-term alanyl-glutamine dipeptide pretreatment in liver ischemia-reperfusion model: effects on microcirculation and antioxidant status in rats. Clin Nutr. 2007:26(5):640-8.
- 6. Ribeiro ME, Yoshida WB. Lesões intestinais decorrentes de isquemia e reperfusão: fisiopatologia e modelos experimentais. J Vasc Br. 2005;4(2):183-94.
- 7. Le Boucher J, Coudray-Lucas C, Lasnier E, Jardel A, Ekindjian OG, Cynober LA. Enteral administration of ornithine alpha-ketoglutarate or arginine alpha-ketoglutarate: a comparative study of their effects on glutamine pools in burn-injured rats. Crit Care Med. 1997;25(2):293-8.
- 8. Raul F, Gossé F,Galluser M, Hasselmann M, Seiler N. Functional and metabolic changes in intestinal mucosa of rats after enteral administration of ornithine [alpha]-ketoglutarate salt. J Parenter Enter Nutr. 2005;19:145-50.
- 9. Duranton B, Schleiffer R, Gossé F, Raul F. Preventive administration of ornithine alpha-ketoglutarate improves intestinal mucosal repair after transient ischemia in rats. Crit Care Med. 1998;26(1):120-5.
- 10. Luyt CE, Meddahi-Pellé A, Ho-Tin-Noe B, Colliec-Jouault S, Guezennec J, Louedec L, Prats H, Jacob MP, Osborne-Pellegrin M, Letourneur D, Michel JB. Low-molecular-weight fucoidan promotes therapeutic revascularization in a rat model of critical hindlimb ischemia. J Pharmacol Exp Ther. 2003;305(1):24-30.
- 11. Schuster H, Blanc MC, Genthon C, Thérond P, Bonnefont-Rousselot D, Le Tourneau A, De Bandt JP, Cynober L. Does dietary ornithine alpha-ketoglutarate supplementation protect the liver against ischemia-reperfusion injury? Clin Nutr. 2005;24(3):375-84.
- 12. Lacey JM, Crouch JB, Benfell K, Ringer SA, Wilmore CK, Maguire D, Wilmore DW. The effects of glutamine-supplemented parenteral nutrition in premature infants. JPEN J Parenter Enteral Nutr. 1996;20(1):74-80.
- 13. Jia CJ, Dai CL, Zhang X, Cui K, Xu F, Xu YQ. Alanylglutamine dipeptide inhibits hepatic ischemia-reperfusion injury in rats. World J Gastroenterol. 2006;12(9):1373-8.
- 14. Armeni T, Ghiselli R, Balercia G, Goffi L, Jassem W, Saba V, Principato G. Glutathione and ultrastructural changes in inflow occlusion of rat liver. J Surg Res. 2000;88(2):207-14.
- 15. Schauer RJ, Kalmuk S, Gerbes AL, Leiderer R, Meissner H, Schildberg FW, Messmer K, Bilzer M. Intravenous administration of glutathione protects parenchymal and non-parenchymal liver cells against reperfusion injury following rat liver transplantation. World J Gastroenterol. 2004;10(6):864-70.
- 16. Hohorst HJD. Glucose-6-phosphate and D-fructose-6-phosphate. Determination with glucose-6-phosphate dehydrogenase and phosphoglucose isomerase. In: Bergmeyer HU. Methods of enzymatic analysis. Weinheim/Academic: London; 1963. p.134-8.
- 17. Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271-8.
- 18. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25(1):192-205.
- 19. Cynober L. Ornithine alpha-ketoglutarate as a potent precursor of arginine and nitric oxide: a new job for an old friend. J Nutr. 2004;134(10 Suppl):2858S-62S.
- 20. Demling RH, Seigne P. Metabolic management of patients with severe burns. World J Surg. 2000;24(6):673-80.
- 21. Schuster H, Blanc MC, Genthon C, Thérond P, Bonnefont-Rousselot D, Le Tourneau A, De Bandt JP, Cynober L. Does dietary ornithine alpha-ketoglutarate supplementation protect the liver against ischemia-reperfusion injury? Clin Nutr. 2005;24(3):375-84.
- 22. Comporti M, Maellaro E, Del Bello B, Casini AF. Glutathione depletion: its effect on other antioxidant systems and hepatocelular damage. Xenobiotica. 1991;21(8):1067-76.
- 23. Kretzschmar M, Pfeifer U, Machnik G, Klinger W. Glutathione homeostasis and turnover in the totally hepatectomized rat: evidence for a high glutathione export capacity of extrahepatic tissues. Exp Toxicol Pathol. 1992;44(5):273-81.
- 24. Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8(9-10):1865-79.
- 25. Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P Jr, Reed RL, Jones DP. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;15;24(5):699-704.
- 26. Hong RW, Rounds JD, Helton WS, Robinson MK, Wilmore DW. Glutamine preserves liver glutathione after lethal hepatic injury. Ann Surg. 1992;215(2):114-9.
Publication Dates
-
Publication in this collection
23 Sept 2011 -
Date of issue
2011