Abstracts
PURPOSE: To develop a model to evaluate the effects of focal pulsed ultrasound (US) waves as a source of heat for treatment of murine subcutaneous implanted Walker tumor. METHODS: An experimental, controlled, comparative study was conducted. Twenty male Wistar rats (160-300 g) randomized in 2 equal groups (G-1: Control and G-2: Hyperthermia) were inoculated with Walker-256 carcinosarcoma tumor. After 5 days G-2 rats were submitted to 45ºC hyperthermia. Heat was delivered directly to the tumor by an ultrasound (US) equipment (3 MHz frequency, 1,5W/cm³). Tumor temperature reached 45º C in 3 minutes and was maintained at this level for 5 minutes. Tumor volume was measured on days 5, 8, 11, 14 e 17 post inoculation in both groups. Unpaired t-test was used for comparison. P<0.05 was considered significant. RESULTS: Tumor volume was significantly greater in day 5 and decreased in days 11, 14 and 17 in treated rats. Rats treated with hyperthermia survived longer than control animals. On the 29th day following tumor inoculation, 40% of control rats and 77.78% of hyperthermia-treated rats remained alive. CONCLUSION: The proposed model is quite simple and may be used in less sophisticated laboratory settings for studying the effects of focal hyperthermia in the treatment of malignant implanted tumours or in survival studies.
Carcinoma 256; Animal Experimentation; Hyperthermia; Rats
OBJETIVO: Desenvolver um modelo para avaliar os efeitos do ultra-som focal pulsado como fonte de calor para o tratamento de tumores de Walker subcutâneos implantados em ratos. MÉTODOS: Um estudo experimental, controlado, comparativo foi realizado. Vinte ratos Wistar machos (160-300 g) divididos em dois grupos (G-1: Controle e G-2: hipertermia) foram inoculados com tumor de Walker carcinossarcoma-256. Após cinco dias os ratos do grupo G-2 ratos foram submetidos a hipertermia (45ºC). O calor foi aplicado diretamente no tumor por um equipamento de ultrassonografia (3 MHz, 1,5 W/cm³). A temperatura no tumor atingiu 45ºC em 3 minutos e foi mantida nesse nível por 5 minutos. O volume do tumor foi medido nos dias 5, 8, 11, 14 e 17 após a inoculação, em ambos os grupos. Teste t não pareado foi utilizado para comparação. P <0,05 foi considerado significante. RESULTADOS: O volume do tumor foi significativamente maior no 5º dia e diminuiu nos dias 11, 14 e 17 nos ratos tratados. Animais submetidos à hipertermia sobreviveram mais tempo que os animais do grupo controle. No 29º dia após a inoculação do tumor, 40% dos ratos do grupo controle e 77,78% dos ratos tratados com hipertermia permaneceram vivos. CONCLUSÃO: Os resultados obtidos mostram que o modelo proposto é bastante simples e pode ser utilizado em laboratórios menos sofisticados para estudar os efeitos da hipertermia focal no tratamento dos tumores malignos implantados ou em estudos de sobrevida.
Carcinoma 256 de Walker; Experimentação Animal; Hipertermia Induzida; Ratos
11 - ORIGINAL ARTICLE
MODELS, BIOLOGICAL
Experimental model of ultrasound thermotherapy in rats inoculated with Walker-236 tumor1 Correspondence: Manoel Odorico de Moraes Filho Rua Professor Costa Mendes, 1608/3º andar, Bloco Didático 60430-140 Fortaleza - CE Brasil Tel.: (55-85)3366-8083 Fax: (55-85)3366-8064 odorico@ufc.br
Modelo experimental de termoterapia ultrassônica em ratos inoculados com tumor de Walker-236
José Antonio Carlos Otaviano David MoranoI; Naylana CordeiroII; Sergio Botelho GuimarãesIII; Francisco Vagnaldo Fechine JamacaruIV; Manoel Odorico de Moraes FilhoV
IFellow PhD degree, Department of Surgery, Postgraduate Program, UFC, Ceara, Brazil. Technical procedures, acquisition and interpretation of data. The article is part of the PhD degree thesis
IIGraduate student, UFC, Ceara, Brazil. Helped with technical procedures, acquisition of data
IIIPhD, Associate Professor, Department of Surgery, Head, LABCEX. UFC, Ceara, Brazil. Manuscript writing, statistical analysis, graphics design
IVResearch Fellow, Department of Physiology and Pharmacology, UFC, Ceara, Brazil. Statistical analysis and critical review of results
VPhD, Associate Professor, Department of Physiology and Pharmacology, UFC, Ceara, Brazil. Tutor, responsible for conception, design, intellectual and scientific content of the study, critical analysis, final approval of manuscript
Correspondence Correspondence: Manoel Odorico de Moraes Filho Rua Professor Costa Mendes, 1608/3º andar, Bloco Didático 60430-140 Fortaleza - CE Brasil Tel.: (55-85)3366-8083 Fax: (55-85)3366-8064 odorico@ufc.br
ABSTRACT
PURPOSE: To develop a model to evaluate the effects of focal pulsed ultrasound (US) waves as a source of heat for treatment of murine subcutaneous implanted Walker tumor.
METHODS: An experimental, controlled, comparative study was conducted. Twenty male Wistar rats (160-300 g) randomized in 2 equal groups (G-1: Control and G-2: Hyperthermia) were inoculated with Walker-256 carcinosarcoma tumor. After 5 days G-2 rats were submitted to 45ºC hyperthermia. Heat was delivered directly to the tumor by an ultrasound (US) equipment (3 MHz frequency, 1,5W/cm3). Tumor temperature reached 45º C in 3 minutes and was maintained at this level for 5 minutes. Tumor volume was measured on days 5, 8, 11, 14 e 17 post inoculation in both groups. Unpaired t-test was used for comparison. P<0.05 was considered significant.
RESULTS: Tumor volume was significantly greater in day 5 and decreased in days 11, 14 and 17 in treated rats. Rats treated with hyperthermia survived longer than control animals. On the 29th day following tumor inoculation, 40% of control rats and 77.78% of hyperthermia-treated rats remained alive.
CONCLUSION: The proposed model is quite simple and may be used in less sophisticated laboratory settings for studying the effects of focal hyperthermia in the treatment of malignant implanted tumours or in survival studies.
Keywords: Carcinoma 256, Walker. Animal Experimentation. Hyperthermia, Induced. Rats.
RESUMO
OBJETIVO: Desenvolver um modelo para avaliar os efeitos do ultra-som focal pulsado como fonte de calor para o tratamento de tumores de Walker subcutâneos implantados em ratos.
MÉTODOS: Um estudo experimental, controlado, comparativo foi realizado. Vinte ratos Wistar machos (160-300 g) divididos em dois grupos (G-1: Controle e G-2: hipertermia) foram inoculados com tumor de Walker carcinossarcoma-256. Após cinco dias os ratos do grupo G-2 ratos foram submetidos a hipertermia (45ºC). O calor foi aplicado diretamente no tumor por um equipamento de ultrassonografia (3 MHz, 1,5 W/cm3). A temperatura no tumor atingiu 45ºC em 3 minutos e foi mantida nesse nível por 5 minutos. O volume do tumor foi medido nos dias 5, 8, 11, 14 e 17 após a inoculação, em ambos os grupos. Teste t não pareado foi utilizado para comparação. P <0,05 foi considerado significante.
RESULTADOS: O volume do tumor foi significativamente maior no 5º dia e diminuiu nos dias 11, 14 e 17 nos ratos tratados. Animais submetidos à hipertermia sobreviveram mais tempo que os animais do grupo controle. No 29º dia após a inoculação do tumor, 40% dos ratos do grupo controle e 77,78% dos ratos tratados com hipertermia permaneceram vivos.
CONCLUSÃO: Os resultados obtidos mostram que o modelo proposto é bastante simples e pode ser utilizado em laboratórios menos sofisticados para estudar os efeitos da hipertermia focal no tratamento dos tumores malignos implantados ou em estudos de sobrevida.
Descritores: Carcinoma 256 de Walker. Experimentação Animal. Hipertermia Induzida. Ratos.
Introduction
The use of hyperthermia changes tumor behavior, resulting in increased radio sensitivity1, better response to chemotherapy2 and genetic therapy modulation3. Ultrasound (US) has been used as a source of heat for malignant tumor thermotherapy4. However, some researchers contraindicate the use of US in the treatment of malignancies. Continuous US applied to murine tumors has resulted in larger and heavier tumor compared with controls. The increase in tumor growth could be due directly to the energy delivered to the tissues by the ultrasonic wave and to the associated tissue temperature rise5. It is known that the therapeutic effects of US are directly related to the heat applied to the tumor6. It has been demonstrated that continuous US treatments are associated with an increase in tissue temperatures and that pulsed US treatments show no tissue temperature elevation4.
Walker 256 carcinosarcoma tumor induction model has been widely described in the literature, showing high take rate and easy handling during laboratory analysis7. The model was discovered in 1928, when George Walker, in his laboratory at Johns Hopkins University Medical School, noticed in mammal gland of an albino, pregnant, female rat a tumor mass, which according to his own description, receded during lactation and broke out anew shortly after8. Considering that there are controversies about the safe use of US in tumor therapy, this study was aimed at developing a model to evaluate the effects of focal US waves as a source of heat for treatment of murine subcutaneous implanted Walker tumor.
Methods
An experimental, controlled, comparative study was conducted following approval for experimental use of laboratory animals, obtained on May 17, 2006 (Protocol #48/05) from the Ethics Committee on Animal Research (CEPA) of the Federal University of Ceará, now Ethics Committee on the Use of Animals (CEUA), in view of the Federal Law No. 11794 of October 8, 2008, http://www.planalto.gov.br/ccivil_03/_Ato2007-2010/2008/Lei/L11794.htm and Decree No. 6689 of July 15, 2009 that regulated the Law 11794, available at: http://www.planalto.gov.br/ccivil_03/_Ato2007-2010/2009/Decreto/D6899.htm.
The study was designed so as to minimize the number of animals required for the experiments.
Twenty male Wistar rats (160-300 g) randomized in 2 equal groups (G-1: Control and G-2: Hyperthermia) were inoculated with Walker-256 tumor and sheltered in a 24º C environment with light/dark cycles of 12 hours. Tap water and dry pellets were offered ad lib. After 5 days G-2 rats were submitted to 45º C hyperthermia during 5 minutes. Heat was delivered directly to the tumor by ultrasound equipment (3 MHz frequency, in a continuous way, adjusted to attain 1,5W/cm3). Within 3 minutes of ultrasound application the tumor temperature reached 45º C and was maintained at this level for 5 minutes. Temperature level was controlled by a digital thermometer utilizing a probe placed under the tumor. Adjacent tissues temperature was not altered. Tumor volume was measured on days 5, 8, 11, 14 e 17 post inoculation in both groups.
Walker tumor cells implant technique
Initially, stock tumor cells were inoculated in two health rats dorsi. One week later the tumor were removed and placed on a Petri plate containing Ringer´s lactate 5.0ml and gentamicyn sulphate solution (10 mg/ml). Next, the tumor was incubated for 5 minutes. Maceration of tumor tissues and sequential dilution were carried out resulting in a suspension of 2.0x106 cells/ml. An aliquot of 1.0 ml was inoculated subcutaneously in each rat dorsum in a point located in an equidistant position from the line of insertion of the anterior and posterior limbs of the animals. Tumor volumes were measured every third day using a manual pachimeter. Two perpendicular diameters, the major (D) and the minor diameters (d) were measured. Tumor final volume (Vol) was calculated using the using the formula [Vol = π/6.D.d2] expressed in cm3, as previously reported. 9
Anesthesia of all rats was obtained with Chloral Hydrate 10% (300 mg/kg) injected intraperitoneally.
Hyperthermia technique
Hyperthermia was applied on the 5th day after tumor implant. The experiment was carried out with the animal under general anaesthesia and placed with the dorsum turned upside on a specially designed table. This table has a device the moves down the applicator until it touches the animal dorsum. A metallic electrode inserted through a small skin incision was placed under the tumor to verify its temperature during the US application. The US equipment (Sonopulse® 3MHz, IBRAMED, Brazil) - has a 3.5 diameter effective radiating area (ERA). Conductive gel (1.0 ml layer) was placed on the skin to improve conductivity (Figure 1). The equipment was adjusted to attain 1,5W/cm3, continuous way. After 3 to 4 minutes, the tumor temperature reached 45ºC, and was maintained at this level for 5 minutes (Figure 1).
Statistical methods
Graphpad Prism 5.0 (GraphPad Software, San Diego,CA, USA, www.graphpad.com) was used for computation and statistical analysis. All data were tested for distribution using Kolmogorov-Smirnov test. All results were expressed as mean±SD. Comparisons between groups (Control versus Hyperthermia) were performed using unpaired t test. The survival analyses were made according to Kaplan-Meier method. Comparisons of survival curves were done by log-rank test. A P-value < 0.05 was considered statistically significant.
Results
Analysis of treatment effects
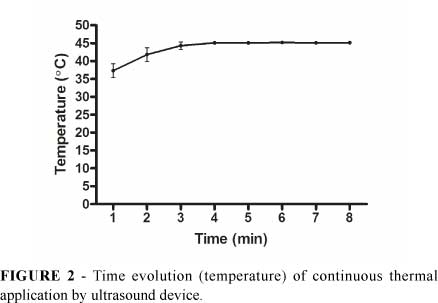
Tumor temperature increased rapidly with US application reaching 45ºC within 3-4 minutes. On the 8th minute the temperature remained at the 45ºC mark (Figure 2). Surrounding tissues temperature was not altered.
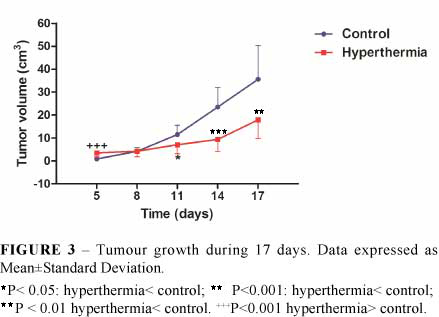
Tumor volume was significantly greater in G-2 rats in day 5. No significant differences were found in day 8. However, in days 11, 14 and 17, tumor volumes in G-2 rats were significantly decreased compared to control group (Figure 3).
Rats treated with hyperthermia survived longer than control animals. On the 29th day following tumor inoculation remained alive 40% of control rats and 77.78% of hyperthermia-treated rats (Figure 4).
Discussion
Recurrent malignancies after previous radiotherapy are often treated with hyperthermia for its potential effects in cancer treatment10. The combination of heat and drugs could improve the treatment outcome of recurrent tumor11. The search for practical and safe models designed to study the effects of hyperthermia induced by US motivated this study. The model presented here uses US in the control of tumor growth and reduction using moderate heat (45ºC) and it is very simple and easily reproducible. Therefore experimental studies with a large series of animals at relatively low cost and consistent results are feasible with this model.
US focal application for five minutes induced tumor growth in the first 5 days of the experiment. Researchers have demonstrated an increase in tumor oxygenation and blood flow after maintaining tumor temperature at 42.5ºC for 30 minutes12. Novak et al.13 developed an external local ultrasound (US) system to induce controlled hyperthermia of subcutaneously implanted tumor in small animals (e.g., mice and rats). There are some similarities with the system used in our paper. However the system developed by those researchers was aimed at targeting tumors of up to 1 cm in diameter, while we aimed at treating larger subcutaneously implanted tumor. Besides, Novak et al.13 used temperatures in the range of 41-43ºC for periods of 65 min on average while we used a fixed temperature of 45ºC for a much shorter period (5 minutes).
The temperature required to induce cell death varies between different cell types. One fundamental observation is that the capability to induce cell death at lower temperatures (42-43 °C) is markedly lower than above 43 °C1416. This explains why we elected to maintain the final temperature used at the 45º C level.
Greater temperature gradients have been used to treat tumor with thermally significant vessels15. Complex US systems have been used to treat patients with malignancies. Smith et al.16 used a 16-channel ultrasound intracavitary array in the treatment of prostate tumor by hyperthermia. The system here presented is quite simple and the results are adequate for experimental studies using small animals. In our experiment the initial tumor growth was followed by a significant reduction in tumor volume in US treated rats. This effect could be related to cell death induced by mild hyperthermia. It is interesting to point out the fact that although the hyperthermotherapy was applied for a short period of time its effects in reducing tumor growth were present for the length of the experiment (17 days).
Another important fact is the prolonged survival of US treated rats. Walker tumor is very aggressive resulting in short time survival. This prolonged survival will ensure the possibility of studying associated drug therapy for a longer time.
In summary, this study demonstrated that the use of focal hyperthermia for 5 minutes promotes an increase in tumor size in the 5th day and a subsequent decrease in size beginning on the 11th day after inoculation and prolonged survival in inoculated rats.
Conclusion
The proposed model is quite simple and may be used in less sophisticated laboratory settings for studying the effects of focal hyperthermia in the treatment of malignant implanted tumors or in survival studies in small rodents.
Conflict of interest: none
Financial source: none
1 Research performed at Experimental Surgery Research Laboratory (LABCEX), Department of Surgery, Federal University of Ceara (UFC), Brazil.
- 1. Jones EL, Prosnitz LR, Dewhirst MW, Marcom PK, Hardenbergh PH, Marks LB, Brizel DM, Vujaskovic Z. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res. 2004;10:4287-93.
- 2. Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia. 2001;17:1-18.
- 3. Myhr G. Multimodal cancer treatment: real time monitoring, optimization, and synergistic effects. Technol Cancer Res Treat. 2008;7:409-14.
- 4. Kramer JF. Ultrasound: evaluation of its mechanical and thermal effects. Arch Phys Med. 1955;65:223-7.
- 5. Sicard-Rosenbaum L, Danoff JV, Guthrie JA, Eckhaus MA. Effects of energy-matched pulsed and continuous ultrasound on tumor growth in mice. Phys Ther. 1998;78:271-7.
- 6. Malyapa RS, Sawada S. Cell-cycle dependence of heat-induced interphase death in mouse L5178Y cells Radiat Res. 1991;125:134-40.
- 7. Moraes SP, Cunha A, Reis Neto JA, Barbosa H, Roncolatto CAP, Duarte RF. Modelo experimental de tumor de Walker. Acta Cir Bras. 2000;15(4):252-6.
- 8. Hard GC. Experimental models for the sequential analysis of chemical induced renal carcinogenesis. Toxicol Pathol. 1986;14:112-22.
- 9. Bianco R, Caputo R, Damiano V, De Placido S, Ficorella C, Agrawal S, Bianco AR, Ciardiello F, Tortora G. Combined targeting of epidermal growth factor receptor and MDM2 by gefitinib and antisense MDM2 cooperatively inhibit hormone-independent prostate cancer. Clin Cancer Res. 2004;10:4858-64.
- 10. Overgaard, J. The rationale for clinical trials in hyperthermia. In: Field SB, Hand, JW, eds. An introduction in the practical aspects of clinical hyperthermia. London: Taylor and Francis; 1990. p.213-41.
- 11. Van Bree C, Rietbroek RC, Shopman EM, Kipp JBA, Bakker JM. Local hyperthermia enhances the effect of cisdiamminedichloro-platinum(i1) on nonirradiated and preirradiated rat solid tumors. Int J Radiat Oncol Biol Phys. 1996;36(1):135-40.
- 12. Song CW, Shakil A, Osborn JL, Iwata K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int J Hyperthermia. 2009;25:91-5.
- 13. Novák P, Moros EG, Parry JJ, Rogers BE, Myerson RJ, Zeug A, Locke JE, Rossin R, Straube WL, Singh AK. Experience with a small animal hyperthermia ultrasound system (SAHUS): report on 83 tumours. Phys Med Biol. 2005; 50:5127-39.
- 14. Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33-56.
- 15. Straube WL, Moros EG, Myerson RJ, Fan X. A two-parameter method for the estimation of ultrasound-induced temperature artifacts. Int J Hyperthermia. 1999;15:187-202.
- 16. Smith NB, Merrilees NK, Dahleh M, Hynynen K. Control system for an MRI compatible intracavitary ultrasound array for thermal treatment of prostate disease. Int J Hyperthermia. 2001;17:271-82.
Publication Dates
-
Publication in this collection
23 Sept 2011 -
Date of issue
2011