Abstracts
PURPOSE: To evaluate the effects of L-alanyl-glutamine (L-Ala-Gln) pretreatment on oxidative stress, glycemic control and inflammatory response in children submitted to palatoplasty. METHODS: Thirty male children scheduled for routine palatoplasty, age range 2-10 years, were randomly assigned to 2 groups (n=15): Group A (saline, control) and Group B (L-Ala-Gln). Group A received normal saline 100 ml, delivered intravenously by infusion pump over 3 hours preceding surgical procedure. Group B was treated with L-Ala-Gln, 20% solution (0.5g/Kg), adding saline to complete 100ml. Peripheral venous blood samples were collected at 5 different time-points: T1- at the beginning of the study, 3 h prior to the surgical procedure; T2- at the end of the infusion (before the surgical procedure), T3- at the end of the surgical procedure, T4- 6 h postoperative and T5- 12 h postoperative. Parameters analyzed included glutathione (GSH), thiobarbituric acid reactive substances (TBARS), glucose, insulin, C-reactive protein (CRP) and interleukin-6 (IL-6). RESULTS: No statistically significant differences were found between groups comparing glucose, insulin, TBARS, GSH and IL-6 levels. However, glucose levels increased (P <0.001) in T4 and T5 as compared to baseline (T1) in control group as opposed to L-Ala-Gln group. IL-6 increased in both groups during the postoperative period, indicating an increased inflammatory response. L-Ala-Gln pretreatment did not suppress the increase of IL-6, but reduced the increase of postoperative CRP levels (T5, p <0.01). CONCLUSION: Pretreatment with L-Ala-Gln in children submitted to palatoplasty attenuates the inflammatory response in early post-operative period and promoted a better glycemic control.
Congenital Abnormalities; Surgical Procedures; Glutamine; Cytokines; Child
OBJETIVO: Avaliar os efeitos do pré-tratamento com L-alanil-glutamina (L-Ala-Gln) sobre o estresse oxidativo, o controle glicêmico e a resposta inflamatória em crianças submetidas à palatoplastia. MÉTODOS: Trinta crianças do sexo masculino, agendadas para palatoplastia, faixa etária 2-10 anos, foram distribuídas aleatoriamente em dois grupos (n = 15): Grupo A (salina, controle) e Grupo B (L-Ala-Gln). O grupo A recebeu solução salina 0,9% 100 ml, administrado por via intravenosa utilizando uma bomba de infusão durante 3 horas anteriores ao procedimento cirúrgico. O grupo B foi tratado com L-Ala-Gln, solução a 20% (0,5 g/kg), acrescentando soro fisiológico até completar 100 ml. Amostras de sangue venoso periférico foram coletadas em cinco momentos diferentes: T1 (3 h antes do procedimento cirúrgico); T2 (no final da perfusão), T3 (no final do procedimento cirúrgico), no pós-operatório, após 6 h (T-4) e 12 h (T5). Os parâmetros analisados foram a glutationa (GSH), ácido tiobarbitúrico (TBARS), glicose, insulina, proteína C-reativa (PCR) e interleucina-6 (IL-6). RESULTADOS: Não houve diferença significante entre os grupos comparando as concentrações de glicose, insulina, TBARS, GSH e IL-6. No entanto, os níveis de glicose aumentaram em T4 e T5, comparado ao basal (T1) (P <0,001) e a IL-6 aumentou em ambos os grupos durante o período pós-operatório, sinalizando o aumento da resposta inflamatória. O pré-tratamento com L-Ala-Gln não suprimiu o aumento de IL-6, mas reduziu o aumento pós-operatório de PCR (T5, p<0,01). CONCLUSÃO: O pré-tratamento com L-Ala-Gln em crianças submetidas à palatoplastia atenua a resposta inflamatória no período pós-operatório imediato, promovendo um melhor controle glicêmico.
Anormalidades Congênitas; Procedimentos Cirúrgicos Operatórios; Glutamina; Citocinas; Criança
15 - ORIGINAL ARTICLE
CLINICAL INVESTIGATION
L-alanyl-glutamine pretreatment attenuates acute inflammatory response in children submitted to palatoplasty1 Correspondence: Prof. Paulo Roberto Leitão de Vasconcelos Rua Professor Costa Mendes, 1608/3º andar 60430-140 Fortaleza - CE Brasil Tel.: (55 85)3366-8083 Fax: (55 85)3366-8064 paulo.vasconcelos@ufc.br
Pré-tratamento com L-alanil-glutamina atenua a resposta inflamatória aguda em crianças submetidas à palatoplastia
José Ferreira da Cunha FilhoI; Isabelle Ivo GonçalvesII; Sergio Botelho GuimarãesIII; Francisco Vagnaldo Fechine JamacaruIV; José Huygens Parente GarciaV; Paulo Roberto Leitão de VasconcelosVI
IFellow Master Degree, Department of Surgery, Postgraduate Program, UFC, Ceara, Brazil. Technical procedures, acquisition and interpretation of data. The article is part of a master degree dissertation
IIGraduate student, University of Fortaleza, UNIFOR, Ceara, Brazil. Helped with technical procedures, acquisition of data
IIIPhD, Associate Professor, Department of Surgery, Head, LABCEX, UFC, Ceara, Brazil. Manuscript writing, statistical analysis, graphics design
IVPhD, Research Fellow, Department of Physiology and Pharmacology, UFC, Ceara, Brazil. Statistical analysis and interpretation of data
VPhD, Associate Professor, Head, Department of Surgery, UFC, Ceara, Brazil. Critical revision and analysis of data
VIPhD, Associate Professor, Coordinator, Postgraduate Program, Department of Surgery, UFC, Ceara, Brazil. Tutor, responsible for conception, design, intellectual and scientific content of the study, critical analysis, final approval of manuscript
Correspondence Correspondence: Prof. Paulo Roberto Leitão de Vasconcelos Rua Professor Costa Mendes, 1608/3º andar 60430-140 Fortaleza - CE Brasil Tel.: (55 85)3366-8083 Fax: (55 85)3366-8064 paulo.vasconcelos@ufc.br
ABSTRACT
PURPOSE: To evaluate the effects of L-alanyl-glutamine (L-Ala-Gln) pretreatment on oxidative stress, glycemic control and inflammatory response in children submitted to palatoplasty.
METHODS: Thirty male children scheduled for routine palatoplasty, age range 2-10 years, were randomly assigned to 2 groups (n=15): Group A (saline, control) and Group B (L-Ala-Gln). Group A received normal saline 100 ml, delivered intravenously by infusion pump over 3 hours preceding surgical procedure. Group B was treated with L-Ala-Gln, 20% solution (0.5g/Kg), adding saline to complete 100ml. Peripheral venous blood samples were collected at 5 different time-points: T1- at the beginning of the study, 3 h prior to the surgical procedure; T2- at the end of the infusion (before the surgical procedure), T3- at the end of the surgical procedure, T4- 6 h postoperative and T5- 12 h postoperative. Parameters analyzed included glutathione (GSH), thiobarbituric acid reactive substances (TBARS), glucose, insulin, C-reactive protein (CRP) and interleukin-6 (IL-6).
RESULTS: No statistically significant differences were found between groups comparing glucose, insulin, TBARS, GSH and IL-6 levels. However, glucose levels increased (P <0.001) in T4 and T5 as compared to baseline (T1) in control group as opposed to L-Ala-Gln group. IL-6 increased in both groups during the postoperative period, indicating an increased inflammatory response. L-Ala-Gln pretreatment did not suppress the increase of IL-6, but reduced the increase of postoperative CRP levels (T5, p <0.01).
CONCLUSION: Pretreatment with L-Ala-Gln in children submitted to palatoplasty attenuates the inflammatory response in early post-operative period and promoted a better glycemic control.
Keywords: Congenital Abnormalities. Surgical Procedures, Operative. Glutamine. Cytokines. Child.
RESUMO
OBJETIVO: Avaliar os efeitos do pré-tratamento com L-alanil-glutamina (L-Ala-Gln) sobre o estresse oxidativo, o controle glicêmico e a resposta inflamatória em crianças submetidas à palatoplastia.
MÉTODOS: Trinta crianças do sexo masculino, agendadas para palatoplastia, faixa etária 2-10 anos, foram distribuídas aleatoriamente em dois grupos (n = 15): Grupo A (salina, controle) e Grupo B (L-Ala-Gln). O grupo A recebeu solução salina 0,9% 100 ml, administrado por via intravenosa utilizando uma bomba de infusão durante 3 horas anteriores ao procedimento cirúrgico. O grupo B foi tratado com L-Ala-Gln, solução a 20% (0,5 g/kg), acrescentando soro fisiológico até completar 100 ml. Amostras de sangue venoso periférico foram coletadas em cinco momentos diferentes: T1 (3 h antes do procedimento cirúrgico); T2 (no final da perfusão), T3 (no final do procedimento cirúrgico), no pós-operatório, após 6 h (T-4) e 12 h (T5). Os parâmetros analisados foram a glutationa (GSH), ácido tiobarbitúrico (TBARS), glicose, insulina, proteína C-reativa (PCR) e interleucina-6 (IL-6).
RESULTADOS: Não houve diferença significante entre os grupos comparando as concentrações de glicose, insulina, TBARS, GSH e IL-6. No entanto, os níveis de glicose aumentaram em T4 e T5, comparado ao basal (T1) (P <0,001) e a IL-6 aumentou em ambos os grupos durante o período pós-operatório, sinalizando o aumento da resposta inflamatória. O pré-tratamento com L-Ala-Gln não suprimiu o aumento de IL-6, mas reduziu o aumento pós-operatório de PCR (T5, p<0,01).
CONCLUSÃO: O pré-tratamento com L-Ala-Gln em crianças submetidas à palatoplastia atenua a resposta inflamatória no período pós-operatório imediato, promovendo um melhor controle glicêmico.
Descritores: Anormalidades Congênitas. Procedimentos Cirúrgicos Operatórios. Glutamina. Citocinas. Criança.
Introduction
Cleft lip and palate are congenital anomalies due to errors in development or in the maturation of embryonic processes1. Surgical repair of theses anomalies is a traumatic event due extensive dissection and bone exposure required for closing the communication between the nasal and oral cavities.
Healing of a surgical wound requires local activation of immune cells and secretion of various anti-inflammatory mediators. The process is tightly regulated by anti-inflammatory mediators. Depending on the magnitude of tissue damage and on the vulnerability of the host, the local immune response may fail to control the damage and restore homeostasis. In patients with more severe injury, release of mediators into the circulation occurs with activation of the immune system and systemic release of both pro-inflammatory and, subsequently, anti-inflammatory cytokines2.
Glutamine (GLN) is a conditionally essential nutrient during sepsis or trauma3. GLN is the most abundant amino acid in plasma and skeletal muscle. However, GLN levels fall dramatically after major injury or infection4. GLN supplementation in patients submitted to elective surgery attenuates the negative postoperative nitrogen balance, diminishing the dive in intracellular concentration of the amino acid in skeletal muscle and enhancing the synthesis of muscle protein5,6. Parenteral nutrition supplemented with the dipeptide alanyl-glutamine in patients in an intensive care unit is associated with a reduction in infection complications and better metabolic tolerance7.
The effect of parenteral GLN treatment has not been assessed in the setting of palatoplasty in children. This study tests the hypothesis that GLN attenuates surgical trauma-induced inflammatory response and oxidative stress in children submitted to surgical palate repair.
Methods
Patients
This prospective, randomized, placebo-controlled, single-blind study was approved by the Hospital Infantil Albert Sabin Ethics Committee, accredited by CONEP - CNS/MS, Protocol Nº. 051/06, May 29, 2006 and conducted in compliance with the Helsinki Declaration of 1975, as revised in 2008 (World Medical Association www.wma.net/e/policy/b3.htm) and Resolution 196/96 of the Brazilian National Health Service (http://conselho.saude.gov.br/resolucoes/reso_96.htm).
Written informed consent was obtained from all patients' parents or legal guardians. Thirty patients (age range: 2-10 years at the time of surgery) undergoing elective surgical repair of cleft lip and palate were included in the study. Exclusion criteria were history of hepatic, renal, gastrointestinal, cardiac, hematological or psychiatric disease, oral and/or systemic infection, anemia and refusal of consent from parents or legal guardians. Allocation of patients to groups A and B was made by software program (www.lee.dante.br). Patients who met inclusion criteria were randomly assigned to receive either 100 ml of normal saline (Group A) or L-Ala-Gln 20% solution (Dipeptiven®), adding saline to complete 100ml, delivered intravenously by infusion pump (LF 2001®, Lifemed Ind. Equip. Artigos Med. Hosp. S/A), over 3 hours preceding the surgical procedure.
Peripheral venous blood samples were collected at 5 different time-points: T1, at the beginning of the study (3 h prior to the surgical procedure); T2, at the end of the infusion (before the surgical procedure); T3, at the end of the surgical procedure; T4, 6 h postoperative and T5, 12 h postoperative. Parameters analyzed included glutathione (GSH), thiobarbituric acid reactive substances (TBARS), glucose, insulin, C-reactive protein (CRP) and interleukin-6 (IL-6).
Surgical procedure
All patients were submitted to the same anesthetic procedure with balanced general anesthesia, orotracheal intubation and controlled ventilation with a semi-open circuit. All surgical procedures were performed at the Albert Sabin Children´s Hospital by a single surgeon (J.F.C.F.) using the same technique (Veau-Wardill-Kilner)8 for all the lip and palate repairs.
Chemicals and drugs
L-Ala-Gln (Dipeptiven®) was purchased from Frenesius Kabi Áustria GmbH Graz/Áustria. All other chemicals were purchased from standard commercial sources and were of the highest quality available.
Biochemical analysis
GSH9, TBARS10 and glucose11 were measured according to biochemical methods published elsewhere. Serum level of insulin was measured by the electrochemiluminescence immunoassay; CRP was measured by an immunonephelometric method and IL6 by ELISA assay.
Statistical analysis
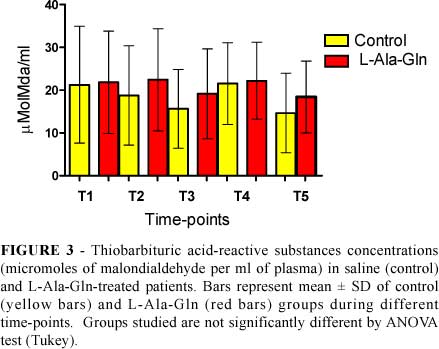
Graphpad Prism 5.0 (GraphPad Software, www.graphpad.com) was used for statistical analysis and graphics design. All data were tested for distribution (Kolmorogov-Smirnov test with Dallal-Wilkinson-Lilliefor P value). Results were expressed as mean±SD. Comparisons between the control and L-alanyl-glutamine groups were made using the t test for non paired variables (parametric data) or the Mann-Whitney U test (non parametric data). Repeated analysis of variance (ANOVA) combined with Tukey test was used compare timepoints. In all cases, the level of significance was set at 5%. P<0.05 was considered statistically significant.
Results
No statistically significant differences were found in glucose levels comparing control and L-Ala-Gln-treated patients. Glucose levels were significantly different in T3, T4 and T5 time-points compared with basal (T1) values in control children (Figure 1) and insulin (Figure 2) assays.
No statistically significant differences were found in insulin levels comparing control and L-ala-Gln-treated patients (Figure 2).
TBARS (Figure 3) and GSH (Figure 4) levels were not significantly different comparing control and L-Ala-Gln-treated patients.
CPR were significantly different in control patients, comparing T-5 with basal values (T-1). CPR levels in L-Ala-Gln treated patients were not different (Figure 5).
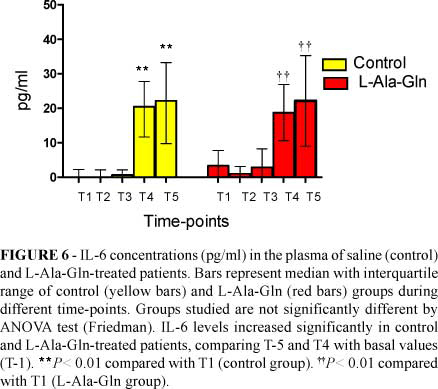
IL-6 levels were significantly different in both groups comparing T4 and T-5 with basal values (Figure 6).
Discussion
Children selected for this study showed a homogeneous profile in relation to sex (all males), age and weight.
Safety of GLN administration has been the aim of several publications6,12. Supplementation with GLN, using doses of 3 to 3.5 g/kg/day, for 10 days, did not have significant adverse effects, when administered parenterally in newborns with low birth weight13. In a recent publication, Ward et al.14 evaluated the severity of mucositis in children undergoing chemotherapy, where 0.65 g/kg/day of glutamine was administered orally for 7 days. There was no adverse and/or cumulative effect attributed to glutamine. In our study children were given L-ala-Gln 0.5g/Kg using a continuous infusion pump. This dose is equivalent to 0.33 g/kg of GLN, per 3-h period or 0.11 g/kg/h of GLN. No adverse effects were observed.
In this study, glucose levels increased in T3, T4 and T5 time-points compared with basal (T1) values. While the levels of glucose increased gradually in the control group, the absence of significant changes in glucose levels in children treated with L-Ala-Gln suggests a better glycemic control in these patients (Figure 1).
CRP is an acute phase reactant protein pool produced by the liver as part of the inflammatory response to tissue injury such as infection or trauma15. Normal plasma level of CRP is less than 10 mg/L in healthy adults. The rapid increase in synthesis within hours of tissue injury suggests that it contributes to host defense, and that it is part of the innate immune response16. Serum levels of CRP can be used for early detection of surgical complications17. In this study the significant increase in CPR levels in control patients (Figure 5), comparing T-5 with basal values (T-1) and the absence of significant differences in CPR levels in L-Ala-Gln treated patients points to a possible attenuation of the surgical trauma response in GLN-treated children.
Il-6 is an important cytokine in the early inflammatory response to trauma. This cytokine is produced and detectable within an hour after trauma and it seems to play a dual role in the inflammatory response by acting as both a pro-inflammatory and anti-inflammatory mediator18,19. Parenteral GLN supplementation had a beneficial effect in reducing the systemic production of IL-6 after abdominal operations. Lower IL-6 levels probably improved nitrogen balance in these patients20. In our study, the increase in IL-6 levels in both groups (Figure 6) during the post-operative period signals increased inflammatory response regardless of pre-operative use of L-Ala-Gln. Therefore pretreatment with L-Ala-Gln did not suppress the rise of the proinflammatory cytokine IL -6.
Conclusion
Based on the observations presented above, there is a rationale for the potential benefit of glutamine. Pretreatment with L-Ala-Gln attenuates the inflammatory response in early post-operative period and promotes a better glycemic control in children undergoing elective cleft lip and palate repair.
Acknowledgment
The authors thank the Hospital Infantil Albert Sabin board for permission to use their facilities to conduct this study.
Conflict of interest: none
Financial source: none
1 Research performed at Walter Cantidio University Hospital and Experimental Surgery Research Laboratory (LABCEX), Federal University of Ceara (UFC), Brazil.
- 1. Krost B, Schubert J. Influence of season on prevalence of cleft lip and palate. Int J Oral Maxillofac Surg. 2006;35:215-8.
- 2. Lenz A, Franklin GA, Cheadle WG Systemic inflammation after trauma. Injury. 2007;38(12):1336-45.
- 3. Lacey JM, Crouch JB, Benfell K, Ringer SA, Wilmore CK, Maguire D, Wilmore DW. The effects of glutamine-supplemented parenteral nutrition in premature infants. JPEN J Parenter Enteral Nutr. 1996;20(1):74-80.
- 4. Bergström J, Fürst P, Norée LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974;36(6):693-7.
- 5. Wilmore DW. Metabolic response to severe surgical illness: overview. World J Surg. 2000;24(6):705-11.
- 6. Wilmore DW. The effect of glutamine supplementation in patients following elective surgery and accidental injury. J Nutr. 2001;131(9 Suppl):2543S-9S; discussion 2550S-1S. Review.
- 7. Déchelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coëffier M, Hecketsweiler B, Merle V, Mazerolles M, Samba D, Guillou YM, Petit J, Mansoor O, Colas G, Cohendy R, Barnoud D, Czernichow P, Bleichner G. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double-blind, multicenter study. Crit Care Med. 2006;34(3):598-604.
- 8. Choudhary S, Cadier MA, Shinn DL, Shekhar K, McDowall RA. Effect of Veau-Wardill-Kilner type of cleft palate repair on long-term midfacial growth. Plast Reconstr Surg. 2003;111(2):576-82; discussion 583-5.
- 9. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25(1):192-205.
- 10. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-10.
- 11. Trinder P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J Clin Pathol. 1969;22(2):246.
- 12. García-de-Lorenzo A, Zarazaga A, García-Luna PP, Gonzalez-Huix F, López-Martínez J, Miján A, Quecedo L, Casimiro C, Usán L, del Llano J. Clinical evidence for enteral nutritional support with glutamine: a systematic review. Nutrition. 2003;19(9):805-11.
- 13. Poindexter BB, Ehrenkranz RA, Stoll BJ, Koch MA, Wright LL, Oh W, Papile LA, Bauer CR, Carlo WA, Donovan EF, Fanaroff AA, Korones SB, Laptook AR, Shankaran S, Stevenson DK, Tyson JE, Lemons JA. Effect of parenteral glutamine supplementation on plasma amino acid concentrations in extremely low-birth-weight infants. Am J Clin Nutr. 2003;77(3):737-43.
- 14. Ward E, Smith M, Henderson M, Reid U, Lewis I, Kinsey S, Allgar V, Bowers D, Picton SV. The effect of high-dose enteral glutamine on the incidence and severity of mucositis in pediatric oncology patients. Eur J Clin Nutr. 2009; 63(1):134-40.
- 15. Jaye DL, Waites KB. Clinical applications of C-reactive protein in pediatrics. Pediatr Infect Dis J. 1997;16(8):735-46.
- 16. Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279(47):48487-90.
- 17. Ackland GL, Scollay JM, Parks RW, de Beaux I, Mythen MG. Pre-operative high sensitivity C-reactive protein and postoperative outcome in patients undergoing elective orthopaedic surgery. Anaesthesia. 2007;62(9):888-94.
- 18. Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101(2):311-20.
- 19. Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127(2):117-26.
- 20. Lin MT, Kung SP, Yeh SL, Liaw KY, Wang MY, Kuo ML, Lee PH, Chen WJ. Glutamine-supplemented total parenteral nutrition attenuates plasma interleukin-6 in surgical patients with lower disease severity. World J Gastroenterol. 2005;11(39):6197-201.
Publication Dates
-
Publication in this collection
23 Sept 2011 -
Date of issue
2011