Abstracts
PURPOSE: Current study is focused on extraction with methanol, purification, labeling with 131I using iodogen method of the yarrow plant and investigating in vivo biological activity using biodistribution and imaging studies on healthy animal models. The aim of the study is to contribute plant extracts to discover new drugs in the diagnosis and treatment of several diseases. METHODS: Nine female and nine male healthy Wistar albino rats, which were approximately 100-150 g in weight, were used for biodistribution studies. For imaging studies four healthy male Balb-C mice were used. Quality control studies were done utilizing thin layer radio chromatography (TLRC) and high performance liquid chromatography (HPLC) methods. For biodistribution studies, 131I radiolabeled Peak 7 (131I-Peak 7) was sterilized and injected into the tail veil of rats and imaging studies were obtained using Kodak FX PRO in vivo Imaging System. RESULTS: The radiolabeling yield of each purified the bioactive extracts of the yarrow plant, seven peaks was between 79 and 92%. The highest radiolabeling yield was calculated for 131I radiolabeled seventh peak (131I-Peak 7) (92.78±5.04, n=5). For this reason the biodistribution and imaging studies were done for 131I-Peak 7. That's why; these studies with Peak 7 were carried out. CONCLUSION: Peak 7 was radiolabeled with 131I in high yield for using imaging and therapeutic studies in nuclear medical applications.
Achillea; Luteolin; Iodine Isotopes; Chromatography, Liquid; Diagnostic Imaging; Rats
OBJETIVO: O atual estudo tem por objetivo a extração com metanol, purificação, marcação com I131 usando o método direto de marcação da planta Achillea, para investigar in vivo a atividade biológica usando biodistribuição e estudos de imagem em modelos animais saudáveis. O objetivo do estudo é contribuir com extratos de plantas para descobrir novas drogas para o diagnóstico e tratamento de várias doenças. MÉTODOS: Nove fêmeas e nove machos ratos Wistar albino saudáveis, com aproximadamente 100 a 150g de peso foram usados para estudos de biodistribuição. Para estudos de imagem, quatro camundongos Balb-C machos e saudáveis foram usados. Estudos de controle de qualidade foram realizados usando métodos de cromatografia de camada fina e cromatografia líquida de alta performance. Para estudos de biodistribuição, pico 5 radiografado com I131 (I131-Peak 7) foi esterilizado e injetado na veia da cauda dos ratos e estudos de imagem foram obtidos usando Sistema de Imagem Kodak FX PRO in vivo. RESULTADOS: O retorno radiomarcado de cada extrato bioativo purificado da planta Achillea sete picos estavam entre 79 e 92%. O retorno com maior marcação foi calculado para I131 sétimo pico (I131-Peak 7) (92,78±5,04, n=5). Por esta razão os estudos de biodistribuição e de imagem foram feitos para I131-Peak 7. CONCLUSÃO: Peak 7 foi radiomarcado com I131 em alto retorno para uso em estudos terapêuticos e de imagens nas aplicações médicas nucleares.
Achillea; Luteolina; Isótopos de Iodo; Cromatografia Líquida; Diagnóstico por Imagem; Ratos
3 - ORIGINAL ARTICLE
MODELS, BIOLOGICAL
Radiolabeling of methanol extracts of yarrow (Achillea millefolium l) in rats1 1 Research performed at the Department of Nuclear Applications, Institute of Nuclear Sciences, Ege University, Izmir-Turkey.
Radiomarcação do extrato metanólico de yarrow (Achillea millefolium l) em ratos
Betul CekicI; Ayfer Yurt KilcarII; Fazilet Zumrut Biber MuftulerIII; Perihan UnakIV; Emin Ilker MedineV
IFellow Master degree, Department of Nuclear Applications, Institute of Nuclear Sciences, Ege University. Carried out the study and drafted the manuscript
IIFellow PhD degree, Research Assistant, Department of Nuclear Applications, Institute of Nuclear Sciences, Ege University. Involved in technical procedures and animal experiments, manuscript writing
IIIAssociate Professor, Department of Nuclear Applications, Institute of Nuclear Sciences, Ege University. Responsible for conception, design, intellectual and scientific content of the study, supervised all phases of the study, manuscript writing and critical revision
IVFull Professor, Department of Nuclear Applications, Institute of Nuclear Sciences, Ege University. Compiled the results, critical revision
VPhD, Research Assistant, Department of Nuclear Applications, Institute of Nuclear Sciences, Ege University. Involved in technical procedures and animal experiments
ABSTRACT
PURPOSE: Current study is focused on extraction with methanol, purification, labeling with 131I using iodogen method of the yarrow plant and investigating in vivo biological activity using biodistribution and imaging studies on healthy animal models. The aim of the study is to contribute plant extracts to discover new drugs in the diagnosis and treatment of several diseases.
METHODS: Nine female and nine male healthy Wistar albino rats, which were approximately 100-150 g in weight, were used for biodistribution studies. For imaging studies four healthy male Balb-C mice were used. Quality control studies were done utilizing thin layer radio chromatography (TLRC) and high performance liquid chromatography (HPLC) methods. For biodistribution studies, 131I radiolabeled Peak 7 (131I-Peak 7) was sterilized and injected into the tail veil of rats and imaging studies were obtained using Kodak FX PRO in vivo Imaging System.
RESULTS: The radiolabeling yield of each purified the bioactive extracts of the yarrow plant, seven peaks was between 79 and 92%. The highest radiolabeling yield was calculated for 131I radiolabeled seventh peak (131I-Peak 7) (92.78±5.04, n=5). For this reason the biodistribution and imaging studies were done for 131I-Peak 7. That's why; these studies with Peak 7 were carried out.
CONCLUSION: Peak 7 was radiolabeled with 131I in high yield for using imaging and therapeutic studies in nuclear medical applications.
Key words: Achillea. Luteolin. Iodine Isotopes. Chromatography, Liquid. Diagnostic Imaging. Rats.
RESUMO
OBJETIVO: O atual estudo tem por objetivo a extração com metanol, purificação, marcação com I131 usando o método direto de marcação da planta Achillea, para investigar in vivo a atividade biológica usando biodistribuição e estudos de imagem em modelos animais saudáveis. O objetivo do estudo é contribuir com extratos de plantas para descobrir novas drogas para o diagnóstico e tratamento de várias doenças.
MÉTODOS: Nove fêmeas e nove machos ratos Wistar albino saudáveis, com aproximadamente 100 a 150g de peso foram usados para estudos de biodistribuição. Para estudos de imagem, quatro camundongos Balb-C machos e saudáveis foram usados. Estudos de controle de qualidade foram realizados usando métodos de cromatografia de camada fina e cromatografia líquida de alta performance. Para estudos de biodistribuição, pico 5 radiografado com I131 (I131-Peak 7) foi esterilizado e injetado na veia da cauda dos ratos e estudos de imagem foram obtidos usando Sistema de Imagem Kodak FX PRO in vivo.
RESULTADOS: O retorno radiomarcado de cada extrato bioativo purificado da planta Achillea sete picos estavam entre 79 e 92%. O retorno com maior marcação foi calculado para I131 sétimo pico (I131-Peak 7) (92,78±5,04, n=5). Por esta razão os estudos de biodistribuição e de imagem foram feitos para I131-Peak 7.
CONCLUSÃO: Peak 7 foi radiomarcado com I131 em alto retorno para uso em estudos terapêuticos e de imagens nas aplicações médicas nucleares.
Descritores: Achillea. Luteolina. Isótopos de Iodo. Cromatografia Líquida. Diagnóstico por Imagem. Ratos.
Introduction
The genus Achillea is represented by about 110-140 species mostly found in Europe and Asia and a few species native to northern Africa and North America1.About forty species of Achillea are located in Turkey2. Edirne is one of the places where members of Achillea family are located in yarrow particularly. Yarrow (Achillea millefolium L. s. l.) which has been used as medicinal herb for a long time, is one of the most important drug used both in folk and official medicine today3. Achillea millefolium plants have been used for centuries for treat spasms, digestive complaints, menstruation disorders, urinary infections, anti inflammatory, spasmolytic, hemostatic, diarrhea, abdominal pain, stomach ache and other ailments4. Also it has been reported Achillea millefolium plants have some pharmacological effects such as antispasmodic, antimicrobial, analgesic, antipyretic, choleretic, cytotoxic, and estrogenic5. It is believed that these effects are mainly attributed to the flavonoid and phenolcarbonic acid complex6. Flavonoids are polyphenolic compounds that can be found in natural products, plants and fruits7.
Yarrow contains many bioactive compounds. Some of them are achilleine, apigenin, luteolin, azulene, camphor, coumarin, inulin, menthol, quercetin, rutin, succinic, salicylic and caffeic acids3. On the other hand, luteolin, quercetin, chrysin, and kaempferol have antiestrogenic effect8.
Iodine131 is an important isotope of iodine which is used in diagnostic and therapeutic nuclear medicine due to its decay properties. Its half life is 8.04 days and decays through beta and gamma emissions9.
In this study; the bioactive components of the collected yarrow samples from Edirne were obtained by extraction, purification methods and MALDI/TOF spectra are used for the identification of them.
The aim of the present study was to radiolabel the bioactive extracts of the yarrow plants with 131I using iodogen method and to investigate their in vivo biological activity using biodistribution and imaging studies in healthy animal models. On the other hand, there is no study using the extracts of yarrow plant which have lots of pharmacologic activity, as radiolabeled compounds in the literature.
Methods
The experimental protocol was approved by the Institutional Animal Review Committee of Ege University, (Number: 2011-205) Izmir, Turkey. The rats and mice were kept according to the ethical principles of the Ege University.
Nine female and nine male healthy Wistar albino rats weighing 100 to 150g and four healthy male Balb-C mice weighing 20 to 25g were used. The animals were kept in light-darkcycles (12/12h) with free access to food and water.
Experimental Procedures
Plant material and extraction
The yarrow plants (Achillea millefolium L.) were collected in Edirne, Turkey. The collected plants were dried and powdered.
Ten grams of the powdered plant material were extracted with 0.43 L of 20% (v/v) methanol under reflux for 120 min. After centrifuging at 2500 rpm for 5 minutes, the procedure was repeated for three times. Supernatant (upper organic phase) was separated out and collected. Methanol was evaporated at 40°C about 12 hours and the remaining aqueous solution was lyophilisated. Crude plant extract was obtained 1.73g, approximately.
All crude plant extract (1.73g) was dissolved with 0.05 L of 20 % (v/v) methanol. For the solid phase extraction, C18-seppak cartridges were used. Finally, all of the fractions were collected and kept at -4°C for using next experimental step10.
Purification of the extract with High Performance Liquid Chromatography (HPLC)
The extract samples of the yarrow were purified by using HPLC. A low pressure gradient HPLC system (LC-10ATvp quaternary pump and SPD-10A/V UV detector with syringe injector equipped with a 20 µL loop and 5-µm RP-C18 column (250×4.6 mm I.D., Macharey-Nagel) was used for analytical and purification experiments. The chromatographic conditions were presented in Table 1.
Radiolabeling procedure of the purified bioactive components of yarrow extract with 131I
Preparation of the iodogen coated tubes
Prior to radiolabeling with 131I, 250µg iodogen (1, 3, 4, 6-tetrachloro-3α, 6α-diphenylglycoluril) was dissolved in 250 µL dichloromethane and then solution was evaporated successively. The solvent was allowed to evaporate forming a thin solid layer on the wall of the reaction vials. These tubes were stored at +4°C until use.
Radiolabeling of the purified bioactive componentsusing131I
Twenty five µg of each purified extracts of yarrow was added into the prepared iodogen coated tubes one by one and then 100 µCi (3.7 MBq) of 131I was added. This mixture was incubated 30 minutes at room temperature. At the end of the incubation time, the Relative front (Rf) values and radiolabeling yield of the each radiolabeled purified extracts of yarrow were calculated by using Thin layer radiochromatography (TLRC) method.
Quality control procedure of the radiolabeled bioactive components
Thin Layer Radio Chromatography (TLRC)
The highest radiolabeling yield was calculated for 131I radiolabeled seventh peak (131I-Peak 7). The radiolabeled purified extracts of the yarrow were assessed by TLRC using cellulose plates. N-butanol/ ethyl alcohol / 0.2 N NH4OH (5:2:1) was used as mobile phase. The cellulose plates were counted by TLC Scanner (BioscanAR 2000). The Relative front (Rf) values and yield of the radiolabeled compounds [131I, oxidated131I (Oxi. 131I) and131I-Peak 7] were calculated.
Structural analysis
MALDI-TOF spectra were taken in order to identify the chemical structure of purified extracts of the yarrow at the Laboratory of Biological Mass Spectrometry at Institute of Technology Faculty of Science Department of Chemistry, Izmir, Turkey. Table 2 shows the bioactive components of the purified extracts and their theoretical LogP values according to ACD/I Lab (Version 6) for supporting the structural analysis. Luteolin compound as standard was used for structural analysis of each purified extracts of the yarrow.
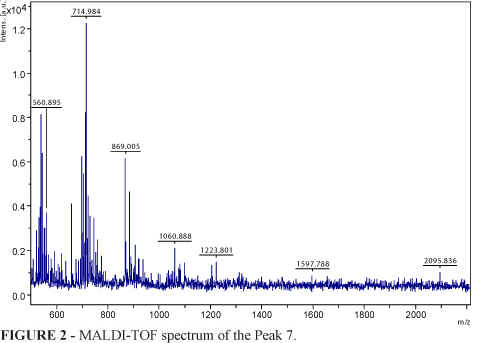
According to Table 2, Peak 7 includes luteolin. MALDI-TOF spectrum of Peak 7 and MALDI-TOF spectrum of Luteolin standart were given in Figures 2 and 3, respectively.
Biodistribution studies of 131I-Peak 7
Animal experiments were carried out under the approval of the relevant Institutional Animal Review Committee of Ege University, (Number: 2011-205) Izmir, Turkey. For blocking of iodine uptake into the thyroid gland, 10 mg of potassium iodide was added to one liter drinking water of the rats. Radiolabeled with 131I Peak 7 (131I-Peak 7) was sterilized by passing through a 0.22 μm membrane filter and was injected into the tail vein of rats. The activity was 5.55 MBq (150 µCi (0.2 mL)/20 µg 131I- Peak 7 per rat) approximately. The rats were euthanized at 30, 60 and 120 minutes post-injection by injection of sodium pentobarbital (200 mg/kg) by intraperitoneal via. After euthanasia tissues of interest were isolated, weighted and counted by Cd(Te) detector. The percentage of injected dose per gram of tissue weight (% ID/g) was determined.
In vivo imaging studies
The imaging studies were performed using the Kodak FX PRO in vivo Imaging System (Metis Biotechnology Ltd, Ankara, Turkey). The 131I labeled product was sterilized by passing through a 0.22 μm membrane filter and injected into the tail vein of male Balb-C mice. The injected mass of 131I-Peak 7 was 20 µg/mice and activity was 5.55MBq (150 µCi/0.2 mL) approximately. A supplemental dose of alfazine and alfamine were used.
Statistical analysis
Data were analyzed statistically by using SPSS 13 program (Univariate Variance Analyses and Pearson Correlation). Probability values <0.05 were considered significant. Pearson correlation was carried out between the organs for 131I-Peak-7.
Results
The chromatogram of the bioactive extract of the yarrow is shown in Figure 1. There are seven peaks which called as Peak 1, Peak 2, Peak 3, Peak 4, Peak 5, Peak 6 and Peak 7, respectively. Retention time (Rt) value of Peak 7 was determined 37.88 minutes at the conditions shown in Table 1.
Basic fragments in yarrow and their theoretical LogP values according to ACD/I Lab (Version 6) was given in Table 3. According to Table 2, it is thought that Peak 7 includes luteolin which have high lipophilicity. This result was also supported by MALDI-TOF structural analysis (Figures 2 and 3).
According to MALDI-TOF spectrum of Peak 7 (as seen in Figure 2), the molecular fragments at m/z 554.45 and 714.58 belong to the fragments which could be two luteolin dimerization products and MALDI-TOF spectrum of luteolin standart was given in Figure 3.
Yurt Kýlçar et al.11 studied on radiolabeling purified the bioactive components of the yarrow with 125I and their effects on the MCF-7, PC-3, A-549 and Caco-2 cell lines. According to unpublished data, the incorporation rate of radiolabeled Peak 7 was calculated 3 times higher than other radiolabeled peaks11.
According to the TLRC results, the Rf values of radiolabeled compounds were calculated for solvent system as presented in Table 3. The radiochemical yield of 131I-Peak 7 for mobile phase [n-butanol/ethyl alcohol/0.2 N NH4OH (5:2:1)] was 92.78±5.04 % (n=5).
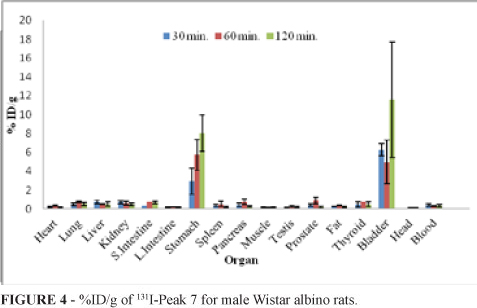
The % ID/g values of the 131I-Peak 7 biodistribution in male and female Wistar Albino rats are illustrated in Figures 4 and 5. As is seen on the biodistribution studies, no thyroid uptake was observed during 1440 minutes following the administration of 131I-Peak 7 and this has also indicated a high radioiodination yield of 131I labeled Peak 7. In both the figures, uptake of stomach and urinary bladder are significantly higher than other tissues. Maximum bladder uptake for male rats is at 120 min. (11.59±6.14) and for female rats is at 60 min. (3.90±2.01). The uptake of stomach reached a maximum value (7.35) at 60 min. for female rats and (8.02) at 120 min. for male rats. The stomach uptakes of 131I-Peak 7 for male and female rats are considerably high for each time periods (p<0.05). The stomach uptake for male rats was 2.95±1.40 at 30 min. and it increased 2.72 fold at 120 min. For female rats it also increased 2.10 fold at 60 min.
Differently, large intestine uptake for female Wistar Albino rats is higher than male rats. Large intestine uptake for male and female rats are 0.21±0.02 and 5.61±2.56 at 60 min., 0.16±0.06 and 5.57±1.67 at 120 min., particularly (p<0.05). In addition there is also an alteration on time response uptake of prostate, pancreas, uterus, breast, and ovary. At 30 min. prostate uptake is 0.43±0.11, at 60 min. 0.89±0.35, at 120 min. 0.17±0.10, respectively. Prostate uptake increased 2.09 fold at 60 min. and decreased at 120 min. 2.50 fold. At 30 min. pancreas uptake for male and female rats are 0.45±0.18 and 0.64±0.24 at 60 min., 0.75±0.29 and 0.22±0.03 at 120 min., 0.25±0.09 and 0.90±0.45, respectively. Maximum uterus and ovary uptake were 0.47±0.17 and 0.37±0.18 at 30 min., respectively. The uptake of 131I-Peak 7 reached the highest value (0.81±0.16) in the breast tissue at 30 min. it showed a decrease during period time. There is no significant alteration on the % ID/g of tissues from heart, lung, liver, small intestine, spleen, muscle, fat, thyroid, testis, head and blood.
Figure 6 showed that static images of Peak 7 radiolabeled with 131I were obtained at 10 and 1440 min on male Balb-C mice. According to static images which were obtained at 10 and 1440 mins on male Balb-C mice, 131I-Peak 7 had high uptake on intestinal system and urinary bladder in 10 mins (Figure 6a). On the other hand 131I-Peak 7 had an uptake of thyroid uptake at 24th hours (Figure 6b).
Discussion
Extraction of yarrow was performed by using Benedek's et al.10 method. In the presence of Peak 7, the molecule might be activated towards electrophilic iodine attack by ortho-position of the dihydroxyphenyl ring on the luteolin molecule.
Some reports indicated that Achillea millefolium includes variety of flavonoid which mainly occurs as mono and diglycosides of apigenin, luteolin and quercetin and phenolic compounds which have antioxidant properties2,6. Furthermore, it was determined that some of flavonoid and polyphenols have estrogenic/antiestrogenic effect by binding intracellular estrogen receptors (ERs)7. In the literature, luteolin, quercetin, chrysin, and kaempferol were demonstrated to be antiestrogenic8. Our results supported that Peak 7 consists of luteolin according to MALDI-TOF and HPLC analyses. Due to luteolin, Peak 7 has phytoestrogenic effect.
Beside these properties of Achillea millefolium, the potent gastric anti-secretory and gastroprotective activity in models of acute and chronic gastric injury are showed12. Also, Pinto et al.5 showed hydroalcoholic extract of Achillea millefolium have gastroprotective effects in rat stomach due to its antioxidant properties. Also, it was determined that the stomach contains sex steroid receptors such as estrogen and androgen13. So, antiestrogenic properties and gastroprotective activity of bioactive compounds in Achillea millefolium could cause high stomach uptake. Because of being estrogen receptors, the uptake of stomach and intestinal system was very high in biodistribution study of 131I-Peak 7 for male and female rats. As expected biodistribution studies results of 131I-Peak 7 supported the imaging studies results.
Experimental results obtained in current study demonstrated that the Peak 7 which is one of the bioactive components of Yarrow extract could be easily radiolabeled with 131I radionuclide which means that should be radiolabeled with other iodine radioisotopes promising a wide use in Nuclear Medicine for both imaging and therapeutic agents.
Due to the recently described study, further insights into the cancer imaging potential of yarrow is supported and confirmed the traditional use and benefit of the yarrow plant. Current study is a contribution to the area of the plant extracts use for medicinal purposes.
Further investigations with animal models and cell culture experiments need to be performed to show that Peak 7 may be used as an imaging and/or a therapeutic agent because of the convenient properties 131I radionuclide and its other radioisotopes. Radiolabeled Peak 7, which is a component of yarrow plant extract, could be used as a novel plant origin agent for diagnosis and therapy of cancer.
Conclusion
Peak 7 was radiolabeled with 131I in high yield for using imaging and therapeutic studies in nuclear medical applications.
Acknowledgements
The authors thank PhD. student Hasan ZORA and MSc. student MeltemOZKAN for technical help, Mr. Ertan TASKIN for providing the yarrow plant, Mr. Muhittin AKSOY for kind assistance in imaging studies from Metis Biotechnology Ltd, Ankara Turkey, Dr. Filiz YESILIRMAK and Dr.AhmetEmin ATIK from the Laboratory of Biological Mass Spectrometryat Institute of Technology Faculty of Science Department of Chemistry Izmir, Turkey for their help with mass measurements.
Received: December 12, 2011
Review: February 14, 2012
Accepted: March 15, 2012
Conflict of interest: none
Financial source: Ege University Research Fund
- 1. Applequist WL, Moerman DE. Yarrow (Achillea millefolium L.): A Neglected Panacea? A Review of Ethnobotany, Bioactivity, and Biomedical Research. Econ Bot. 2011;65(2):209-5.
- 2. Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sökmen A, Akpulat HA. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J Ethnopharmacol. 2003; 87:215-20.
- 3. Bocevska M, Sovov H. Supercritical CO2 extraction of essential oil from yarrow. J Supercrit Fluid .2007;40:360-7.
- 4. Jonsdottir G, Omarsdottir S, Vikingsson A, Hardardottir I, Freysdottir J. Aqueous extracts from Menyanthes trifoliate and Achillea millefolium affect maturation of human dendritic cells and their activation of allogeneic CD4+ T cells in vitro. J Ethnopharmacol. 2011;136:88-93.
- 5. Potrich FB, Allemand A, Silva LM, Santos AC, Baggio CH, Freitas CS, Mendes DAGB, Andre E, Werner MFP, Marques MCA. Antiulcerogenic activity of hydroalcoholic extract of Achillea millefolium L.: involvement of the antioxidant system. J Ethnopharmacol. 2010;130:85-92.
- 6. Trumbeckaite S, Benetis R, Bumblauskiene L, Burdulis D, Janulis V, Toleikis A, Viškelis P, Jakštas V. Achillea millefolium L. s.l. herb extract: Antioxidant activity and effect on the rat heart mitochondrial functions. Food Chem. 2011;127:1540-8.
- 7. Huang Z, Fang F, Wang J, Wong CW. Structural activity relationship of flavonoids with estrogen-related receptor gamma. FEBS Lett. 2010;584:22-6.
- 8. Pinto B, Caciagli F, Riccio E, Reali D, Saric A, Balog T, Likic S, Scarpato R. Antiestrogenic and antigenotoxic activity of bee pollen from Cystusincanus and Salix alba as evaluated by the yeast estrogen screen and the micronucleus assay in human lymphocytes. Eur J Med Chem. 2010;45:4122-8.
- 9. Morita T, Niwa K, Fujimoto K, Kasai H, Yamada H, Nishiutch K, Sakamoto T, Godo W, Taino S, Hayashi Y, Takeno K, Nishigaki T, Fujiwara K, Aratake H, Kamonoshita S, Hashimoto H, Kobayashi T, Otosaka S, Imanaka T. Detection and activity of iodine-131 in brown algae collected in the Japanese coastal areas. Sci Total Environ. 2010;408(16):3443-7.
- 10. Benedek B, Kopp B, Melzig FM. Achillea millefolium L. s.l. - Is the anti-inflammatory activity mediated by protease inhibition? J Ethnopharmacol. 2007;113:312-7.
- 11. Yurt Kýlçar A, Cekic B, Medine EÝ, BiberMüftüler FZ, Unak P. Extraction of Yarrow, Purification, Radiolabeling, in vitro Evaluation on Cell Cultures and imaging on Balb-C Mice. 3rd East Mediterranean ICLAS Symposium Abstrack Book 2011 June; 35.
- 12. Cavalcanti AM, Baggio CH, Freitas CS, Rieck L, Sousa RS, Santos JES, Vela SM, Marques MCA. Safety and antiulcer efficacy studies Achillea millefolium L. after chronic treatment in Wistar rats. J Ethnopharmacol. 2006;107:277-84.
- 13. Biber Muftuler FZ, Unak P, Teksoz S, Acar C, Yolcular S, Yurekli Y. 131I labeling of tamoxifen and biodistribution studies in rats. Appl Radiat Isotopes. 2008;66:178-87.
Publication Dates
-
Publication in this collection
01 June 2012 -
Date of issue
May 2012
History
-
Received
12 Dec 2011 -
Accepted
15 Mar 2012 -
Reviewed
14 Feb 2012