Abstracts
PURPOSE: To investigate synergistic suppression of donor liver pre-perfusion with recipient serum (RS) and cobra venom factor (CVF) treatment on hyperacute rejection (HAR) following liver xenotransplantation. METHODS: Guinea-pigs (GP, n=24) and Sprague-Dawley rats (SD, n=24) were recruited. Before transplantation, serum was collected from SD rats and used for preparation of inactivated complements. GP and SD rats were randomly assigned into four groups (n=6), respectively: RS group, CVF group, RS+CVF group and control group. Orthotopic liver xenotransplantation was performed with modified two-cuff technique. The survival time and liver function of recipients, morphological and pathological changes in rat livers were investigated. RESULTS: There was no piebald like change in the recipient livers in all experiment groups. The survival time of recipients in all experiment groups was longer than that in control group (p<0.05). Moreover, the survival time in the RS+CVF group was markedly longer than that in the RS group (p<0.01) and CVF group (p<0.05). The serum ALT level in all experiment groups were lower than that in the control group (p<0.05). Furthermore, the ALT level in the RS+CVF group was significantly lower than that in the CVF group (p<0.05) and RS group (p<0.01). The histological damages were significantly improved when compared with the control group, and the histological damages in the RS+CVF group were milder than those in the remaining groups (p<0.05) CONCLUSION: Pre-perfusion of donor liver with recipient serum and cobra venom factor treatment can exert synergistic suppressive effects on the hyperacute rejection following liver xenotransplantation.
Transplantation, Heterologous; Liver; Hypersensitivity, Immediate; Guinea Pigs; Rats
OBJETIVO: Investigar a supressão sinérgica da pré-perfusão do doador de fígado com soro do receptor (SR) e tratamento com fator veneno de cobra (FVC) na rejeição hiperaguda (RHA) após o xenotransplante de fígado. MÉTODOS: Foram utilizados Cobaias (GP, n=24) e ratos Sprague-Dawley (SD, n=24). Antes do transplante foram coletadas amostras de soro dos ratos SD e usados para a preparação dos complementos inativados. Cobaias GP e ratos SD foram randomicamente distribuídos em quatro grupos (n=6), respectivamente: grupo RS, grupo FVC, grupo SR+FVC e grupo controle. Xenotransplante ortotópico do fígado foi realizado com a técnica de dois cuffs modificados. Foram investigados o de tempo de sobrevida, a função hepática dos receptores e alterações morfopatológicas em fígados de ratos. RESULTADOS: Não houve alteração na coloração do parênquima dos fígados nos receptores. O tempo de sobrevida dos receptores em todos os grupos experimentais foi mais longo do que o grupo controle (p<0,05). Além disso, o tempo de sobrevida do grupo SR+ FVC foi marcadamente maior do que o grupo SR (p<0,01) e o grupo FVC (p<0,05). O nível sérico ALT foi menor em todos os grupos experimentais do que o grupo controle (p<0,05). O nível de ALT no grupo SR+ FVC foi significantemente menor do que no grupo FVC (p<0,05) e o grupo SR (p<0,01). As alterações histológicas foram significantemente melhoradas quando comparado com o grupo controle, e os danos histológicos no grupo SR+ FVC foram mais moderados do que nos grupos restantes (p<0,05). CONCLUSÃO: Pré-perfusão do fígado doador com soro do receptor e fator veneno de cobra pode exercer efeito supressor sinérgico da rejeição hiperaguda após xenotransplante de fígado.
Transplante Heterólogo; Fígado; Hipersensibilidade Imediata; Cobaia; Ratos
4 - ORIGINAL ARTICLE
MODELS, BIOLOGICAL
Synergistic suppression of pre-perfusion of donor livers with recipient serum and cobra venom factor treatment on hyperacute rejection following liver xenotransplantation1 1 Research performed at Department of Sugery, 1 st Clinical Center of Guangdong Medical College, Zhanjiang, Guangdong, China.
Supressão sinérgica da rejeição hiperaguda no xenotransplante hepático com uso da pre-perfusão dos fígados doadores tratados com soro do receptor e com fator veneno de cobra
Baohua ZhuI; Chuanming TongII; Weitao GuoIII; Rong PuII; Guoping ZhangIV; Lantian WangV; Mingyi LiV
IMD, Department of Sugery, 1st Clinical Center of Guangdong Medical College, Zhanjiang, Guangdong, China. Main author, study design and manuscript writing
IIMD, Department of Sugery, 1st Clinical Center of Guangdong Medical College, Zhanjiang, Guangdong, China. Acquisition and interpretation of data
IIIMD, Department of Sugery, 1st Clinical Center of Guangdong Medical College, Zhanjiang, Guangdong, China. Responsible for conception of the study and critical revision
IVMD, Department of Sugery, 1st Clinical Center of Guangdong Medical College, Zhanjiang, Guangdong, China. Critical revision
VMD, Department of Sugery, 1st Clinical Center of Guangdong Medical College, Zhanjiang, Guangdong, China. Statistical analysis
Correspondence Correspondence: Prof. Baohua Zhu 1 st Clinical Center of Guangdong Medical College Zhanjiang, Guangdong 524023, China zhuless@126.com
ABSTRACT
PURPOSE: To investigate synergistic suppression of donor liver pre-perfusion with recipient serum (RS) and cobra venom factor (CVF) treatment on hyperacute rejection (HAR) following liver xenotransplantation.
METHODS: Guinea-pigs (GP, n=24) and Sprague-Dawley rats (SD, n=24) were recruited. Before transplantation, serum was collected from SD rats and used for preparation of inactivated complements. GP and SD rats were randomly assigned into four groups (n=6), respectively: RS group, CVF group, RS+CVF group and control group. Orthotopic liver xenotransplantation was performed with modified two-cuff technique. The survival time and liver function of recipients, morphological and pathological changes in rat livers were investigated.
RESULTS: There was no piebald like change in the recipient livers in all experiment groups. The survival time of recipients in all experiment groups was longer than that in control group (p<0.05). Moreover, the survival time in the RS+CVF group was markedly longer than that in the RS group (p<0.01) and CVF group (p<0.05). The serum ALT level in all experiment groups were lower than that in the control group (p<0.05). Furthermore, the ALT level in the RS+CVF group was significantly lower than that in the CVF group (p<0.05) and RS group (p<0.01). The histological damages were significantly improved when compared with the control group, and the histological damages in the RS+CVF group were milder than those in the remaining groups (p<0.05)
CONCLUSION: Pre-perfusion of donor liver with recipient serum and cobra venom factor treatment can exert synergistic suppressive effects on the hyperacute rejection following liver xenotransplantation.
Key words: Transplantation, Heterologous. Liver. Hypersensitivity, Immediate. Guinea Pigs. Rats.
RESUMO
OBJETIVO: Investigar a supressão sinérgica da pré-perfusão do doador de fígado com soro do receptor (SR) e tratamento com fator veneno de cobra (FVC) na rejeição hiperaguda (RHA) após o xenotransplante de fígado.
MÉTODOS: Foram utilizados Cobaias (GP, n=24) e ratos Sprague-Dawley (SD, n=24). Antes do transplante foram coletadas amostras de soro dos ratos SD e usados para a preparação dos complementos inativados. Cobaias GP e ratos SD foram randomicamente distribuídos em quatro grupos (n=6), respectivamente: grupo RS, grupo FVC, grupo SR+FVC e grupo controle. Xenotransplante ortotópico do fígado foi realizado com a técnica de dois cuffs modificados. Foram investigados o de tempo de sobrevida, a função hepática dos receptores e alterações morfopatológicas em fígados de ratos.
RESULTADOS: Não houve alteração na coloração do parênquima dos fígados nos receptores. O tempo de sobrevida dos receptores em todos os grupos experimentais foi mais longo do que o grupo controle (p<0,05). Além disso, o tempo de sobrevida do grupo SR+ FVC foi marcadamente maior do que o grupo SR (p<0,01) e o grupo FVC (p<0,05). O nível sérico ALT foi menor em todos os grupos experimentais do que o grupo controle (p<0,05). O nível de ALT no grupo SR+ FVC foi significantemente menor do que no grupo FVC (p<0,05) e o grupo SR (p<0,01). As alterações histológicas foram significantemente melhoradas quando comparado com o grupo controle, e os danos histológicos no grupo SR+ FVC foram mais moderados do que nos grupos restantes (p<0,05).
CONCLUSÃO: Pré-perfusão do fígado doador com soro do receptor e fator veneno de cobra pode exercer efeito supressor sinérgico da rejeição hiperaguda após xenotransplante de fígado.
Descritores: Transplante Heterólogo. Fígado. Hipersensibilidade Imediata. Cobaia. Ratos.
Introduction
Activation of complements in recipients is a critical step in hyperacute rejection (HAR) following xenotransplantation1. There is evidence showing that cobra venom factor (CVF) can clear complements in recipients and inhibit the occurrence of HAR in non-coordinated transplantation2-5. Miyagawa et al.6 postulated that an alternative pathway of complement activation is involved in the HAR in guinea pigs receiving xenotransplantation, in which no natural antibodies are required. However, other researchers also found the involvement of nature antibodies in this process7. Our previous study revealed pre-perfusion of donor liver with recipient serum (RS) could block xenoantigens of the donor through natural antibodies in recipients, which then inhibited the HAR following liver xenotransplantation. The present study further investigated the synergistic suppressive effect of pre-perfusion of donor liver with RS and CVF treatment on HAR following liver xenotransplantation.
Methods
Animals and main reagents
Donors were healthy PG (male or female) weighing 200~240g. Recipients were adult healthy Sprague-Dawley (SD) rats (male) weighing 240~280g. Animals were purchased from the Experimental Animal Center of Guangdong Medical College and housed in a specific pathogen free environment. CVF was purchased from Kunming Beijie Biotechnology Co., Ltd. Ether, atropine, Ringer's solution, heparin sodium and 5% sodium bicarbonate were purchased from Beijing Fresenius Kabi Pharmaceutical Co. Ltd.
Collection and inactivation of RS
Adult SD rats were anesthetized with ether followed by laparotomy under an aseptic condition. Blood was collected from the abdominal aorta and then kept at room temperature for 2h. The serum was collected and kept at 45ºC for 30 min to inactivate complements. Then, the serum was stored at 4ºC for use.
Pre-perfusion of donor liver with RS
The RS above was added to pre-cold Ringer's solution to prepare 0.1% RS solution (blocking solution) which was then stored at 4ºC. The donor animals (PGs) were anesthetized with ether followed by laparotomy, and 100 U heparin in 2 ml of normal saline was injected through the distal inferior vena cava. The liver was separated followed by portal vein catheterization and 10 ml of 0~4ºC Ringer's solution (12.5 U of heparin in each ml) were used to perfuse the liver at 2.5 ml/min. When the liver became white, the inferior vena cava under the liver and the thoracic inferior vena cava were opened to excrete the Ringer's solution. Then, 10 ml of blocking solution were used for perfusion again for 5 min and the liver was collected and immersed in blocking solution. The liver was trimmed and the portal vein and inferior vena cava cannulas were placed. Subsequently, the liver was stored at 4ºC and liver transplantation was performed within 1h.
Orthotopic liver transplantation
PGs and SD rats served as donors and recipients, respectively. Eight hours before transplantation, animals received food and water deprivation and were intramuscularly treated with 0.03 mg of atropine at 15 min before transplantation. Animals were anesthetized with ether and orthotopic liver transplantation was performed with modified two-cuff technique8,9. Transplantation was performed by the same investigator. The cold ischemia time of the liver was 50.3±10.1 min and warm ischemia time was almost 0 min. In addition, the liver free time was 21.5±2.0 min and the time for surgery was 72.0±16.9 min.
Grouping and treatments
PG (n=24) and SD rats (n=24) were matched randomly and then divided into 4 groups (n=6 per group), respectively. (1) RS group: Before transplantation, the donor liver was perfused with Ringer's solution and then with 0.1% RS solution to block donor liver. (2) CVF group: The recipients were intravenously treated with CVF (50 µg/kg) via the tail vein at 24 h before transplantation. Other treatments were similar to those in (1). (3) RS+CVF group: Treatments in both (1) and (2) were simultaneously done in donor liver and recipients, respectively. (4) Control group: Before transplantation, the donor livers were perfused with Ringer's solution. Other treatments were similar to those above.
Observations
(1) Observation of survival time: Following liver transplantation, the survival time was recorded since the wound closure. Survival was defined as spontaneous breathing and heart beating, and death as absence of respiration and heart beating. The mean survival time was calculated. (2) Morphological examination of transplanted livers: Since the opening of portal vein, the blood supply to the liver was observed: blood filling, liver color and intrahepatic thrombosis. (3) Histological examination of transplanted liver: Once the breathing and heart beating was discontinued, the transplanted livers were sampled at different sites followed by fixation, sectioning and HE staining. The histological examination was done under a light microscope. According to the pathological damages to the liver (microthrombi, interstitial bleeding and hepatocyte swelling), scoring was performed: 1, mild damage; 2, moderate damage; 3, severe damage. The sum of different scores was calculated (0~9). (4) Detection of liver function of the recipients: Once breathing was discontinued, blood was collected from the heart and kept at room temperature for 30 min followed by centrifugation at 3000 r/min and collection of serum. The ALT was measured by using CL-7200 automatic biochemical analyzer (Shimadzu, Japan).
Statistical analysis
Data were expressed as mean ± standard deviation, and statistical analysis was performed with SPSS version 13.0. One way analysis of variance was employed for comparisons of differences among different groups and LSD method for comparisons between two groups. A value of p<0.05 was considered statistically significant.
Results
Hyperacute rejection following orthotopic liver transplantation and pathological findings
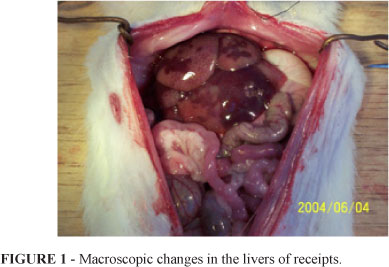
The cold ischemia time of the liver was 50.3±10.1 min and warm ischemia time was almost 0 min. In addition, the liver free time was controlled at 21.5±2.0 min and the time for surgery was 72.0±16.9 min. When the portal vein was opened, the blood filling was uneven. Part of liver became dark purple, and incomplete perfusion and edema were also present and piebald-like (Figure 1).
At 15 min after transplantation, the receipts developed tachycardia, tachypnea and systemic seizure. At 45.3±13.9 min after transplantation, respiratory arrest and cardiac arrest were observed and animals died. Pathological examinations of transplanted livers showed evident microthrombi, interstitial bleeding and edema, but obvious cell infiltration was absent (Figure 2).
Effects of pre-perfusion of donor liver with RS and CVF on reperfusion of the liver following liver transplantation
Following liver re-perfusion, the blood filling in the liver was uneven in the control group and typical piebald-like changes were found about 5~10 min after re-perfusion. In the RS group, the blood filling was not completely even, and the liver became dark purple but evident thrombosis was absent. In the CVF group and RS+CVF group, the blood filling was rapid and even and the liver remained red at 10~15 min after re-perfusion.
Effects of pre-perfusion of donor liver with RS and CVF on the survival time following liver transplantation
After transplantation, the survival time in the experiment groups was significantly longer than that in the control group (p<0.05). In addition, the survival time in the RS+CVF group was marked prolonged when compared with RS group and CVF group (p<0.05) (Table 1).
These findings suggest pre-perfusion of donor liver with RS can enhance the suppression of CVF on the HAR following liver xenotransplantation.
Effects of pre-perfusion of donor liver with RS and CVF on the liver function following liver transplantation
The ALT level in all experiment groups were significantly lower than that in the control group (p<0.05). In addition, the ALT level in the RS+CVF group was markedly lower than that in the RS group and CVF group (p<0.05) (Table 2).
These findings suggest pre-perfusion of donor liver with RS in combination with CVF treatment can favorably alleviate the HAR induced damage to the liver function following liver xenotransplantation.
Effects of pre-perfusion of donor liver with RS and CVF on the liver tissues following liver transplantation
In all experiment groups, the pathological damages to the transplanted liver including microthrombi, interstitial bleeding and hepatocyte swelling were significantly alleviated when compared with the control group, and thus the pathological score (sum) in all experiment groups was markedly lower than that in the control group (p<0.05). Moreover, there was no significant difference in the pathological score between RS group and CVF group (p>0.05), but the pathological score in the RS+CVF group was dramatically reduced when compared with RS group and CVF group (p<0.05) (Table 3).
These findings suggests pre-perfusion of donor liver with RS and CVF treatment can significantly improve the HAR induced pathological damages to the liver following liver xenotransplantation.
Discussion
The complement activation in the recipients is an important step in the development of HAR following xenotransplantation3. The complement activation can be mediated by the binding of heterogeneous natural antibodies to EC antigens in the graft, and the complex is then adherent to C1q activating the classic complement activation pathway. In addition, in the absence of natural antibodies, C3b is produced and binds to B factor eliciting the activation of alternative pathway. The activated complements can transmit signals to the EC and then induces a series of changes in the metabolism and structure in EC. Important changes include shedding of Heparan sulfate from the surface of EC, synthesis of tissue factors, inactivation of thrombomodulin, morphological changes in EC leading to enlargement of intercellular space and P2 selectin (platelet ligand) expression on the EC4. These changes finally result in a series of pathological changes including platelet aggregation, production of fibrin, adhesion of neutrophils to EC, interstitial edema, bleeding and intravascular coagulation.
Therefore, suppressing complement activity and/or inhibiting complement activation is a key to avoid HAR. CVF can clear complements, which may be attributed to the binding of CVF to B factor in the serum forming a C3 protein-converting enzyme. This enzyme can not be regulated by other complements but can bind to C3 to form a highly stable enzyme complex. This complex may replace the endogenous C3b and activate the alternative pathway of complement activation, which leads to the consumption of C3, C5 and B factor and subsequent reduction of serum complements. Thus, the classic and alternative pathways of complement activation were nonfunctional. It has been confirmed3-5 that CVF can significantly inhibit the HAR following xenotransplantation. In the present study, our findings also revealed CVF could inhibit the occurrence of HAR following liver xenotransplantation.
During the occurrence of HAR following xenotransplantation, the pathways in which complements are activated and the role of classic and alternative pathways may vary among different donors and species. Miyagawa et al.6 postulated that the HAR following heart xenotransplantation between PG and SD rats had the involvement of alternative pathway of complement activation, in which the natural antibodies were not required. Other researchers also found the survival time of grafts prolonged with the reduction of natural antibody titer in the recipients. Sun et al.7,9 investigated the liver xenotransplantation between PG and rats. Their findings indicated the deposition of IgM in the endothelial cells and IgG in the sinusoids. Further study revealed perfusion of donor antibody before transplantation could elicit the antibody outbreak in the recipients, and the recipients had xenogenic natural antibody (XNA) against endothelial cell antigen in the PG which was mainly IgM. Moreover, CsA could prevent this antibody outbreak but had no influence on the survival time. These findings indicate that binding of natural antibody to xenogenic antigens may induce the activation of classic pathway of complement activation, which plays an important role in the HAR in the animal model above. Our previous study showed pre-perfusion of donor liver (PG) with XNA containing serum (rats) could block the EC antigens in the liver, which then blocked the activation of classic complement pathway following binding of natural antibodies to xenogenic antigens after liver xenotransplantation. This finding revealed the suppressive effect of pre-pefusion of donor liver with RS on the HAR following liver xenotransplantation, which was consistent with results in the present study.
Taken together, our results demonstrate that CVF can interrupt the cascade of complement activation during the HAR through exhausting serum complements, and RS can block the initiation of classic complement pathway during HAR. Thus, CVF and RS can exert synergistic suppressive effect on the HAR following xenotransplantation through different mechanisms. Our findings reveal pre-perfusion of donor liver with RS and CVF treatment can prolong the survival time of graft, alleviate the live damage and improve the liver function. The suppressive effect of both RS and CVF on HAR is superior to that of RS and CVF alone, demonstrating the synergistic of RS and CVF. Our results further confirm that classic pathway of complement activation plays an important role in the HAR following liver xenotransplantation between PG and rats, and pre-perfusion of donor liver with RS in combination with CVF may become a strategy for the prevention of HAR after xenotransplantation. More studies are required to confirm our findings.
Conclusion
Pre-perfusion of donor liver with recipient serum and cobra venom factor treatment can exert synergistic suppressive effects on the hyperacute rejection following liver xenotransplantation.
Received: December 19, 2011
Review: February 21, 2012
Accepted: March 15, 2012
Conflict of interest: none
Finance source: Nature and Science Fund of Guangdong Province in China (NSFGP)
- 1. Dalmasso AP. The complement system in xenotransplantation. Immunopharmacology. 1992, 24:149-60.
- 2. Meyer zu Vilsendorf A, Link C, Jörns A, Nagel E, Köhl J. Preconditioning with the prostacyclin analog epoprostenol and cobra venom factor prevents reperfusion injury and hyperacute rejection in discordant liver xenotransplantation. Xenotransplantation. 2001;8:41-7.
- 3. Li ZD, Shi CQ, Zhang QH. Mechanisms of purified cobra venom factor in preventing hyperacute rejection following discordant liver xenotransplantation in rats. Nat Med J China. 2004,84(23):2007-10.
- 4. Chen G, Sun QY, Wang WY. Prevention of hyperacute rejection of guinea pig-to-rat cardiac xenografts by depletion of complement with cobra venom factor. Chin J Organ Transplant. 2002, 23(5):263-5.
- 5. Lan G, Yang K, Bai Y. Inhibition effects of complement depletion with CVF on cardiac allografts rejection in inbred strain rats. Chin J Organ Transplant. 2006;27(12):744-7.
- 6. Miyagawa S, Hirose H, Shirakura R, Naka Y, Nakata S, Kawashima Y, Seya T, Matsumoto M, Uenaka A, Kitamura H. The mechanism of discordant xenograft rejection. Transplantation. 1988;46:825-30.
- 7. Sun P, Cai R, Zhang QH. Effect of xenoreactive natural antibody in the liver xenotransplantation from guinea pig to rat. Chin J Exp Surg. 2002;19(2):120-1.
- 8. Harihara Y, Sanjo K, Idezuki Y. A modified cuff technique for suprahepatic vena cava anastomosis in rat liver transplantation. Transplantation. 1992;53:707-8.
- 9. Sun P, Cai R, Zhang QH. Experimental study of guinea pig to rat liver xenotransplantation model. Chin J Organ Transplant. 2001;13(4):218-9.
Publication Dates
-
Publication in this collection
01 June 2012 -
Date of issue
May 2012
History
-
Received
19 Dec 2011 -
Accepted
15 Mar 2012 -
Reviewed
21 Feb 2012