Abstracts
PURPOSE: Tumor markers are substances found in blood and other biological fluids if tumor is present in the body. They can be produced by tumor itself or can be results of cancer - body relation. They may be used in the follow-up of cancer patients to identify tumor recurrence. Pre-treatment levels have prognostic tool and could signalize persistence of minimal residual disease despite radical surgery. METHODS: We operated on 52 patients with upper GI malignancy (32 with gastric cancer and 20 with pancreatic cancer). Blood samples were taken before surgery and peritoneal samples immediately after laparotomy before any manipulation with tumor. All samples were examined by standard biochemical technique and the level was compared with a stage of the disease. RESULTS: Patients suffering from gastric carcinoma of stage I and II had higher level of both markers in sera then in the peritoneal cavity, however most of them were within physiological range. Patients in stage III and IV had average marker levels in the peritoneal cavity higher than in sera. Number of positive findings was increasing according to the stage of the disease. The peritoneal levels of both markers varied extremely in higher stages. In patients suffering from pancreatic carcinoma the CEA levels both in sera and peritoneal cavity were parallel but peritoneal levels were slightly higher in stages III and IV. Ca 19 - 9 was more sensitive for pancreatic cancer. The percentage of positive findings was higher in sera but the level of Ca 19 - 9 was higher in the peritoneal cavity. The number of positive findings again correlated with the stage of the disease. CONCLUSIONS: Levels of tumor markers in sera could signalize inoperability of tumor (Ca 19 - 9 in cases of pancreatic carcinoma); peritoneal levels could predict R1 resection especially in gastric cancer patients and risk of early peritoneal recurrence of the disease. Difference between the levels in the peritoneum and sera may signalize the route of dissemination (hematogenous and intraperitoneal).
Stomach Neoplasms; Pancreatic Neoplasms; Tumor Markers, Biological; Neoplasm, Residual
OBJETIVO: Os marcadores tumorais são substâncias encontradas no sangue e outros fluidos biológicos em pacientes com doenças oncológicas. São produzidos pelo próprio tumor ou ser resultado da interação entre o tumor e o organismo. Podem ser usados no seguimento de pacientes com câncer para identificar recidiva tumoral. Os níveis pré-tratamento têm valor prognóstico e podem sinalizar persistência de doença residual mínima após cirurgia radical.. MÉTODOS: Foram operados 52 pacientes com tumores do trato gastroinstestinal superior (32 com câncer do estômago e 20 do pâncreas). Amostras sanguineas foram colhidas no préoperatório e amostras peritoneais imediatamente após a laparotomia, antes de qualquer manipulação do tumor. Todas as amostras foram examinadas bioquímicamente e os resultados foram comparados entre si e em face ao progresso da doença. RESULTADOS: Os pacientes com câncer de estômago nos estadios I e II apresentaram níveis sanguineos mais elevados de ambos os marcadores tumorais do que no peritônio, mas a maioria dos valores encontrava-se dentro dos limites fisiológicos. Já nos estadios III e IV os níveis dos marcadores tumorais foram mais elevados no peritônio do que no sangue. O número de exames positivos aumentou de acordo com o estadio da doença. Nos estádios avançados, observou-se elevada variabilidade nos níveis de ambos os marcadores analisados no peritônio. Os doentes com carcinoma de pâncreas tiveram níveis de CEA semelhantes no sangue e no peritônio, mas os níveis peritoneais foram ligeiramente mais elevados nos estadios III e IV. Ca 19 - 9 foi muito mais sensível para o câncer do pâncreas. A porcentagem de exames positivos foi mais elevada no sangue, mas o níveis do Ca19-9 foram mais elevados no peritônio.A porcentagem de exames positivos também teve correlação com o estadio da doença. CONCLUSÕES: Os níveis de marcadores tumorais no sangue podem indicar inoperabilidade do tumor. No peritônio podem indicar o tipo de ressecção, especialmente nos doentes com câncer gástrico, e o risco de recidiva peritoneal precoce. A diferença entre os níveis no peritônio e sangue podem sinalizar a via de disseminação, hematogênica ou intra-peritoneal.
Neoplasias Gástricas; Neoplasias Pancreáticas; Marcadores Biológicos de Tumor; Neoplasia Residual
9 - ORIGINAL ARTICLE
CLINICAL INVESTIGATION
Levels of CEA and Ca 19 - 9 in the sera and peritoneal cavity in patients with gastric and pancreatic cancers1 1 Research performed at Department of Surgery, University Hospital Bulovka, Charles University Prague, Czech Republic.
Níveis de CEA e Ca 19 - 9 em soro e cavidade peritoneal em pacientes com câncer do estômago e pâncreas
David HoskovecI; Jozef VargaII; Ellen KonečnáIII; Frantiek AntoIV
IMD, Fellow PhD degree, Postgraduate Program of Experimental Surgery, Charles University Prague, Czech Republic. Assistant Professor of Surgery, 1st Medical Faculty Czech Rep. Main author; study concept and design; acquisition, analysis and interpretation of data; drafting and revision of the manuscript; intellectual content; study supervision; responsible for English language
IIMD, Department of Surgery, 1st Medical Faculty Charles University, Czech Republic. Assistant Professor of Surgery. Concept, design, intellectual content, acquisition of data and critical revision
IIIMD, Fellow PhD degree, Postgraduate Program of Experimental Surgery, Charles University Prague, Czech Republic. Assistant Professor of Surgery, 1st Medical Faculty Czech Rep. Concept, design, intellectual content, acquisition of data and critical revision
IVProfessor of Surgery, Charles University Prague, Czech Republic. MD, PhD, former Head of Department of Surgery, Hospital Bulovka, Prague, Czech Republic, Consultant and Supervisor of PhD programs. Concept, design, intellectual content, drafting and revision of the manuscript, responsible for English language
Correspondence Correspondence: David Hoskovec, MD 1 st Department of Surgery General University Hospital U nemocnice 2 12000 Prague 2 Czech Republic Phone/Fax: (00 420)224963377 david.hoskovec@vfn.cz
ABSTRACT
PURPOSE: Tumor markers are substances found in blood and other biological fluids if tumor is present in the body. They can be produced by tumor itself or can be results of cancer body relation. They may be used in the follow-up of cancer patients to identify tumor recurrence. Pre-treatment levels have prognostic tool and could signalize persistence of minimal residual disease despite radical surgery.
METHODS: We operated on 52 patients with upper GI malignancy (32 with gastric cancer and 20 with pancreatic cancer). Blood samples were taken before surgery and peritoneal samples immediately after laparotomy before any manipulation with tumor. All samples were examined by standard biochemical technique and the level was compared with a stage of the disease.
RESULTS: Patients suffering from gastric carcinoma of stage I and II had higher level of both markers in sera then in the peritoneal cavity, however most of them were within physiological range. Patients in stage III and IV had average marker levels in the peritoneal cavity higher than in sera. Number of positive findings was increasing according to the stage of the disease. The peritoneal levels of both markers varied extremely in higher stages. In patients suffering from pancreatic carcinoma the CEA levels both in sera and peritoneal cavity were parallel but peritoneal levels were slightly higher in stages III and IV. Ca 19 9 was more sensitive for pancreatic cancer. The percentage of positive findings was higher in sera but the level of Ca 19 9 was higher in the peritoneal cavity. The number of positive findings again correlated with the stage of the disease.
CONCLUSIONS: Levels of tumor markers in sera could signalize inoperability of tumor (Ca 19 9 in cases of pancreatic carcinoma); peritoneal levels could predict R1 resection especially in gastric cancer patients and risk of early peritoneal recurrence of the disease. Difference between the levels in the peritoneum and sera may signalize the route of dissemination (hematogenous and intraperitoneal).
Key words: Stomach Neoplasms. Pancreatic Neoplasms. Tumor Markers, Biological. Neoplasm, Residual.
RESUMO
OBJETIVO: Os marcadores tumorais são substâncias encontradas no sangue e outros fluidos biológicos em pacientes com doenças oncológicas. São produzidos pelo próprio tumor ou ser resultado da interação entre o tumor e o organismo. Podem ser usados no seguimento de pacientes com câncer para identificar recidiva tumoral. Os níveis pré-tratamento têm valor prognóstico e podem sinalizar persistência de doença residual mínima após cirurgia radical..
MÉTODOS: Foram operados 52 pacientes com tumores do trato gastroinstestinal superior (32 com câncer do estômago e 20 do pâncreas). Amostras sanguineas foram colhidas no préoperatório e amostras peritoneais imediatamente após a laparotomia, antes de qualquer manipulação do tumor. Todas as amostras foram examinadas bioquímicamente e os resultados foram comparados entre si e em face ao progresso da doença.
RESULTADOS: Os pacientes com câncer de estômago nos estadios I e II apresentaram níveis sanguineos mais elevados de ambos os marcadores tumorais do que no peritônio, mas a maioria dos valores encontrava-se dentro dos limites fisiológicos. Já nos estadios III e IV os níveis dos marcadores tumorais foram mais elevados no peritônio do que no sangue. O número de exames positivos aumentou de acordo com o estadio da doença. Nos estádios avançados, observou-se elevada variabilidade nos níveis de ambos os marcadores analisados no peritônio. Os doentes com carcinoma de pâncreas tiveram níveis de CEA semelhantes no sangue e no peritônio, mas os níveis peritoneais foram ligeiramente mais elevados nos estadios III e IV. Ca 19 9 foi muito mais sensível para o câncer do pâncreas. A porcentagem de exames positivos foi mais elevada no sangue, mas o níveis do Ca19-9 foram mais elevados no peritônio.A porcentagem de exames positivos também teve correlação com o estadio da doença.
CONCLUSÕES: Os níveis de marcadores tumorais no sangue podem indicar inoperabilidade do tumor. No peritônio podem indicar o tipo de ressecção, especialmente nos doentes com câncer gástrico, e o risco de recidiva peritoneal precoce. A diferença entre os níveis no peritônio e sangue podem sinalizar a via de disseminação, hematogênica ou intra-peritoneal.
Descritores: Neoplasias Gástricas. Neoplasias Pancreáticas. Marcadores Biológicos de Tumor. Neoplasia Residual.
Introduction
Gastric cancer is the fourth abundant malignancy worldwide. Even when surgical resection is possible, the long-term survival is observed only in minority of patients with an overall five-year survival less than 30%1,2.
The most important prognostic factor influencing survival of patients with stomach cancer is the extent of disease as assessed by tumor stage.
The incidence of pancreatic cancer rises3. Pancreatic ductal adenocarcinoma is the fourth leading cause of cancer death in men and women in the USA and has the lowest survival rate for any solid cancer. Similar mortality figures are reported in the UK, with only 2-3 % of patients surviving 5 years after a diagnosis of pancreatic cancer. One important reason for this poor survival is that only 10-15 % of patients are diagnosed with small, resectable cancers4. However surgery is the only way to cure these tumors.
The aim of surgical treatment is R0 resection. It means that no tumor cells are left behind. Peritoneal lavage examination is one option to establish R0 resection. The importance of cytology from the peritoneal lavage is well known especially in cases of gastric tumors. The positive peritoneal lavage cytology shifts the stage of the disease to stage IV according to classification of Japanese Research Society for Gastric Cancer (JRSGC)1. The American Joint Committee for Cancer (AJCC) uses the peritoneal cytology in the same manner for pancreatic tumors5,6. TNM classification does not use cytology. Intraperitoneal levels of tumormarkers have similar prognostic value as peritoneal cytology. The most frequently used marker is CEA. But there are fewer reports focusing on this problem in comparison with the reports on cytology. Peritoneal lavage biochemistry has better sensitivity and specificity than cytology according to some authors7. Nearly all data dealing with peritoneal biochemistry were recorded in patients with gastric tumors.
A tumor marker has been defined, as a naturally occurring molecule measured in serum or plasma, or in other body fluids or tissue extracts, or in paraffin-embedded tissue to identify the presence of cancer and to assess patient's prognosis, or to monitor a patient's to therapy with the overall goal of improving the clinical management of the patient response4. Tumor markers comprise a wide spectrum of biomacromolecules synthesized in excess concentration by a wide variety of neoplastic cells. The markers could be endogenous products of highly active metabolic malignant cells, or the products of newly switched on genes, which remained unexpressed in early life, or newly acquired antigens at cellular and sub-cellular levels. The appearance of tumor markers and their concentration is related to the genesis and growth of malignant tumors in patients8. These markers may be detected in exfoliated or distributed cells, or as circulating agents within the peripheral blood or plasma. Other surrogate biological specimens, typically bodily fluids (e.g., urine, saliva, sputum, cerebrospinal fluid, or effusions) may also carry tumor markers. Tumor markers are often present in low concentrations in serum of healthy persons9.
Carcinoembryonic antigen
Carcinoembryonic antigen (CEA) first described in 1965 by Gold and Freedman is characterized as an oncofetal acid glycoprotein of 200 KD. CEA is present in the periphery of tumor cell membrane from where it is released into surrounding body fluids. Probably it plays a role in cell adhesion and inhibition of apoptosis in physiologic state, so it is expressed in normal mucosal cells and over-expressed in adenocarcinoma (colorectal, gastric, pancreatic, breast, lung and others)8,10-13.
Cancer antigen 19-9
Ca 19 9 is an intracellular adhesion molecule. The marker is 210 KD tumor associated glycoprotein antigen present as carbohydrate determinant on glycolipid and glycoprotein. Since the reference to antibody detection for Ca 19 9 published in 1979, it has been found to be the most useful tumor marker for pancreatic adenocarcinoma and gall bladder carcinoma too. Patients who are negative for Lewis antigen (a−, b−) do not synthesize Ca 19 9, and this constitutes 4 to 15 % of the population8,11-13.
Methods
We examined 52 patients with malignant disease (32 with gastric cancer and 20 with pancreatic cancer). There were 19 men and 11 women in group with gastric cancer and 12 men and eight women in group with pancreatic cancer. The average age of the patients in the group with gastric cancer was 67 years (range 37 87 years), in group with pancreatic cancer was 63 years (range 48 79 years) (Table 1).
Comparison of the levels of CEA and Ca 19 9 in sera and peritoneal lavage in patients suffering from gastric and pancreatic carcinoma was performed. Blood samples were collected after diagnosis of the disease. Peritoneal samples were taken immediately after laparotomy. When ascites was present, 20 ml were sent to biochemistry laboratory. In cases without ascites the peritoneal cavity was lavaged with 100 ml saline solution and the samples were collected 5 minutes later. Levels of the CEA and Ca 19 9 were examined. Physiological range was the same for peritoneal fluid and sera. CEA ranges were 0 4, 6 μg/l, Ca 19 9 levels were normal within 0 37 U/l.
Patients were stratified according to the stage of the disease.
Results
Tumor markers sensitivity and specificity (Tables 2 and 3)
Sensitivity and specificity for microscopic intraperitoneal cancer dissemination were evaluated in group of patient without confirmation of the malignant tumors and in patients with serosal infiltration or serosal metastases (we expected 100% prevalence of tumor cells in the peritoneal fluid in these patients and 100% positivity of the tumor markers).
CEA sensitivity was 38% and Ca 19-9 42%, respectively. Combination of both markers achieved 53% (we repeatedly found elevation only ones of both markers). Specificity of the biochemistry examination was 100%.
Gastric cancer
CEA levels in sera were within normal ranges in nearly all patients in stages I and II (with only one exception in patients with chronic kidney failure) and in nearly 70% of patients in stage III and IV. When the elevation was present it was not any extremely high value. Peritoneal levels of CEA were normal in all cases of stages I and II. Patients in stage III displayed normal CEA levels in about 65%. More positive finding in the peritoneal cavity was in stage IV (9:6). The variance of the levels in stage IV was high (0 - 5500 μg/l) (Figure 1).
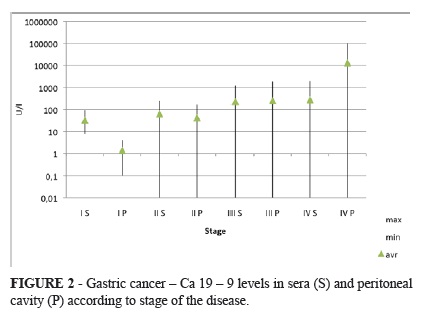
Ca 19 9 levels in sera and in peritoneum was nearly the same as CEA in stage I and II except for one patient the levels were within the normal ranges. Elevation of sera Ca 19 9 in stage III occurred in 25 % and in stage IV in 50% of patients. Peritoneal positivity was higher. 25% of patients in stage III and nearly 70 % in stage IV were positive. Value of Ca 19 9 levels varied extremely in stage IV (0 101 066 U/l) (Figure 2).
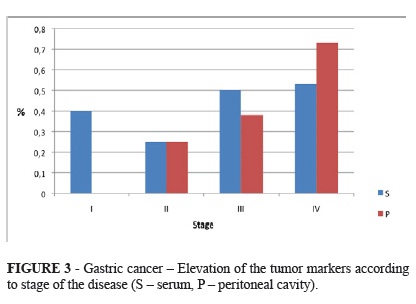
The positivity of at least one tumor markers was increasing according to the stage of the disease (Figure 3).
To sum-up, the percentage of negative findings of both markers in sera and peritoneal cavity was decreasing according to the stage of the disease. Positivity of both markers was increasing reversely and Ca 19 9 was probably more sensitive than CEA (Figure 4).
Pancreatic carcinoma
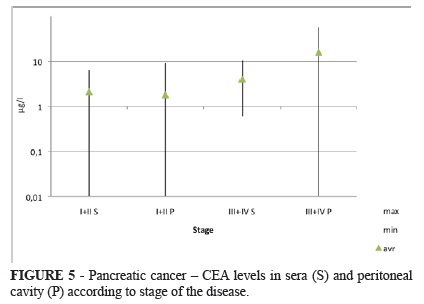
Patients with pancreatic carcinoma had normal levels of CEA in stages I and II (except for one patient). Positivity in stage III and IV is much higher. 20 % of patients had elevated levels of CEA in sera and 43 % displayed elevation in the peritoneal cavity (Figure 5).
Ca 19 9 was more sensitive in cases of pancreatic cancer. Elevation in sera was found in 66 % of cases in stage I and II and in the peritoneal cavity it was in 33%. In stages III and IV the findings of Ca 19 9 were positive in 85% in sera and in 60% in the peritoneal cavity (Figure 6).
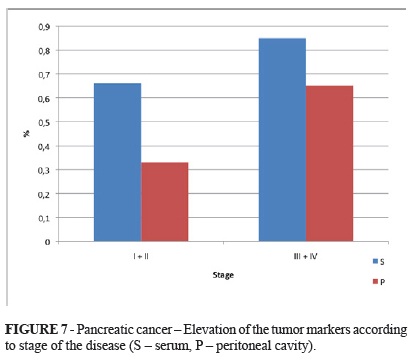
Similarly as in gastric cancer patients, the positive finding of at least one tumor marker is higher with the higher stage of the disease (Figure 7).
In summary, physiological ranges of both markers were less frequent than in cases of gastric carcinoma. Only 30% of patients had negative finding in sera in stages I and II. Negativity of both markers in higher stages was about 15%. The intraperitoneal finding was similar, but there were more negative patients in stages I and II (Figure 8).
Discussion
There are few publications focusing on comparison of levels of various tumor markers in sera and in peritoneal cavity. All demonstrate that higher level of tumor markers is associated with advanced disease, poor prognosis and early recurrence. In cases of gastric cancer peritoneal recurrence is one of the most important reasons of the treatment failure. Examination of peritoneal fluid by cytology, biochemistry or RT PCR could predict this relapse of the disease. The cytological examination is widely used in East Asian countries for diagnosis of microscopic peritoneal dissemination which is undetectable by other diagnostic tools in time of laparotomy or laparoscopy. Patients with positive cytology are classified as advanced disease with distant metastasis. All reports confirm worsening of the patient's prognosis with positive cytology despite various detection rates14-18.
Positive peritoneal cytology could predict the site of recurrence. It is an independent prognostic factor of the intraperitoneal dissemination (sensitivity 56%, specificity 97%)19.
However, the sensitivity and specificity of cytology is very high in Asian countries, nonetheless in the western countries the results are worse. In our previous study we published that cytology had sensitivity only 34% and specificity 80%. Biochemistry for combination of CEA and Ca 19 9 proved sensitivity of 53% and specificity 100%20. Similar data were published by other western centres. Our data show that gastric cancers with infiltration of the serosal layer or beyond associate with increasing frequency of positivity of tumor markers. CEA and Ca 19 9 levels can show the amount of tumor cells in the peritoneal cavity. Early gastric carcinoma may reveal increased temporal marker in the peritoneal washing, suggesting the presence of penetration pathways in the stomach wall by cancer cells, without invasion through continuation into the serosa1,21. Lymphatic channels or metastases in lymph nodes are examples of these possible pathways. This notion is partially supported by the fact that peritoneal recurrence takes place in around 1% of patients with early gastric carcinoma15.
In cases of pancreatic cancer free tumor cells in the peritoneal fluid predict unresectable, aggressive disease with early metastatic seeding and short survival time22,23. Positive peritoneal cytology is classified as metastatic disease according to the 6th edition of the Cancer staging manual AJCC3. It is presumed that positive cytology indicates ventral spreading of the tumor and it is a sign of tumor penetration through the peritoneum of the omental burse21. Free cancer cells in the peritoneal cavity are the only sign of generalization in 12 30% patients3,22,24. Positive cytology is more often found in tumors larger than 4 cm and tumors of the pancreatic body and tail23-25. Free tumor cells are diagnosed more often after fine needle biopsy (17: 28%)22. Similar to our previous published study, here again the biochemistry of peritoneal fluid is more sensitive to the presence of cancer cells in peritoneal cavity and could signal more reliably the advanced stages of the disease than conventional examinations (CT, EUS). We found lymph nodes metastases in all cases in stage II with elevation of the peritoneal Ca 19 9 lymph nodes metastasis.
Conclusions
Elevated levels of tumor markers both in peritoneal cavity and sera could signalize advanced stage of the disease than it is established by CT and EUS. Patients with a higher level of the markers in sera are usually treated as high risk patients for treatment failure. But the same signalize higher level of tumor markers in the peritoneal fluid. These patients are in risk of peritoneal and locoregional recurrence and further treatment should be focused to prevent this recurrence. Unfortunately there is no satisfactory procedure how to prevent and treat peritoneal cancer dissemination caused by gastrointestinal cancers. According to contemporary knowledge the best possibility now is probably the combination of surgery (peritonectomy) and some type of chemotherapy.
Received: January 18, 2012
Review: March 14, 2012
Accepted: April 16, 2012
Conflict of interest: none
Financial source: none
- 1. Aiko T, Sasako M. The new Japanese clasification of gastric carcinoma: points to be revised. Gastric Cancer. 1998;1:25-30.
- 2. Wells Jr SA. Gastric cancer. Curr Probl Surg. 2006;43:558-669.
- 3. Stefanidis D, Grove KD, Schwesinger WH, Thomas CR Jr. The current role of staging laparoscopy for adenocarcinoma of the pancreas: a review. Ann Oncol. 2006;17:189-99.
- 4. Goggins M, Koopmann J, Yang D, Canto MI, Hruban R. National Academy of Clinical Biochemistry (NACB) Guidelines for the Use of Tumor Markers in Pancreatic Ductal Adenocarcinoma, Available from http://www.aacc.org/SiteCollectionDocuments/NACB/LMPG/tumor/chp3i_pancreatic.pdf
- 5. Liu RC, Traverso LW. Laparoscopic staging should be used routinely for locally extensive cancer of the pancreatic head. J Gastrointest Surg. 2004;8:923-4.
- 6. Greene FL, Page DL, Fleming ID, Fritz A, Balch ChM, Haller DG, Morrow M. AJCC Cancer Staging Manual. 6ed. Available from www.cancerstaging.org
» link - 7. Li JK, Zheng M, Miao CW, Zhang JH, Ding GH, Wu WS. Peritoneal lavage cytology and carcinoembryonic antigen determination in predicting peritoneal metastasis and prognosis of gastric cancer. World J Gastroenterol. 2005;46:7374-7.
- 8. Malati T. Tumour markers: an overview. Ind J Clin Biochem. 2007;2:17-31.
- 9. Urban D, Catane R. Serum tumor markers in oncology. Isr Med Assoc J. 2009;11:103-4.
- 10. Bonfrer JMG, Louhimo L. National Academy of Clinical Biochemistry Guidelines for the Use of Tumor Markers in Gastric Cancer, http://www.aacc.org/SiteCollectionDocuments/NACB/LMPG/tumor/chp3g_gastric.pdf
- 11. Perkins GL, Evan D, Slater D, Sanders GK, Prichard JG. Serum tumor markers. Am Fam Physician. 2003;68:1075-82.
- 12. Scientific Committee of the Association of Clinical Biochemists in Ireland (ACBI) Guidelines for the Use of Tumour Markers, http://www.acbi.ie/Downloads/Guidelines-for-the-Use-of-Tumour-Markers-2005.pdf accessed Juni 15, 2011.
- 13. Schlieman MG, Hung H, Bold RJ. Utility of tumor markers in determining resectability of pancreatic cancer. Arch Surg. 2003;138:951-6.
- 14. Bold JR, Ota MD, Ajani JA, Mansfield PF. Peritoneal and serum tumor markers predict recurrence and survival of patients with resectable gastric cancer. Gastric Cancer. 1999;2:1-7.
- 15. Crepaldi-Filho R, Palma RT, Marcelo Franchini Giusti MF, de Assis Galveo Bueno M, da Silva PSL, Waisberg J. Levels of carcinoeembryonic antigen and CA 19-9 in the sera and peritoneal washing of patients undergoing surgical treatment for gastric carcinoma. Arq Gastroenterol. 2008;45:219-24.
- 16. Majima T, Ichikura T, Mochizuki H. Prognostic significance of the cytologic features of free cancer cells in the peritoneal cavity of patients with gastric cancer. Surg Today. 2002;32:35-9.
- 17. Manzoni G, Verlato G, Di Leo A, Tomezzoli A, Pedrazzani C, Pasini F, Piubello Q, Cordiano C. Peritoneal cytology does not increase the prognostic information provided by TNM in gastric cancer. World J Surg. 2006;30:579-84.
- 18. Ribeiro U Jr, Gama-Rodrigues JJ, Safatle-Ribeiro AV, Bitelman B, Ibrahim RE, Ferreira MB, Laudanna AA, Pinotti HW. Prognostic significance of intraperitoneal free cancer cells obtained by laparoscopic peritoneal lavage in patients with gastric cancer. J Gastrointest Surg. 1998;2:244-9.
- 19. Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T, Nishimura G, Miwa K. Intraoperative lavage for cytological examination in 1297 patients with gastric carcinoma. Am J Surg. 1999;178:256-62.
- 20. Hoskovec D, Varga J, Anto F, Dytrych P, Benková K. Peritoneal lavage cytology and biochemistry in cases of gastric and biliopancreatic tumors. Gastroent hepatol. 2008;62(Suppl 3):15. Available from www.medvik.cz/link/bmc09002002
- 21. Hermanek P. Pathology and biology of pancreatic ductal adenocarcinoma. Langenbeck´s Arch Surg. 1998;383:116-20.
- 22. Jimenez RE, Warshaw AL, Fernandez-del Castillo C. Laparoscopy and peritoneal cytology in the staging of pancreatic cancer. J Hepatobiliary Pancreat Surg. 2000;7:15-20.
- 23. Jimenez RE, Warshaw AL, Ratner DW, Willett CG, McGrath D, Fernandez-del Castilo C. Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch Surg. 2000;135:409-15.
- 24. Warshaw AL. Implications of peritoneal cytology for staging of early pancreatic cancer. Am J Surg. 1991;161:26-30.
- 25. Traverso LW. Pancreatic cancer: surgery alone is not sufficient. Surg Endosc. 2006;20:446-
Correspondence:
Publication Dates
-
Publication in this collection
04 June 2012 -
Date of issue
June 2012
History
-
Received
18 Jan 2012 -
Accepted
16 Apr 2012 -
Reviewed
14 Mar 2012