Abstracts
PURPOSE: To investigate the osteoconductive properties and biological performance of Poly L-lactic acid (PLLA) with omentum in bone defects. METHODS: PLLA nanofiber scaffolds were prepared via electrospinning technique. Forty four New Zealand white female rabbits randomly divided into three groups of 18 rabbits each. Created defects in right tibias were filled in group I with omentum, in group II with PLLA nanofiber scaffold and in group III with combination of the omentum and PLLA. The same defects were created in left tibia of all groups but did not receive any treatment (control group). Histological and histomorphometric evaluations were performed at two, four and six weeks after the implantation. RESULTS: Histological changes on all groups along with the time course were scored and statistical analysis showed that the average scores in group III were significantly higher than the other groups. CONCLUSION: Histomorphometric analysis of bone healing was shown to be significantly improved by the combined PLLA with omentum compared with the other groups, suggesting this biomaterial promote the healing of cortical bone, presumably by acting as an osteoconductive scaffold.
Lactic Acid; Omentum; Bone Regeneration; Rabbits
OBJETIVO: Investigar as propriedades de osteocondução e desempenho biológico do ácido L láctico-Poly (PLLA) com omento em defeitos ósseos. MÉTODOS: Andaimes PLLA nanofibras foram preparados via eletrofiação técnica. Cinquenta e quatro coelhos fêmeas Nova Zelândia brancos foram distribuídos aleatoriamente em três grupos de 18 coelhos cada. Defeitos criados em tíbias direitas foram preenchidos no grupo I com omento, no grupo II com PLLA nanofibras e no grupo III com a combinação do omento e PLLA. Os mesmos defeitos foram criados na tíbia esquerda de todos os grupos, mas não receberam qualquer tratamento (grupo controle). As avaliações histológicas e histomorfométricas foram realizadas em duas, quatro e seis semanas após a implantação. RESULTADOS: As alterações histológicas em todos os grupos, juntamente com o curso de tempo foram marcados e análise estatística mostrou que as pontuações médias do grupo III foram significativamente mais elevadas do que os outros grupos. CONCLUSÃO: Análise histomorfométrica da cicatrização óssea mostrou-se significativamente melhor com o PLLA combinado com omento em comparação com os outros grupos, sugerindo que este biomaterial promove a cicatrização do osso cortical, provavelmente atuando como osteocondutor.
Ácido Láctico; Omento; Regeneração Óssea; Coelhos
5 - ORIGINAL ARTICLE
WOUND HEALING
Combination of poly L-lactic acid nanofiber scaffold with omentum graft for bone healing in experimental defect in tibia of rabbits1 1 Research performed at Department of Experimental Surgery, Faculty of Veterinary, Islamic Azad University (IAU), Kahnooj Branch.
Combinação de poli L-ácido láctico nanofibras com enxerto de omento para reparo ósseo em defeito experimental em tíbia de coelhos
Amir SotoudehI; Gholamreza JahanshahiII; Amirali JahanshahiIII; Mohammad Ashrafzadeh TakhtfooladilII; Iman ShabaniIV; Masoud SoleimaniIV
IAssistant Professor, Department of Veterinary Science, Kahnooj Branch, Islamic Azad University, Kerman, Iran. Design, supervised all phases of the study, analysis and interpretation of data, manuscript writing
IIAssociate Professor, Esfahan University of Medical Science, School of Dentistry, Department of Oral Pathology, Esfahan, Iran. Histological analysis
IIIPhD student, Department of Surgery, Science and Research Branch, Islamic Azad University, Tehran, Iran. Helped with technical procedures, collection and processing of study informations
IVPhD student, Nanotechnology and Tissue Engineering Department, Stem Cell Technology Research Center, Tehran, Iran. Scaffold fabrication
VAssociate Professor, Hematology Department, Faculty of Medical Science, Tarbiat Modares University, Tehran, Iran. Helped with technical procedures and collection of study information
Correspondence Correspondence: Amir Sotoudeh Islamic Azad University Kahnooj Branch Kahnooj, Iran Tel.: 00979121768066 Fax: 00973495230203 dramirsotoudeh@kahnoojiau.ac.ir
ABSTRACT
PURPOSE: To investigate the osteoconductive properties and biological performance of Poly L-lactic acid (PLLA) with omentum in bone defects.
METHODS: PLLA nanofiber scaffolds were prepared via electrospinning technique. Forty four New Zealand white female rabbits randomly divided into three groups of 18 rabbits each. Created defects in right tibias were filled in group I with omentum, in group II with PLLA nanofiber scaffold and in group III with combination of the omentum and PLLA. The same defects were created in left tibia of all groups but did not receive any treatment (control group). Histological and histomorphometric evaluations were performed at two, four and six weeks after the implantation.
RESULTS: Histological changes on all groups along with the time course were scored and statistical analysis showed that the average scores in group III were significantly higher than the other groups.
CONCLUSION: Histomorphometric analysis of bone healing was shown to be significantly improved by the combined PLLA with omentum compared with the other groups, suggesting this biomaterial promote the healing of cortical bone, presumably by acting as an osteoconductive scaffold.
Key words: Lactic Acid. Omentum. Bone Regeneration. Rabbits.
RESUMO
OBJETIVO: Investigar as propriedades de osteocondução e desempenho biológico do ácido L láctico-Poly (PLLA) com omento em defeitos ósseos.
MÉTODOS: Andaimes PLLA nanofibras foram preparados via eletrofiação técnica. Cinquenta e quatro coelhos fêmeas Nova Zelândia brancos foram distribuídos aleatoriamente em três grupos de 18 coelhos cada. Defeitos criados em tíbias direitas foram preenchidos no grupo I com omento, no grupo II com PLLA nanofibras e no grupo III com a combinação do omento e PLLA. Os mesmos defeitos foram criados na tíbia esquerda de todos os grupos, mas não receberam qualquer tratamento (grupo controle). As avaliações histológicas e histomorfométricas foram realizadas em duas, quatro e seis semanas após a implantação.
RESULTADOS: As alterações histológicas em todos os grupos, juntamente com o curso de tempo foram marcados e análise estatística mostrou que as pontuações médias do grupo III foram significativamente mais elevadas do que os outros grupos.
CONCLUSÃO: Análise histomorfométrica da cicatrização óssea mostrou-se significativamente melhor com o PLLA combinado com omento em comparação com os outros grupos, sugerindo que este biomaterial promove a cicatrização do osso cortical, provavelmente atuando como osteocondutor.
Descritores: Ácido Láctico. Omento. Regeneração Óssea. Coelhos.
Introduction
Acceleration of bone healing in fracture site has always been a major problem. Fresh autologous spongy bone transplantation is considered as the gold standard for bone grafts1,2. As compared with other methods the progenitor cells provided without stimulation of immune system response. But it has some problems, including donor site damage and limitation of harvested amount. These restrictions encourage researchers to use synthetic bone graft substitutes3,4.
Matrix-based tissue-engineering approaches aim to use structural implants and/or materials to replace the defective bone5,6. These approaches depend on the recruitment of endogenous osteoinductive factors and migration of osteogenic cells to regenerate bone defect7,8. If the scaffold could induce cells from the surrounding tissues, pre-expansion of the cells for the transplantation would not be required9. After insertion of a material to damage site, it will be exposed to adjacent cells, resulting in captured repair cells and the possibility of leakage and non-homogeneous scatter will be decreased.
Various materials1,2,10 have been developed for these purposes: ceramics11,12, polymers13,14, metals15,16 and composites1,17,18.
However about the use of these materials, complete success has not yet achieved14,15 because their physical structures are different from bones. Furthermore, they may have osteoconductive but normally not osteoinductive properties and can only act as a matrix for new osseous ingrowth and slow creeping substitution. The time-consuming process of resorption is also a problem14.
PLLA is an organic polymer from L-lactate that dissolves in H2O and CO2. PLLA was used as a material for human orthopedic implants19. In vitro studies showed that PLLA scaffolds had slow absorption rates and good mechanical20 and biocompatible properties21.
According to recent researches omentum contains growth factors such as vascular endothelial growth factor (VEGF), Basic fibroblast growth factor (bFGF) and transforming growth factor beta-1 (TGF-b1)22,23. It has been demonstrated that VEGF activity is essential for appropriate callus architecture and mineralization in response to bone injury24-27. Both bFGF28-32 and TGF-b33-36 are reported to have positive effects on bone repair.
Given this, combined omenton with Synthetic bone graft substitutes may improve the bone tissue formation and repair of bone defects. The aim of this study was to investigate the osteoconductive properties and biological performance of PLLA combined with omentum in bone defects.
Electrospinning is one of the approaches that allow the fabrication of synthetic materials into fibrous structures in the micro- and nanometer scale21. In this research PLLA nanofiber Scaffolds were prepared via electrospinning technique, and then these biomaterials alone and with omentum were implanted in experimental bone defects. Histological and histomorphmetric evaluations were performed at two, four and six weeks after the implantation.
Methods
All animal of the present research were cared according to the norms of the Islamic Azad University Faculty of Veterinary Sciences laboratory of animal experimentations; this investigation was approved by the Committee of Ethics in Research with animals too.
Scaffold preparation
Nanofibrous PLLA scaffolds were prepared via electrospinning technique. A 4 % (wt/wt) solution of PLLA (Sigma-Aldrich, St. Louis, MO, USA) in dichloromethane/dimethylformamide (Merck, Germany) was placed in a 5 mL syringe which was connected to a blunted 21- gauge needle through an extension tube. A steel grounded collector was used to collect the electrospun nanofibers. The solution was fed through the tube into the needle by a syringe pump with a rate of 0.5 mL/h. Application of high voltage (20 kV) between the needle and collector, forced the solution droplet to leave the needle and deposit on the cylinder in the form of nanofibers. In order to modify surface characteristics of prepared scaffolds, surface plasma treatment was performed by a low frequency plasma generator of 40 kHz frequency with a cylindrical quartz reactor (Diener Electronics, Germany). Pure oxygen was introduced into the reaction chamber at 0.4 mbar pressure and then the glow discharge was ignited for three min.
Scanning electron microscopy
The surface morphology of scaffolds was characterized using a scanning electron microscope (SEM, Philips XL30, Netherlands) after specimens were coated with gold using a sputter coater.
Scaffold implantation
Forty four New Zealand white adult female rabbits were used with following ethical approval as previously reported. The rabbit's age was eight months and the weight 3000-3500g. They were randomly divided into three groups of 18 rabbits each. The rabbits were anesthetized with an intramuscular injection of ketamine hydrochloride (30 mg/Kg of body weight) and xylazine (5 mg/Kg of body weight). The animals were immobilized, and the bilateral tibias were shaved, washed and disinfected with povidone-iodine. A 4 cm incision was made on the proximal-anterior part of tibiae. The incision penetrated epidermis, dermis and the fascial layers. An additional medial-anterior incision was made through the periosteum. The periosteum was elevated with a periosteal elevator and retained by a selfretaining retractor. A cortical hole of 3mm diameter and depth in each tibia was drilled. The bone cavities were washed with saline during and after the drilling.
Additionaly in group I and III, 3*3 mm2 of the free end of the greater omentum obtained from a 1 cm ventral midline incision midway between the umbilicus and pelvic inlet. Created defects in right tibias were filled in group I with omentum, in group II with PLLA and in group III with combination of the omentum and PLLA. The same defects were created in left tibia of all groups but did not receive any treatment and will be considered as control group. After implantation, Muscles, fascia and skin were sutured separately.
To reduce the perioperative infection risk, prophylactic antibiotic (Terramycines) was administered postoperatively by subcutaneous injection. The animals received a postoperative pain medication and were allowed to move without any restrictions immediately after recovery from the anesthesia.
Histological analysis
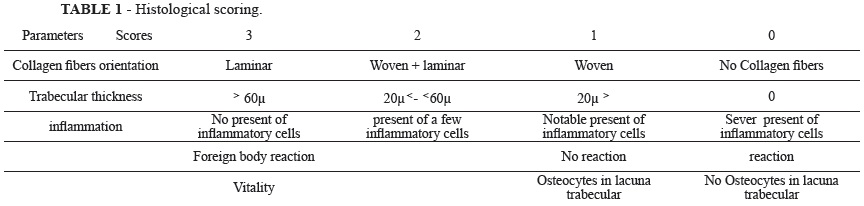
Six of the animals in each group were respectively sacrificed at the end of two, four and six weeks. The right and left tibias were harvested at the designated time points. The specimens were harvested with the surrounding tissue and fixed in 10% neutral formalin and were decalcified with 10% nitric acid, dehydrated, and then embedded in paraffin, after that were sectioned at 5 µm and stained with Hemotoxylin-eosin for histological observation (Table 1). Assessment was performed by a blinded assessor while considering parameters in table 1, with an Olympus BH-2 light microscope (Tokyo, Japan).
Histomorphometric analysis
Measurements were made on two sections for each sample. All sections of specimens were used for histomorphometrical evaluation using computer based image analysis techniques (Leicas Qwin Pro-image analysis system, Wetzlar, Germany). Digital analysis determined the total bone formation (%). This parameter was subdivided in: (A) bone inside the implant (BII), defined as bone formed within the implant edges; (B) bone outside the implant (BOI) defined as newly formed bone deposited outside the implant.
Statistical analysis
Analysis of variance (ANOVA) was used to evaluate the differences between groups as well as between different time points in a group. Statistical analysis was performed using the SPSS statistics package version 15.0.15.
Results
Characterization of nanofibers
SEM micrographs of PLLA scaffolds are shown in Figure 1. The results indicate that fabricated PLLA scaffolds have highly porous structure with interconnected pores and bead-free nanofibers. An ideal scaffold should mimic the physical and biochemical properties of natural extracellular matrix like good porosity and nano-sized structure1. As can be seen in the SEM micrographs, electrospun PLLA nanofibers have porous and nano-scale structure so have the potential to be used as scaffold in tissue engineering.
Histological evaluations
After two weeks
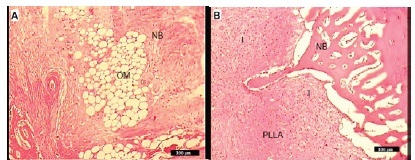
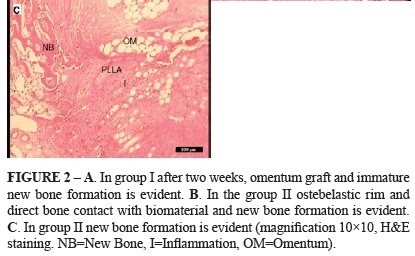
In Group I, there was very little inflammation and new bone formation was already. Contact with bone graft material was also seen, Figure 2A. In group II, no evidence of foreign body response to the PLLA was present but the inflammation in this group was remarkable, Figure 2B. In Group III newly formed woven bone (BII) early appeared and bone bonding between the implants and host bone were observed, Figure 2C.
After four weeks
The woven bone was formated in group I and partial direct contact between the trabeculae and the garft occurred, Figure 3A. Group II was also observed woven bone formation and there was a little inflammation in this group. Bone growth was BOI and BII but contact with the host bone was not well. No macrophages were found around the implants, Figure 3B. Mixed lamellar and woven bone formation was seen in group III and inflammation was very low compared to the second week. Foreign body reaction was negative. Bone growth was observed as well as the BOI and BII. Bone lacunas contained osteocyte that demonstrated bone tissue was active and vital, Figure 3C.
After six weeks
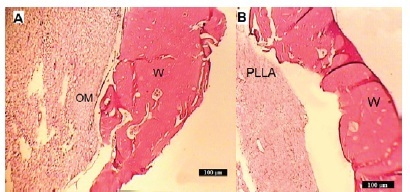
In group I mature bone formation with acceptable trabecular thickness were seen. There was no inflammation feature, Figure 4A. In Group II, lamellar bone formation as BOI was observed and trabecular thickness was remarkable. The inflammation and foreign body reaction were negative. Partially contact with PLLA and host bone was also seen, Figure 4B. In group III formation of mature bone with significant trabecular thickness was observed. There were no inflammation and giant cells that suggest foreign body reaction is negative. There was clear evidence of remodelling around the implant. In all regions of inspection, excellent bone bonding between the host bone and implant was displayed, Figure 4C.
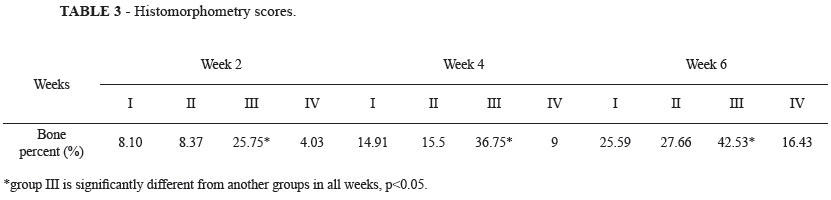
Detailed results of Histological evaluations are summarized in Table 2. Whereas Histological changes on all groups along with the time course were scored and statistical analysis showed that the total average scores in group III were significantly higher than the other groups (p<0.05).
Histomorphometrical evaluations
The results of the histomorphometrical analysis are shown in Table 3. In all four groups the formation of new bone increased all the time periods. Comparison between groups I and II showed no significantly difference in formation of new bone after two, four and ten weeks (p<0.05).
After two weeks in the group III, formation of new bone was 25.75% (BII: 25.75%; BOI: 0.0%). After four weeks it was 36.75% (BII: 29.14%; BOI: 7.61%) and it increased to 42.53 (BII: 32.44%; BOI: 10.09%) after 6 weeks. Statistical testing revealed that these increases were significantly different in formation of new bone after two, four and ten weeks (p<0.05) compared with other groups.
Discussion
Biomaterial scaffolds, osteogenic cells, and bone formation- inductive factors are three essential elements for tissue engineering strategies to achieve repair and restoration of damaged bone tissues37-42. Scaffolds are central components of many tissue engineering strategies and there is a significant challenge in the design and manufacture of scaffolds that possess both a highly porous structure and the ability to control the release kinetics of growth factors over the period of tissue regeneration43. An ideal biomaterial for bone tissue engineering should be non-immunogenic, highly effective in osteoinduction, ready for rapid vascular and mesenchymal cell invasion, carvable and providing mechanical support when needed43.
Various types of biomaterials have been utilized as Scaffold. Currently, bioactive glasses in the form of granules are used to enhance bone healing. However, the clinical handling properties of them are limited45. Bone cements have been shown to conduct bone growth, but will also induce foreign-body cell reactions46. Poor mechanical strength and insufficient adhesion between the bone and the cement as well as low degradation rate restricting the use of some bone filler materials47,48. Metals are difficult to shape and have a problem of corrosion which leads to inflammatory responses of surrounding tissue49,50.
Scaffold porosity and interconnections or pathways between the pores are vital for new bone integration into the material51. The high ratio of surface to volume and high porosity of three dimensional nanofibers scaffolds support binding and migration of mesenchymal stem cells into the scaffold that mainly due to the chemical and morphological similarities of them to natural extracellular matrix. These scaffolds readily allowed invasion of the requisite proteins and cells for bone formation and resorption52 and can support growth and differentiation of MSC53,54 in vitro as well as in vivo55.
The purpose of this study was to evaluate a short term bone response to nanofiber scaffold of PLLA with combination of omentum.
In this study, the surface plasma treatment was used to improve the hydrophilicity of prepared hydrophobic PLLA nanofibers. Oxygen plasma treatment introduces oxygen containing functional groups on the surface of nanofibers which leads to higher hydrophilicity. Increased hydrophilicity facilitates cell adhesion and proliferation on the scaffold surface56.
PLLA is a biodegradable semi-crystalline polymer derived from lactic acid (C3H6O3). The main advantages of these polymers are their mechanical strength, combined with biodegradability and biocompatibility57. In a similar study, PLLA nanofibers scaffolds with growth factors were examined in the calvariom bone defect and results showed that scaffold had bone conductivity properties and also induced osteoinduction58.
Electrospun hydroxyapatite-PLLA scaffold was evaluated on sternal bone healing and histological evaluation demonstrated presence of newly formed bone trabeculae among scaffold fibers21. In the same manner PLLA nanofiber scaffolds were shown to facilitate cell immigration and thus to achieve high cell densities and the histological evaluations showing an improved healing and tissue remodeling higher degree of maturity in the simultaneous application of PLLA nanofiber scaffold and omentum, with respect to the other group specially six weeks after implantation that suggesting this biomaterial combination has bone conduction properties. The most successful use of this material maybe due to bone regeneration stimulation of growth factors of omentum. Olumi et al.59 reported that autogenous free omental graft accelerated collagen synthesis in experimental tibial defects in rabbit. In another study the greater omentum were used for femoral defects healing in rabbits and histological results showed bone formation process were faster than the control group.
In this study we demonstrated the use of this scaffold in weight bearing area and similar results were seen. The short 6-week follow-up period was a limitation of this study. However, the aim of this study was to evaluate early histological changes, long-term biocompatibility of the composite needs to be evaluated in a separate study.
Conclusions
The omentum and PLLA alone had little effects on the formation of new bone as demonstrated by histomorphometry but their results were better than control group. Histomorphological analysis of the bone healing was shown to be significantly improved by the combined PLLA with omentum compared with the other groups, suggesting that this biomaterial promote the healing of cortical bone, presumably by acting as an osteoconductive scaffold.
Conflict of interest: none
Financial source: Islamic Azad University
Received: May 15, 2012
Review: July 16, 2012
Accepted: August 18, 2012
- 1. Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84:454-64.
- 2. Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop. 2000;371:10-27.
- 3. Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995;20:1055-60.
- 4. Kurtz LT, Garfin SR, Booth Jr RE. Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14:1324-31.
- 5. Reddi AH. Symbiosis of biotechnology and biomaterials: application in tissue engineering of bone and cartilage. J Cell Biochem. 1994;56:192-5.
- 6. Sepulveda P, Ortega FS, Innocentini MDM, Pandolfelli VC. Properties of highly porous hydroxyapatite obtained by the gelcasting of foams. J Am Chem Soc. 2001;83:3021-4.
- 7. Bruder SP, Fox BS. Tissue engineering of bone: cell based strategies. Clin Orthop. 1999;367:S68-83.
- 8. Le Guehennec L, Layrolle P, Daculsi G. A review of bioceramics and fibrin sealant. Eur Cells Mater. 2004;8:1-11.
- 9. Inui A, Kokubu T, Fujioka H, Nagura I, Sakata R, Nishimoto H. Application of layered poly (L-lactic acid) cell free scaffold in a rabbit rotator cuff defect model. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:29.
- 10. Costantino PD, Friedman CD. Synthetic bone graft substitutes. Otolaryng Clin North Am. 1994;27:1037-74.
- 11. Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop. 2002;395:44-52.
- 12. LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop. 2002;395:81-98.
- 13. Palissery V, Taylor M, Browne M. Fatigue characterization of a polymer foam to use as a cancellous bone analog material in the assessment of orthopaedic devices. J Mater Sci Mater Med. 2004;15:61-7.
- 14. Bilkay U. Comparing the osteogenic capacities of bone substitutes: hydroxyapatite, high-density porous polyethylene, and bone collagen: a biochemical and histological analysis. J Craniofac Surg. 2004;15:585-93.
- 15. Zou X, Li H, Bunger M, Egund N, Lind M, Bunger C. Bone ingrowth characteristics of porous tantalum and carbon fiber interbody devices: an experimental study in pigs. Spine J. 2004;4:99-105.
- 16. Kujala S, Ryhanen J, Danilov A, Tuukkanen J. Effect of porosity on the osteointegration and bone ingrowth of a weight-bearing nickel-titanium bone graft substitute. Biomaterials. 2003;24:4691-7.
- 17. Sammarco VJ, Chang L. Modern issues in bone graft substitutes and advances in bone tissue technology. Foot Ankle Clin. 2002;7:19-41.
- 18. Wang M. Developing bioactive composite materials for tissue replacement. Biomaterials. 2003;24:2133-55.
- 19. Brand JC Jr, Nyland J, Caborn DN, Johnson DL. Soft-tissue interference fixation: bioabsorbable screw versus metal screw. Arthroscopy. 2005;21:911-6.
- 20. Li WJ, Cooper JA Jr, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly (alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2:377-85.
- 21. Rainer A, Spadaccio C, Sedati P, Marco F, Carotti S. Electrospun hydroxyapatite-functionalized PLLA scaffold: potential applications in sternal bone healing. Ann Biomed Eng. 2011;7:1882-90.
- 22. Bikfalvi A, Alterio J, Inyang AL, Dupuy E. Basic fibroblast growth factor expression in human omental microvascular endothelial cells and the effect of phorbol ester. J Cell Physiol. 1990;144:151-8.
- 23. Zhang Q-X, Magovern CJ, Mack CA, Budenbender KT, Wilson K, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum- mediated angiogenesis. J Surg Res. 1997;67:147-54.
- 24. Vadasz Z, Misselevich I, Norman D, Peled E, Boss JH. Localization of vascular endothelial growth factor during the early reparative phase of the rats' vessels deprivation-induced osteonecrosis of the femoral heads. Exp Mol Pathol. 2004;77:145-8.
- 25. Street J. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci. 2002;99:9656-61.
- 26. Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, Huard J. Synergisitic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751-9.
- 27. Carano RAD, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003;8:980-9.
- 28. Nakamura K, Kurokawa T, Aoyama I, Hanada K, Tamura M, Kawaguchi H. Stimulation of bone formation by intraosseous injection of basic fibroblast growth factor in ovariectomised rats. Int Orthop. 1998;22:49-54.
- 29. Liang H, Pun S, Wronski TJ. Bone anabolic effects of basic fibroblast growth factor in ovariectomized rats. Endocrinology. 1999;140:5788-9.
- 30. Kawaguchi H, Kurokawa T, Hanada K, Hiyama Y, Tamura M, Ogata E, Matsumoto T. Stimulation of fracture repair by recombinant human basic fibroblast growth factor in normal and streptozotocin-diabetic rats. Endocrinology. 1994;135:774-81.
- 31. Bland YS, Crithchlow MA, Ashhurtst DE. Exogenous fibroblast growth factors-1 and -2 do not accelerate fracture healing in the rabbit. Acta Orthop Scand. 1995;66:543-8.
- 32. Andreshak JL, Rabin SI, Patwardhan AG, Wezemn FH. Tibial segmental defect repair: chondrogenesis and biomechanical strength modulated by basic fibroblast growth factor. Anat Rec. 1997;248:198-204.
- 33. Joyce ME, Jingushi S, Bolander ME. Transforming growth factor-beta in the regulation of fracture repair. Orthop Clin North Am. 1990;21:199-209.
- 34. Noda M, Camilliere JJ. In vivo stimulation of bone formation by transforming growth factorbeta. Endocrinology. 1989;124:2991-4.
- 35. Rosier RN, O'Keefe RJ, Hicks DG. The potential role of transforming growth factor beta in fracture healing. Clin Orthop Relat Res. 1998;(355Suppl):S294-300.
- 36. Bostrom MP, Asnis P. Transforming growth factor beta in fracture repair. Clin Orthop Suppl. Relat Res. 1998;(355 Suppl): S124-31.
- 37. Stubbs D, Deakin M, Chapman-Sheath P, Bruce W, Debes J, Gillies RM. In vivo evaluation of resorbable bone graft substitutes in a rabbit tibial defect model. Biomaterials. 2004;25:5037-44.
- 38. Khan SN, Cammisa Jr FP, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone grafting. J Am Acad Orthop Surg. 2005;13:77-86.
- 39. Mistry AS, Mikos AG. Tissue engineering strategies for bone regeneration. Adv Biochem Eng Biotechnol. 2005;94:1-22.
- 40. Cowan CM, Soo C, Ting K, Wu B. Evolving concepts in bone tissue engineering. Curr Top Dev Biol. 2005;66:239-85.
- 41. Orban JM, Marra KG, Hollinger JO. Composition options for tissueengineered bone. Tissue Eng. 2002;8:529-39.
- 42. Fisher JP, Reddi AH. Functional tissue engineering of bone: signals and scaffolds. In: Ashammakhi N, Ferretti P, editors. Topics tissue Engineering, 2003. E-book /http://www.oulu.fi/spareparts/ebook_topics_in_t_e/index.htmlS, Chapter 5.
- 43. Abedi G, Sotoudeh A, Soleymani M, Shafiee A, Mortazavi P. Aflatoonian M. A collagen-poly (vinyl alcohol) nanofiber scaffold for cartilage repair. J Biomater Sci Polym Ed. 2011;18:2445-55.
- 44. Ripamonti U, Duneas N. Tissue engineering of bone by osteoinductive biomaterials. MRS Bull. 1996;21:36-9.
- 45. Närhi TO, Jansen JA, Jaakkola T, de Ruijter A, Rich J, Seppälä J, Yli-Urpo A. Bone response to degradable thermoplastic composite in rabbits. Biomaterials. 2003;24:1697-704.
- 46. Dupraz A, Delecrin J, Moreau A, Pilet P, Passuti N. Long-term bone response to particulate injectable ceramic. J Biomed Mater Res. 1998;42:368-75.
- 47. Gauthier O, Bouler JM, Weiss P, Bosco J, Daculsi G, Aguado E. Kinetic study of bone ingrowth and ceramic resorption associated with the implantation of different injectable calciumphosphate bone substitutes. J Biomed Mater Res. 1999;47:28-35.
- 48. Brown GD, Mealey BL, Nummikoski PV, Bifano SL, Waldrop TC. Hydroxyapatite cement implant for regeneration of periodontal osseous defects in humans. J Periodontol. 1998;69:146-57.
- 49. Costantino PD, Friedman CD, Lane A. Synthetic biomaterials in facial plastic and reconstructive surgery. Facial Plast Surg. 1993;9:1-15.
- 50. Kobayashi S, Hara H, Okudera H, Takemae T, Sugita K. Usefulness of ceramic implants in neurosurgery. Neurosurgery. 1987;21:751-5.
- 51. Walsh WR, Vizesi F, Michael D. b-TCP bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials. 2008;29:266-271.
- 52. Komaki H, Tanaka T, Chazono M, Kikuchi T. Repair of segmental bone defects in rabbit tibiae using a complex of b-tricalcium phosphate, type I collagen, and fibroblast growth factor-2. Biomaterials. 2006;27:5118-26.
- 53. Hosseinkhani H, Hosseinkhani M, Tian F, Kobayashi H, Tabata Y. Ectopic bone formation in collagen sponge self-assembled peptide-amphiphile nanofibers hybrid scaffold in a perfusion culture bioreactor. Biomaterials. 2006;27:5089-98.
- 54. Hosseinkhani H, Hosseinkhani M, Tian F, Kobayashi H, Tabata Y. Osteogenic differentiation of mesenchymal stem cells in self-assembled peptideamphiphile nanofibers. Biomaterials. 2006;27:4079-86.
- 55. Hosseinkhani H, Hosseinkhani M, Kobayashi H. Proliferation and differentiation of mesenchymal stem cells using self-assembled peptide amphiphile nanofibers. Biomed Mater. 2006;1:8-15.
- 56. Shabani I, Haddadi-Asl V, Soleimani M, Seyedjafari E, Babaeijandaghi F, Ahmadbeigi N. Enhanced infiltration and biomineralization of stem cells on collagen-grafted three-dimensional nanofibers. Tissue Eng Part A. 2011;17:1209-18.
- 57. Fernando Cruz. Fabrication of HA/PLLA Composite scaffolds for bone tissue engineering using additive manufacturing technologies. Biopolymers. 2012;11:227-42.
- 58. Li J, Hong J, Zheng Q, Guo X, Lan S, Cui F. Repair of rat cranial bone defects with nHAC/PLLA and BMP-2-related peptide or rhBMP-2. J Orthop Res. 2011;11:1745-52.
- 59. Kos J, Nadini V, Huljev D, Nadinic I. Healing of bone defect by application of free transplant of greater omentum. Veterinaryski Arhv. 2006;5:367-79.
Publication Dates
-
Publication in this collection
27 Sept 2012 -
Date of issue
Oct 2012
History
-
Received
15 May 2012 -
Accepted
18 Aug 2012 -
Reviewed
16 July 2012