Abstracts
PURPOSE: To investigate the differences in Langerhans cells (LCs) populations between HIV-positive and negative anal squamous cell carcinomas patients. METHODS: Twenty five patients (14 HIV-positive and 11 HIV-negative) were evaluated. Paraffin-block transversal thin sections from biopsies of anal squamous cell carcinomas (ASCC) were stained using the anti-CD1A antibody that identifies activated LCs. LCs counts were performed using histometry at 20 different sites, at baseline in the ASCC cases. These were then compared with LCs counts in anal canal specimens from HIV-negative and positive patients without ASCC (controls groups). RESULTS: In patients with ASCC, the LC count was greater among HIV-negative individuals than among HIV-positive individuals (p<0.05). The LC count was greater in the control HIV-negative group than in HIV-positive patients with ASCC (p<0.05). CONCLUSION: There was a lower amount of activated LCs in HIV-positive patients with anal squamous cell carcinomas than in HIV-negative patients, thereby suggesting worsening of the immune response.
Acquired Immunodeficiency Syndrome; Carcinoma, Squamous Cell; Anal Canal; HIV; Langerhans Cells
OBJETIVO: Comparar a quantidade de células de Langerhans (CL) em pacientes portadores do carcinoma espinocelular (CEC) do canal anal HIV-positivo e negativo. MÉTODOS: Avaliamos 25 pacientes, sendo 11 HIV-negativo e 14 HIV-positivo portadores do CEC do canal anal. Realizamos estudo com a coloração imunoistoquímica anti-CD1A para avaliar as CL ativadas. Utilizamos as lâminas coradas e pelo método da histometria contamos em 20 campos diferentes as células coradas na camada basal da lâmina própria, onde era evidente a disseminação tumoral. Realizamos dois grupos controles compostos por pacientes submetidos à biopsia anal sem neoplasia (sete pacientes HIV-negativo e quatro HIV-positivo). Comparamos as contagens de CL. RESULTADOS: A quantidade de CL foi superior nos pacientes portadores do CEC do canal anal soronegativo para o HIV, em relação aos soropositivos (p<0,05). A quantidade de CL foi superior no grupo controle HIV-negativo em relação ao grupo composto por pacientes soropositivos portadores do CEC do canal anal (p<0,05). CONCLUSÃO: Houve aumento das células de Langerhans ativadas na área peritumoral dos pacientes soropositivos para o HIV, o que sugere diminuição da resposta imune local.
Síndrome de Imunodeficiência Adquirida; Carcinoma de Células Escamosas; Canal Anal; HIV; Células de Langerhans
9 - ORIGINAL ARTICLE
CLINICAL INVESTIGATION
Evaluation of Langerhans cells counts comparing HIV-positive and negative anal squamous cell-carcinoma patients1 1 Research performed at Division of Colon and Rectum, Department of Surgery, Santa Casa Faculty of Medical Sciences of Sao Paulo (FCMSCSP) and Division of Colon and Rectum of Emilio Ribas Infectology Institute (IIER), Sao Paulo, Brazil. Part of PhD thesis, Postgraduate Program in Research Surgery of Santa Casa Faculty of Medical Sciences of Sao Paulo. Tutor: Sidney Roberto Nadal.
Avaliação das células de Langerhans no carcinoma espinocelular do canal anal em pacientes HIV-positivo e negativo
Sylvia Heloisa Arantes CruzI; Sidney Roberto NadalII; Carmen Ruth Manzione NadalIII; Edenilson Eduardo CaloreIV
IFellow PhD degree, Postgraduate Program in Research Surgery, FCMSCSP, Sao Paulo, Brazil. Main author, conception and design of the study, analysis and interpretation of data, manuscript writing
IIPhD, Assistant Professor, Postgraduate Program in Research Surgery, FCMSCSP, Sao Paulo, Brazil. Conception, design, intellectual and scientific content of the study; critical revision
IIIPhD, Assistant Professor, IIER, Sao Paulo, Brazil. Conception and design of the study, critical revision
IVPhD, Assistant Professor, IIER, Sao Paulo, Brazil. Conception and design of the study, acquisition of data, critical revision
Correspondence Correspondence: Sylvia Heloisa Arantes Cruz Rua Martinico Prado, 26/111 01224-010 São Paulo-SP Brasil Tel.: (55 11)3337-2558 sylviahacruz@hotmail.com
ABSTRACT
PURPOSE: To investigate the differences in Langerhans cells (LCs) populations between HIV-positive and negative anal squamous cell carcinomas patients.
METHODS: Twenty five patients (14 HIV-positive and 11 HIV-negative) were evaluated. Paraffin-block transversal thin sections from biopsies of anal squamous cell carcinomas (ASCC) were stained using the anti-CD1A antibody that identifies activated LCs. LCs counts were performed using histometry at 20 different sites, at baseline in the ASCC cases. These were then compared with LCs counts in anal canal specimens from HIV-negative and positive patients without ASCC (controls groups).
RESULTS: In patients with ASCC, the LC count was greater among HIV-negative individuals than among HIV-positive individuals (p<0.05). The LC count was greater in the control HIV-negative group than in HIV-positive patients with ASCC (p<0.05).
CONCLUSION: There was a lower amount of activated LCs in HIV-positive patients with anal squamous cell carcinomas than in HIV-negative patients, thereby suggesting worsening of the immune response.
Key words: Acquired Immunodeficiency Syndrome. Carcinoma, Squamous Cell. Anal Canal. HIV. Langerhans Cells.
RESUMO
OBJETIVO: Comparar a quantidade de células de Langerhans (CL) em pacientes portadores do carcinoma espinocelular (CEC) do canal anal HIV-positivo e negativo.
MÉTODOS: Avaliamos 25 pacientes, sendo 11 HIV-negativo e 14 HIV-positivo portadores do CEC do canal anal. Realizamos estudo com a coloração imunoistoquímica anti-CD1A para avaliar as CL ativadas. Utilizamos as lâminas coradas e pelo método da histometria contamos em 20 campos diferentes as células coradas na camada basal da lâmina própria, onde era evidente a disseminação tumoral. Realizamos dois grupos controles compostos por pacientes submetidos à biopsia anal sem neoplasia (sete pacientes HIV-negativo e quatro HIV-positivo). Comparamos as contagens de CL.
RESULTADOS: A quantidade de CL foi superior nos pacientes portadores do CEC do canal anal soronegativo para o HIV, em relação aos soropositivos (p<0,05). A quantidade de CL foi superior no grupo controle HIV-negativo em relação ao grupo composto por pacientes soropositivos portadores do CEC do canal anal (p<0,05).
CONCLUSÃO: Houve aumento das células de Langerhans ativadas na área peritumoral dos pacientes soropositivos para o HIV, o que sugere diminuição da resposta imune local.
Descritores: Síndrome de Imunodeficiência Adquirida. Carcinoma de Células Escamosas. Canal Anal. HIV. Células de Langerhans.
Introduction
Anal squamous cell carcinomas (ASCC) are 20 to 30 times less frequent than colorectal carcinomas1. According to their location, they can be divided into anal canal tumors, present in 85% of such patients, and those of the anal verge, which are similar to skin carcinomas2.
ASCC most frequently affects women in their sixties, in proportions of 5:1 in relation to men3,4. However, a change in this epidemiological profile has been taken place since the onset of the AIDS epidemics. The incidence of this kind of tumor has increased among HIV-infected men aged 30 to 40 years who practice anal receptive sex5,6. In this group of patients, ASCC is 25 to 50 times more frequent than among HIV-negative men of the same age7, thus suggesting that immunological suppression and HIV infection are important in carcinogenesis.
ASCC appears to be associated with human papillomavirus (HPV) infection8, which induces dysplasia in the anal mucosa of anal sex practitioners9-11. These anal dysplasias are nowadays named anal intraepithelial neoplasia (AIN) and may be low-grade (LAIN) or high-grade (HAIN)12,13. They are most common among HIV-infected individuals14,15, even during highly active antiretroviral therapy (HAART)16. Although such treatment improves systemic immunity, apparently it does not influence local immunity in patients with an incomplete response17,18.
Langerhans cells (LCs), which are located in the suprabasal portion of the epidermis, are the immature dendritic cells of the cutaneous immune system. They capture antigens that enter through the skin and, by stimulating proinflammatory cytokines, lose their adherence to epidermal cells and migrate to the regional lymph nodes through lymphatic vessels. Activated LCs are stable and become resistant to tumor-related suppressor factors, and they show an increasing ability to induce an immune response5. Immunodeficiency decreases the LC population and prevents their immediate activation4. By provoking local immunosuppression, HIV causes a defective immune response to virus infection, thereby explaining the increased rates of dysplasia and cancer in these patients' anogenital region19. Furthermore, inhibition of LC migration caused by tumor-derived factors prevents LCs from promoting anti-tumoral immunity20.
Several studies have shown low density of LCs in SCC of the skin21, uterine cervix22 and anal mucosa23, and recent research on cervical SCC has suggested that the decreased density of LCs is secondary to low E-cadherin expression21. On the other hand, HPV increases the number of LCs in the anal mucosa of HIV-negative individuals24.
All of these findings lead us to conclude that immunosuppression caused by HIV infection is important in ASCC development. Moreover, we did not find any studies on LC density that compared ASCC patients with and without HIV infection. Therefore, we decided to evaluate local immunity by quantifying active LC comparatively between these two groups of patients performing immunohistochemical reaction with anti-CD1A antibody.
Methods
This study was approved by the Ethics in Research Committee of FCMSCSP (CEP/FCMSCSP nº 435/09).
We evaluated paraffin blocks containing diagnostic biopsy material from ASCC in 25 patients. All of these patients were in stage II and IIIB, according to the TNM classification of the American Joint Committee on Cancer (AJCC)25. We studied two groups of patients with ASCC, GROUP A and B. The GROUP A had 11 HIV-negative patients (nine women and two men) from Division of Colon and Rectum of Department of Surgery of Santa Casa Faculty of Medical Sciences of São Paulo. The GROUP B had 14 HIV-positive men from the Division of Colon and Rectum of Emilio Ribas Infectology Institute. They were treated between 2001 and 2010. All of the GROUP B patients were under treatment with HAART, and had T CD4 lymphocyte counts higher than 200/μL, with an undetectable viral HIV load. Patients with anal canal SCC in stages I, III and IV, or in situ carcinoma, or anal margin SCC, and those with other, related perianal diseases, were excluded.
Seven HIV-negative patients and four HIV-positive patients who underwent hemorrhoidectomy were used as controls. The HIV-negative control group consisted of four men and three women from Division of Colon and Rectum of Department of Surgery of Santa Casa Faculty of Medical Sciences of Sao Paulo. The HIV-positive control group consisted of three men and one woman all of them were under treatment with HAART, and had T CD4 lymphocite counts higher than 200/μL, with an undetectable viral HIV load from Division of Colon and Rectum of Emilio Ribas Infectology Institute. A fragment of the mucosa from the mucocutaneous specimen containing the hemorrhoidal tissue was obtained, in order to ascertain the number of LCs in patients without ASCC. This material was also embedded in paraffin and tested negative for HPV.
Diagnostic confirmation of ASCC in the paraffin-embedded material was obtained by means of hematoxylin-eosin (HE) staining before undertaking the immunohistochemical method. The paraffin was removed and the samples were processed with xylol and alcohol at increasing hydration rates, sequentially from absolute alcohol 99% to 90%, 70% and, lastly, water. After inhibiting endogenous peroxidase with a 10-volume solution of H2O2, the samples were rinsed in phosphate-buffered saline (PBS) solution, and were exposed to citrate-buffered solution at high temperature. The immunohistochemical reaction was performed with anti-CD1A antibody as the primary reagent. The solutions were left overnight in the refrigerator at a temperature of 4ºC. The samples were then rinsed in PBS and placed in the development buffer with the secondary anti-biotin antibody, coupled with peroxidase in order to cause staining. The process was concluded with diaminobenzidine, which causes a brown color when the reaction is positive.
After preparing transversal histological thin sections, activated LCs from 20 different sites on each section were identified and counted by means of histometry. This procedure was used for both groups and for the controls. The sections were viewed at a magnification of 600x, only in the basal layer of the section, where the tumor was obvious. In order to study the basal layer in the controls groups sections were cut at locations where there were no abnormalities in the epithelium.
The results were compared and subjected to statistical analysis using the non-parametric Mann Whitney test. Results were taken to be significant when p<0.05. Box plots were created using the SPSS software, release 9.0, in order to compare the sample graphically.
Results
The Group A had an average age of 57 years ranging from 37 to 82 years old. The Group B had an average age of 41 years ranging from 29 to 55 years old. The HIV-negative control group had an average age of 40.3 years ranging from 29 to 54 years old. The HIV-positive control group had an average age of 38 years ranging from 20 to 46 years old.
The average LC population in the control HIV-negative group was 27.71 cells, and the average per site was 1.38 cells. The average in the control HIV-positive group was 19.12 cells, and average per site was 0.96 cells. For Groups A and B, the LC counts were 30.54 and 4.14 cells, respectively, and average per site was 1.52 and 0.21 cells, respectively.
Figure 1 show the comparison of LC counts between Group A and the HIV-negative control group. The Group A sample is concentrated between the 50th and 75th percentiles of the control sample and the lower value in Group A was higher than the median value for the control HIV-negative group. Furthermore, there were two outlying values in Group A. However, statistical analysis using the nonparametric Mann Whitney Kruskal-Wallis test showed no difference between these distributions (p = 0.791).
The comparison between distributions of the control HIV-negative group and Group B can be seen in Figure 2, and indicates that the Group B sample was below the 50th percentile in relation to the HIV-negative control group, and its lower limit was zero. The maximum value for patient distribution in Group B was lower than the median value of the control HIV-negative group, and its median value was close to zero. The Mann Whitney test confirmed this difference between the two groups (p = 0.002).
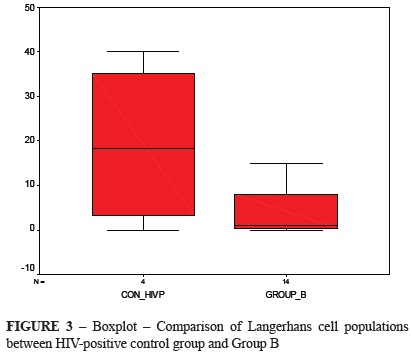
Figure 3 shows the comparison of LC counts between control HIV-positive group and Group B. The Group B sample was lower than the median value of the control HIV-positive group, and its median value was close to zero. The Mann Whitney test showed no difference between these distributions (p = 0.0219).
The comparison between the controls can be seen in Figure 4, and indicates that the HIV-negative control group is concentrated between the 50th and 75th percentiles. The Mann Whitney test showed no difference between the two groups (p = 0.219).
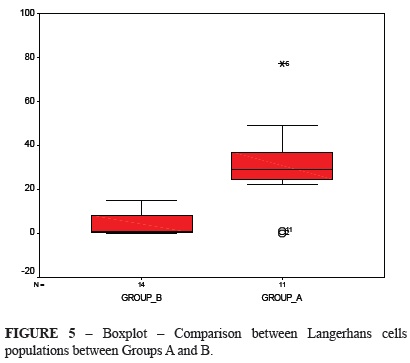
Figure 5 shows the comparison between groups A and B. There was no intersection areas, thus meaning that they were different, except for the outlying values of Group A. Active LC counts in these two groups were compared using the same nonparametric test as above, which showed a statistical difference (p = 0.002).
Discussion
The change in the epidemiological profile of ASCC patients seem to be related to HIV infection and immunosuppression2,26. This can be seen from the fact that, whereas this tumor usually occurs in women29,30, it presents increased frequency among HIV-infected men who have sex with men (MSM)2. Our study also shows this, since ASCC appeared among HIV-positive MSM who were younger than the HIV-negative women that we treated. Nonetheless, if HPV is confirmed as an etiological agent for ASCC, this tumor can be considered to be a sexually transmitted disease2,31. We did not include the viral types of our patients' tumors because this would not change the results or conclusions presented in this study.
We chose to include only anal canal SCC patients in AJCC stage II and IIIB, in an attempt to use groups that were as homogeneous as possible. We also excluded individuals with anal margin SCC, because the behavior of this kind of cancer is similar to that of cutaneous SCC.
We included the controls groups in order to ascertain what the LC population in the anal mucosa would be in a population that was as close as possible to normality, in order to make comparisons with the results from the ASCC patients. We used part of the anal mucosa from hemorrhoidectomy specimens from HIV-negative and positive patients, in order to avoid taking biopsies from volunteers with no anal diseases. The average LC count for the HIV-negative controls was 1.38 per site, which is similar to what has been reported in the literature24. We included a control group of HIV-infected people without HPV infection and there was no difference between the controls groups.
LCs are the main antigen-presenting cells in skin, and they function as a defense mechanism against infections and tumors such as these. We decided to study them in order to evaluate local immunity. We used anti-CD1A antibody staining because it provides a better view of active LCs than other molecules32. We chose to use the immunohistochemical technique described by Hsu33, which facilitates antigen identification in sections stained by CD1A.
We observed that the median sample in HIV-negative patients with ASCC was a little higher than in the control HIV-negative group, but there was no statistical difference. This was unexpected, because tumor development should stimulate LC activation for the immune response, as shown in patients with high-grade cervical neoplasia associated with HPV infection22. One possible explanation for this may be that this group of patients included elderly people and, according to the literature, such individuals must have some degree of immunodeficiency34.
The LC count observed among HIV-positive patients in our study was lower than that of the control HIV-negative group. The same has been seen among HIV-positive patients with cervical SCC, thereby contributing towards the progression of HPV-related lesions35. This was already expected, because HIV-positive patients had lower LC populations and, consequently, an impaired antigen-antibody response. When we compared the LC counts of ASCC patients infected or not by the HIV, we noticed the count was lower among the HIV-positive patients, possibly due to the local and systemic immunosuppression. Tumor cell growth should stimulate the production of such cells, which thus should appear in larger numbers in order to protect the anal mucous tissue32. However, immunosuppression provoked by HIV infection decreases dendritic cells counts in peripheral blood of children who have an incomplete response to HAART36. On the other hand, no studies have yet been conducted to investigate whether the same happens in relation to the local immunity of adults. This could be another theory that might explain why only a few HIV-infected patients among those with anogenital HPV-related lesions will develop ASCC, even if HAART is in use.
HIV-positive patients are believed to have more LCs that are infected with HIV. Thus, activation and migration to the local lymph nodes are inhibited, because they do not receive the proper chemokine signal for migration37, thus worsening local immune response38 and resulting in persistent HPV infection, with an increased risk of neoplasia37,39.
However, there may be several reasons why the immune system is unable to eradicate tumor cells40. Firstly, most tumors are derived from host cells and resemble normal tissues, expressing a few antigens that cannot be recognized and are consequently only weakly immunogenic. Secondly, the rapid growth and spread of tumors may overwhelm the capacity of the immune system to eradicate tumor cells. Thirdly, many tumors have specialized mechanisms for evading host immune responses40.
There is still much to learn about the activation mechanism of LCs. However, its regulation is directly related to immunity41. Tumor evolution is believed to inhibit the action of LCs, with mediators that have still not been described but which predispose towards dysplasia formation. New immunological mediators that stimulate the activation and migration of LCs, thereby causing improvement of the innate immune response42, might be effective in the future for preventing and treating this kind of cancer.
Conclusions
HIV-positive patients with anal squamous cell carcinomas (ASCC) present decreased activated Langerhans cells counts compared with those of HIV-negative patients with ASCC. Furthermore, it may be inferred that there is a decrease in local immunity among HIV-positive patients.
Conflict of interest: none
Financial source: Co-ordination of Improvement for Higher Academic Staff (CAPES)
Received: May 11, 2012
Review: July 12, 2012
Accepted: August 14, 2012
- 1. Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer Clin. 1999;49:8-31.
- 2. Gervaz P, Allal AS, Villiger P, Bühler L, Morel P. Squamous cell carcinoma of the anus: another sexually transmitted disease. Swiss Med Wkly. 2003;133:353-9.
- 3. Chauvienc L, Buthaud X, Falcou MC, Mosseri V, De la Rochefordiere A, Pierga JY. Anal canal cancer treatment: practical limitations of routine prescription of concurrent chemotherapy and radiotherapy. Br J Cancer. 2003;89:2057-61.
- 4. Stearns MW Jr., Urmacher C, Sterberger SS, Woodruff J, Attiyeh F. Cancer of the anal canal. Curr Probl Cancer. 1980;4:1-44.
- 5. Esiashvili N, Landry J, Matthews RH. Carcinoma of the anus: strategies in management. Oncologist. 2002;7:188-99.
- 6. Breese PL, Judson FN, Penley KA, Douglas JM Jr. Anal human papillomavirus infection among homosexual and bisexual men: prevalence of type-specific infection and association with human immunodeficiency virus. Sex Transm Dis. 1995;22:7-14.
- 7. Vatra B, Sobhani I, Aparicio T, Girard PM, Puy Montbrun TD, Housset M. Anal canal squamous-cell carcinomas in HIV positive patients: clinical features, treatments and prognosis. Gastroenterol Clin Biol. 2002; 26:150-6.
- 8. Arany I, Yen A, Tyring SK. p53, WAF1/CIP1 and mdm2 expression in skin lesions associated with human papillomavirus and human immunodeficiency virus. Anticancer Res. 1997;17(2B):1281-5.
- 9. Stadler RF, Gregorcyk SG, Euhus DM, Place RJ, Huber PJ, Simmang CL. Outcome of HIV-infected patients with invasive squamous cell carcinoma of the anal canal in the era of highly active anti retroviral therapy. Dis Colon Rectum. 2004;47:1305-9.
- 10. Moscicki AB, Hills NK, Shiboski S, Darragh TM, Jay N, Powell K. Risk factors for abnormal anal cytology in young heterosexual women. Cancer Epidemiol Biomarkers Prev. 1999;8:173-8.
- 11. Devaraj B, Cosman BC. Expectant management of anal squamous dysplasia in patients with HIV. Dis Colon Rectum. 2006;49(1):36-40.
- 12. Longacre TA, Kong CS, Welton ML. Diagnostic problems in anal pathology. Adv Anat Pathol. 2008;15:263-78.
- 13. Palefsky J. Human papillomavirus and anal neoplasia. Curr HIV/AIDS Rep. 2008;5:78-85.
- 14. Palefsky JM, Holly EA, Ralston ML, Jay N, Berry JM, Darragh TM. High incidence of anal high-grade squamous intra-epithelial lesions among HIV-positive and HIV-negative homosexual and bisexual men. AIDS. 1998;12:495-503.
- 15. Manzione CR, Nadal SR, Calore EE. Postoperative follow-up of anal condylomata acuminata in HIV-positive patients. Dis Colon Rectum. 2003;46(10):1358-65.
- 16. Palefsky JM, Holly EA, Ralston ML, Da Costa M, Bonner H, Jay N. Effect of highly active antiretroviral therapy on the natural history of anal squamous intraepithelial lesions and anal human papillomavirus infection. J Acquir Immune Defic Syndr. 2001;28:422-8.
- 17. Chehimi J, Azzoni L, Farabaugh M, Creer SA, Tomescu C, Hancock A. Baseline viral load and immune activation determine the extent of reconstitution of innate immune effectors in HIV-1-infected subjects undergoing antiretroviral treatment. J Immunol. 2007;179(4):2642-50.
- 18. Nadal SR, Manzione CR, Horta SH. Comparison of perianal diseases in HIV-positive patientes in periods before and after HAART use. Dis Colon Rectum. 2008;51(10):1491-4.
- 19. Sobhani I, Vuagnat A, Walker F, Vissuzaine C, Mirin B, Hervatin F. Prevalence of high-grade dysplasia and cancer in the anal canal in human papillomavirus-infected individuals. Gastroenterology. 2001;120(4):857-66.
- 20. Weber F, Byrne SN, Le S, Brown DA, Breit SN, Scolyer RA. Transforming growth factor-β1 immobilises dendritic cells within skin tumours and facilitates tumour escape from the immune system. Cancer Immunol Immunther. 2005;54:898-906.
- 21. Galan A, Ko CJ. Langerhans cells in squamous cell carcinoma vs. pseudoepitheliomatous hyperplasia of the skin. J Cutan Pathol. 2007;34(12):950-2.
- 22. Campaner AB, Nadais RF, Galvão MA, Santos RE, Aoki T. Evaluation of density of Langerhans cells in human cervical intraepithelial neoplasia. Acta Obstet Gynecol Scand. 2007;86(3):361-6.
- 23. Sobhani I, Walker F, Roudot-Thoraval F, Abramowitz L, Johanet H, Hénin D. Anal carcinoma: incidence and effect of cumulative infections. AIDS. 2004;18(11):1561-9.
- 24. Sobhani I, Walker F, Aparicio T, Abramowitz L, Henin D, Cremieux AC. Effect of anal epidermoid cancer-related viruses on the dendritic (Langerhans') cells of the human anal mucosa. Clin Cancer Res. 2002;8:2862-9.
- 25. American Joint Committee on Cancer. Anal Cancer. In: Greene FL, Page DL, Fleming ID. AJCC cancer staging manual. New York: Springer-Verlag; 2001. p.139.
- 26. Fleshner PR, Chalasani S, Chang GJ, Levien DH, Hyman NH, Buie WD. Practice parameters for anal squamous neoplasms. Dis Colon Rectum. 2008;51:2-9.
- 27. Wexler A, Berson AM, Goldstone SE, Waltzman R, Penzer J, Maisonet OG. Invasive anal squamous-cell carcinoma in the HIV-positive patient: outcome in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2007;51(1):73-81.
- 28. Goldstone SE, Winckler B, Ufford LJ, Alt E, Palefsky JM. High prevalence of anal squamous intraepithelial lesions and squamous-cell carcinoma in men who have sex with men as seen in surgical practice. Dis Colon Rectum. 2001;44:690-8.
- 29. Adams V, Moll C, Schmid M, Rodrigues C, Moos R, Briner J. Detection and typing of human papillomavirus in biopsy and cytological specimens by polymerase chain reaction and restriction enzyme analysis: a method suitable for semiautomation. J Med Virol. 1996;48:161-70.
- 30. Crook T, Vousden KH. Properties of p53 mutations detected in primary and secondary cervical cancers suggest mechanisms of metastasis and involvement of environmental carcinogens. EMBO J. 1992;11(11):3935-40.
- 31. Gervaz P, Calmy A, Durmish Y, Allal AS, Morel P. Squamous cell carcinoma of the anus-an opportunistic cancer in HIV-positive male homosexuals. World J Gastroenterol. 2011;17(25):2987-91.
- 32. Peña-Cruz V, Ito S, Dascher CC, Brenner MB, Sugita M. Epidermal Langerhans cells efficiently mediate CD1a-dependent presentation of microbial lipid antigens to T cells. J Invest Dermatol. 2003;121(3):517-21.
- 33. Hsu SM, Raine L, Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol. 1981;75:816-21.
- 34. Grewe M. Chronological ageing and photoageing of dendritic cells. Clin Exp Dermatol. 2001;26(7):608-12.
- 35. Taube JM, Nichols AD, Bornman LS, Bornman DM, Jackson JB. Langerhans cell density and high-grade vulvar intraepithelial neoplasia in women with human immunodeficiency virus infection. J Cutan Pathol. 2007;34(7):565-70.
- 36. Usuga X, Montoya CJ, Landay AL, Rugeles MT. Characterization of quantitative and functional innate immune parameters in HIV-1-infected Colombian children receiving stable highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;49(4):348-57.
- 37. Santegoets LA, van Seters M, Heijmans-Antonissen C, Kleinjan A, van Beurden M, Ewing PC, Kühne LC, Beckmann I, Burger CW, Helmerhorst TJ, Blok LJ. Reduced local immunity in HPV-related VIN: expression of chemokines and involvement of immunocompetent cells. Int J Cancer. 2008;123(3):616-22.
- 38. Nandwani R, Gazzard BG, Barton SE, Hawkins DA, Zemelman V, Staughton RC. Does HIV disease progression influence epidermal Langerhans cell density? Br J Dermatol. 1996;134(6):1087-92.
- 39. Munoz-Bongrand N, Poghosian T, Zohar S, Gerard L, Chirica M, Quero L. Anal carcinoma in HIV-infected patients in the era of antirretroviral therapy: a comparative study. Dis Colon Rectum. 2011;54:729-35.
- 40. Jager D, Jager E, Knuth A. Immune responses to tumor antigen: implications for antigen specific immunotherapy of cancer. J Clin Pathol. 2001;54:669-74.
- 41. Manickam A, Sivanandham M, Tourkova IL. Immunological role of dendritic cells in cervical cancer. Adv Exp Med Biol. 2007;601:155-62.
- 42. Fahey LM, Raff AB, Da Silva DM, Kast WM. Reversal of human papillomavirus-specific T cell immune suppression through TLR agonist treatment of Langerhans cells exposed to human papillomavirus type 16. J Immunol. 2009;182(5):2919-28.
Publication Dates
-
Publication in this collection
27 Sept 2012 -
Date of issue
Oct 2012
History
-
Received
11 May 2012 -
Accepted
14 Aug 2012 -
Reviewed
12 July 2012