Abstracts
PURPOSE: To evaluate the capacity of natural latex membrane to accelerate and improve the regeneration quality of the of rat sciatic nerves. METHODS: Forty male adult Wistar rats were used, anesthetized and operated to cut the sciatic nerve and receive an autograft or a conduit made with a membrane derived from natural latex (Hevea brasiliensis). Four or eight weeks after surgery, to investigate motor nerve recovery, we analyzed the neurological function by walking pattern (footprints analysis and computerized treadmill), electrophysiological evaluation and histological analysis of regenerated nerve (autologous nerve graft or tissue cables between the nerve stumps), and anterior tibial and gastrocnemius muscles. RESULTS: All functional and morphological analysis showed that the rats transplanted with latex conduit had a better neurological recovery than those operated with autologous nerve: quality of footprints, performance on treadmill (p<0.01), electrophysiological response (p<0.05), and quality of histological aspects on neural regeneration. CONCLUSION: The data reported showed behavioral and functional recovery in rats implanted with latex conduit for sciatic nerve repair, supporting a complete morphological and physiological regeneration of the nerve.
Nerve Regeneration; Latex; Tissue Engineering; Rats
OBJETIVO: Avaliar a capacidade de uma membrana de látex natural em acelerar e melhorar a qualidade da regeneração do nervo ciático seccionado de ratos. MÉTODOS: Foram utilizados 40 ratos machos adultos da linhagem Wistar, anestesiados e operados com autoenxerto ou com interposição de um tubo confeccionado com uma membrana derivada do latex natural (Havea brasiliensis). Quatro ou oito semanas após a cirurgia, para investigar a recuperação motora do nervo, foram analisadas a função neurológica através do padrão da marcha (análise das pegadas e esteira computadorizada), avaliação eletrofisiológica e análise histológica do nervo regenerado (enxerto de nervo autólogo ou formação de nervo novo entre os cotos nervosos) e músculos gastrocnêmio e tibial anterior. RESULTADOS: Todas as análises morfológicas e funcionais demonstraram que os ratos transplantados com o conduto de látex tiveram recuperação melhor do que aqueles operados com nervo autólogo: qualidade das pegadas impressas, desempenho em esteira (p<0,01), resposta eletrofisiológica (p<0,05), e qualidade histológica da regeneração nervosa. CONCLUSÃO: Os dados apresentados demonstraram recuperação comportamental e funcional nos ratos implantados com o conduto de látex para a reparação do nervo ciático por meio de uma completa regeneração morfológica e fisiológica do nervo.
Regeneração Nervosa; Látex; Engenharia Tecidual; Ratos
10 ORIGINAL ARTICLE
EXPERIMENTAL NEUROLOGY
Sciatic nerve regeneration in rats by a nerve conduit engineering with a membrane derived from natural latex1 1 Research performed at Laboratory of Neuroanatomy, Department of Surgery and Anatomy, School of Medicine of Ribeirao Preto, University of Sao Paulo (USP), Ribeirao Preto-SP, Brazil. Part of Master thesis degree in Clinical Surgery Postgraduate Program. Tutor: Luiza da Silva Lopes.
Regeneração do nervo ciático em ratos através de um conduto confeccionado com uma membrana de látex natural
Marcos Vinícius Muniz GangaI, Joaquim Coutinho-NettoII, Benedicto Oscar ColliIII, Wilson Marques JuniorIV, Carlos Henrique Rocha CatalãoV, Ricardo Torres SantanaVI, Marcos Roberto Pedron OltramariVII, Kleber Tadeu CarraroVIII, João-José LachatIX, Luiza da Silva LopesX
IFellow Master degree, Postgraduate Program in Clinical Surgery, Department of Surgery and Anatomy, School of Medicine of Ribeirao Preto, USP, Ribeirao Preto-SP, Brazil. Acquisition and analysis of data, manuscript writing
II Associate Professor, In memorian, Department of Biochemistry and Immunology, School of Medicine of Ribeirao Preto, USP, Ribeirao Preto-SP, Brazil. Responsible for engineering the latex conduit
IIIFull Professor, Division of Neurosurgery, Department of Surgery and Anatomy, School of Medicine of Ribeirao Preto, USP, Ribeirao Preto-SP, Brazil. Conception of the study, involved with technical procedures
IVAssociate Professor, Division of Neurology, Department of Neurosciences and Behavioral Sciences, School of Medicine of Ribeirao Preto, USP, Ribeirao Preto-SP, Brazil. Electromyography of the animals
VFellowMaster degree, Clinical Surgery Postgraduate Program, Department of Surgery and Anatomy, School of Medicine of Ribeirao Preto, USP, Ribeirao Preto-SP, Brazil. Responsible for manuscript preparation
VIAssistant Professor, Department of Clinical Surgery, Faculty of Medicine of Amazonas, Federal University of Amazonas (UFAM), Amazonas-AM, Brazil. Involved with technical procedures
VIIScientific initiation student, Department of Surgery and Anatomy, School of Medicine of Ribeirao Preto, USP, Ribeirao Preto-SP, Brazil. Acquisition of data
VIIIFellow PhD degree, Clinical Surgery Postgraduate Program, Department of Surgery and Anatomy, School of Medicine of Ribeirao Preto, USP, Ribeirao Preto-SP, Brazil.Acquisition of data and and interpretation of data
IXAssociate Professor, Division of Neuroanatomy, Department of Surgery and Anatomy, School of Medicine of Ribeirao Preto, USP, Ribeirao Preto-SP, Brazil. Analysis and interpretation of data
XAssistant Professor, Division of Neuroanatomy, Department of Surgery and Anatomy, School of Medicine of Ribeirao Preto, USP, Ribeirao Preto-SP, SP, Brazil. Conception, design and scientific content of the study, critical revision
Correspondence Correspondence: Luiza da Silva Lopes Departmento de Cirurgia e Anatomia Faculdade de Medicina de Ribeirão Preto Universidade de São Paulo USP Avenida Bandeirantes, 3.900 14040-905 Ribeirão Preto SP Brasil Tel.: (55 16)3602-4690 luslopes@fmrp.usp.br
ABSTRACT
PURPOSE: To evaluate the capacity of natural latex membrane to accelerate and improve the regeneration quality of the of rat sciatic nerves.
METHODS: Forty male adult Wistar rats were used, anesthetized and operated to cut the sciatic nerve and receive an autograft or a conduit made with a membrane derived from natural latex (Hevea brasiliensis). Four or eight weeks after surgery, to investigate motor nerve recovery, we analyzed the neurological function by walking pattern (footprints analysis and computerized treadmill), electrophysiological evaluation and histological analysis of regenerated nerve (autologous nerve graft or tissue cables between the nerve stumps), and anterior tibial and gastrocnemius muscles.
RESULTS: All functional and morphological analysis showed that the rats transplanted with latex conduit had a better neurological recovery than those operated with autologous nerve: quality of footprints, performance on treadmill (p<0.01), electrophysiological response (p<0.05), and quality of histological aspects on neural regeneration.
CONCLUSION: The data reported showed behavioral and functional recovery in rats implanted with latex conduit for sciatic nerve repair, supporting a complete morphological and physiological regeneration of the nerve.
Key words: Nerve Regeneration. Latex. Tissue Engineering. Rats.
RESUMO
OBJETIVO: Avaliar a capacidade de uma membrana de látex natural em acelerar e melhorar a qualidade da regeneração do nervo ciático seccionado de ratos.
MÉTODOS: Foram utilizados 40 ratos machos adultos da linhagem Wistar, anestesiados e operados com autoenxerto ou com interposição de um tubo confeccionado com uma membrana derivada do latex natural (Havea brasiliensis). Quatro ou oito semanas após a cirurgia, para investigar a recuperação motora do nervo, foram analisadas a função neurológica através do padrão da marcha (análise das pegadas e esteira computadorizada), avaliação eletrofisiológica e análise histológica do nervo regenerado (enxerto de nervo autólogo ou formação de nervo novo entre os cotos nervosos) e músculos gastrocnêmio e tibial anterior.
RESULTADOS: Todas as análises morfológicas e funcionais demonstraram que os ratos transplantados com o conduto de látex tiveram recuperação melhor do que aqueles operados com nervo autólogo: qualidade das pegadas impressas, desempenho em esteira (p<0,01), resposta eletrofisiológica (p<0,05), e qualidade histológica da regeneração nervosa.
CONCLUSÃO: Os dados apresentados demonstraram recuperação comportamental e funcional nos ratos implantados com o conduto de látex para a reparação do nervo ciático por meio de uma completa regeneração morfológica e fisiológica do nervo.
Descritores: Regeneração Nervosa. Látex. Engenharia Tecidual. Ratos.
Introduction
Traumatic injury to peripheral nerves is not an uncommon casualty and results from trauma due to vehicle accidents and less commonly from penetrating trauma, falls, and industrial accidents. It can lead to considerable disability and high financial costs across the world1.Even with advances in repair techniques and new technologies involved, the surgical results are often disappointing especially when there is total transected nerve or when the nerve injury resulted in substance loss between the two nerve stumps2. These facts led to the development of techniques using nerve grafts3 or conduits that bridge the ends and guide the growth of nerve fibers between the stumps, including the use of natural or synthetic materials2,4,5. A membrane engineered from natural latex (extracted from Hevea brasiliensis), developed at the Department of Biochemistry and Immunology, Faculty of Medicine of Ribeirao Preto of University of Sao Paulo, was used initially in an experimental study in dogs as a prosthesis for replacement of cervical esophagus. It was observed that the new material was able to induce the neoformation of esophageal wall. Later, other studies such as femoral arterioplasty in dogs, replacement of the pericardium in dogs, reconstruction of the ocular conjunctiva in rabbits, treatment of chronic skin ulcers and regeneration of the tympanic membrane in humans, demonstrated the effects of that biomaterial to induce the neoformation of tissues, which provides motivation for further research with other applications6,7. Since the latex membrane has a potential for regeneration of several tissues, and a satisfactory conduit for guiding the repair of peripheral nerves is not yet developed, the purpose of this study was to evaluate the ability of a conduit engineer with a membrane derived from natural latex to accelerate and improve the regeneration quality of rat sciatic nerves.
Methods
All animals were treated in accordance with guidelines by the COBEA (Brazilian College of Animal Experimentation) and protocols were approved by the local animal ethics committee (CETEA - School of Medicine of Ribeirao Preto, University of Sao Paulo USP), protocol # 080/2005. We used adult male Wistar rats that were bred locally. The rats were clinically healthy and weighed 250g (± 20g). They were distributed in four experimental groups, each group with an equal number of rats (n=10), anesthetized and operated with transplantation of autograft or a conduit made of a membrane derived from natural latex (Hevea brasiliensis). The latex membrane was made with non-vulcanized rubber (cold polymerization). The endpoints of experiments were four or eight weeks after the transplantation surgery.
Surgical procedures
Animals were anesthetized with 10% Ketamine 90mg/Kg (Ketamina® - Pfizer do Brasil Ltda) associated with Xylazine 10mg/Kg (Rompum® - Bayer do Brasil Ltda), administered via intraperitoneal injection. The right hind legs were shaved, the skin disinfected and aseptic techniques used to ensure sterility. After skin incision and dissection of the muscle planes, the sciatic nerve was identified, sectioned, and 10mm of nerve was removed. In the autograft rats the nerve was excised, inverted, and reimplanted between the proximal and distal stumps of the nerve and sutured to the epineurium with 10-0 nylon. In the groups of latex conduit the rats were transplanted with a 12mm conduit made of a membrane derived from natural latex, interposed between the proximal and distal stumps with 10-0 nylon. The conduit dressed the nerve stumps, leaving a 10mm gap between nerve stumps. The muscle layer was re-approximated with 4-0 nylon sutures, and the skin closed with 4-0 sutures. Each rat received one implant that was removed at different time points (four or eight weeks post surgery).
Additional five rats were transplanted with latex conduit and had its content harvested to semi-thin sections.
Walking track and footprints analysis
The rats were allowed to walk up a small inclining gangway lined with white paper after their both hind paws were carefully pressed onto inkpads. The track built for walking8 consists of a long corridor with 120cm, 10cm wide and 15 tilt in the horizontal plane. The animal was placed at the beginning of the walking alley and usually walked to the dark goal box, thereby leaving its footprints on the paper. The footprints of rats were obtained (four or eight weeks) after the surgical procedure (transplantation of autograft nerve or latex conduit), and the footprints of the operated side were compared to those of the non operated side.
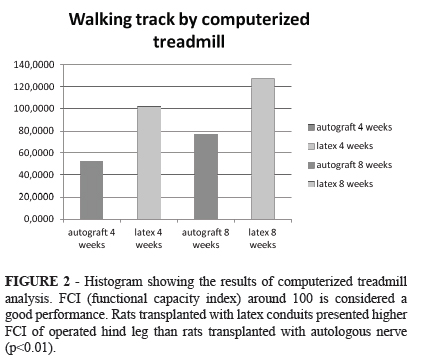
Walking track by computerized treadmill
A computerized treadmill for small rodents was designed and manufactured locally. The track surface was coated with a stainless steel mesh capable of conducting electrical impulses. The speed of the treadmill (12m/min) was controlled electronically and its inclination adjusted 15 degrees to the horizontal plane. Metallic electrodes were fixed to both rat hind feet. The animals were then placed on the track, and during the march, each touch of the electrode on the mesh belt corresponded to a contact (one step). Computer software dedicated to the system was created in an integrated environment to work with Windows XP® operating system. The software was based on data acquisition from the universal serial port (USB) which was connected to a wireless mouse. The left and right feet of animals were connected to the left and right mouse buttons, respectively. Thus, the electric signals originated by circuit closure were filtered, instantly generating the number of contacts (NC), the total contact time (TCT) and time of each contact (DC), of left and right foot individually. Functional impairment was defined as the deficiency presented by the operated foot to contribute to the animal progress on the treadmill for 5 minutes, and was expressed by functional capacity index (FCI), calculated according to the formula:
FCI= (PLF/PRF) x100PLF= performance of the left foot (NC x TCT)PRF= performance of the right foot (NC x TCT)
The quantification of functional capacity index of rats was obtained and groups of autologous implant or latex conduit implant were compared, four or 8 weeks post surgery.
Assessment by electromyogram
Postsurgical recording of muscle activity was performed four or eight weeks after surgical implants. Electrical stimuli were applied to the sciatic nerve trunk at its proximal portion in a pre section point and latency and amplitude were recorded in the gastrocnemius muscle at the ipsilateral side. At the endpoints of the experiments (four or eight weeks), animals were anesthetized with intraperitoneal Ketamine/Xylazine (same doses used for the surgical procedure), to perform the functional evaluation by electromyography.
Both stimulation and recording were performed with subdermal needle electrodes. The record was made in the gastrocnemius muscle. The active recording electrode was placed on the muscle motor point, while the reference was placed distally on the same muscle tendon. Stimulation proximal to the suture site was performed by stimulating (cathode) the trunk of the sciatic nerve in the sciatic foramen, with the anode placed approximately 2cm lateral to that point. The following parameters were analyzed: amplitude and latency of compound muscle action potential (PAMC).
Histological processing and morphological analysis
Histopathological analysis was conducted on samples corresponding to the regions proximal upper stump, distal lower stump of the cut nerve, medial part (M1 and M2) of the latex conduit content or autograft, and gastrocnemius and anterior tibial muscles. At the endpoint of the experiments (four or eight weeks post surgery), rats were euthanized by overdose of anesthesia and exsanguination. The surgical incision was reopened; the implant (latex conduit and its content or inverted nerve) removed with 3mm margins of nerve before and after the implant limits. The segment related to the implant was divided transversely into two halves (M1 and M2) and all samples obtained (a nerve segment proximal to the implant, M1 and M2 related to the implant, a nerve segment distal to the implant, and fragments of gastrocnemius and anterior tibial muscles) immersion fixed in 3% buffered paraformaldehyde, dehydrated in a sequence of alcohols and xylene, and embedded in paraffin. The embedded samples were oriented to transversal cross section of the nerve and muscle fibers. Sections (5μm thickness) were stained with 1% Toluidine Blue.
The nerve samples harvested from additional five rats that received latex conduit implants were immersed in a fixative solution (2% paraformaldehyde and 1% glutaraldehyde in 0.1M phosphate buffer). After the fixation, the samples were postfixed in 0.5% osmium tetroxide, dehydrated, embedded in epoxy resin; semi-thin sections were cut on an ultramicrotome, and stained with 1% Toluidine Blue.
The histological slides were digitized using a Carl Zeiss Axiophot microscope at 40x-magnification and Carl Zeiss Kontron 2.0 software. We evaluated the presence of regenerated nerve within the conduit and in the autograft (M1 and M2), the conditions of the nerve segment before de proximal point of surgery (P), the presence of nerve regeneration in the distal portion of the implant (D), degree of myelination and density of the regenerated nerve fibers within M1 and M2, and presence of nerve fibers interspersed within the muscle fibers.
All data are presented as mean ± standard error of the mean and were analyzed to confirm a normal distribution. Using one-way ANOVA followed by a Tukey post test comparison, data (mean ± SEM) were considered to be statistically significant when p-value was p<0.05. A non-parametric test was chosen when data did not pass normality test. Software used was GraphPad Software's InStat, version 3.06 for Windows (San Diego, USA).
Results
We did evaluate the following regeneration parameters: (1) macroscopic tissue regeneration; (2) electrodiagnostically detectable muscle reinnervation at four or eight weeks after surgery; (3) functional recovery four or eight weeks after surgery by walking track and footprints analysis or computerized treadmill, and (4) quality of regenerating nerve fibers four or eight weeks after surgery under light microscopy.
Functional recovery
Walking track and footprints analysis
The implanted animals (with autografts or latex conduits) showed a tendency for auto-mutilation of their digits in the operated limb, making this analysis extremely difficult. Some animals from the autotransplanted nerve group dragged their paws of the operated side. So, only the qualitative appearance of footprints was evaluated. The records showed better function of paw in groups of four or eight weeks with use of the latex membrane than the groups of autotransplanted nerves. Even greater benefit was seen in the group of eight weeks that received latex membrane, noted by the best quality of footprints (Figure 1).
Walking track by computerized treadmill
The results are represented by mean ± SEM and summarized in Figure 2. The rats of latex group four weeks after surgery presented better results (102.29 ± 6.697) than rats of four weeks autotransplanted group (52.44 ± 9.055) (p<0.01). The rats of latex group eight weeks after surgery had already better results (127.38 ± 9.916) than rats of eight weeks autotransplanted group (77.35 ± 7.71) (p<0.01).
Electrophysiological evaluation
Such results can be best observed in Figure 3. Latency and amplitude results are represented by mean (± SEM). After four weeks of surgery, the autograft group presented latency of 1.967 (± 0.205), and the group of latex membrane obtained 1.248 (± 0.111). Groups of eight weeks of post-operative care that received autografts had latency of 1.655 (± 0.122), and the group that received latex biomembrane had 1.218 (± 0.082). The group of latex conduit had minor latency than group of autotransplanted graft (p<0.05), for both four or eight weeks after surgery. The amplitude in the group of four weeks after receiving the autograft implant was 0.1095 (± 0.031) and the group that used the latex biomembrane obtained 0.40175 (± 0.045). The groups of 8 weeks of post-operative care which used the autograft obtained 0.27775 (± 0.039), while the group that used the latex biomembrane obtained amplitude of 0.549 (± 0.55). Both four or eight weeks after surgery, groups of latex conduit had higher amplitude than the group of autotransplanted graft (p<0.05).
Improvement of sciatic nerve repair and local environment
No adverse inflammatory reactions were observed in the site of the implants, both autotransplanted nerve and latex conduit. All the implanted latex conduits were intact with no swelling, deformation or rupture of their walls in the site of suture. Still, the regenerated nerve could be seen through the semitransparent latex conduits. No nerve dislocation was noted in the rats that received autotransplanted nerves.
Morphological analysis
Histological aspects of nerve and gastrocnemius muscle samples, with the use of latex conduit or autotransplanted nerve, four or eight weeks after surgery, are represented in Figures 4 and 5. There was greater neural regeneration in latex conduit groups than in autotransplanted nerve group, in both four or eight weeks after surgery. Muscles of latex conduit groups (four or eight weeks after surgery), show better quality of muscle fibers, with presence of vessels and reinnervated motor units. The muscles of autotransplanted groups usually had muscular cells atrophy and fibrosis surrounding them. The quality of nerve fibers and motor units in muscles is even better in latex conduit group with eight weeks of surgery than in group with four weeks of surgery.
Discussion
Different techniques and materials have been tested with the intent to promote, accelerate or optimize the regeneration of injured peripheral nerves4,9-18. Although numerous conduits for bridging nerve gaps have been developed, nerve autograft remains as the gold standard for treating peripheral nerves lesions18. The sural nerve is commonly used as autologous nerve graft, but the technique is limited by complications, such as donor site morbidity and muscle atrophy prior to complete regeneration.
Considering the already demonstrated potential of a membrane derived from natural latex in facilitating or even speeding up the regeneration process of several tissues, such as skin, eardrum, cornea, esophagus6,7,19, its use in the regeneration of rat's sectioned sciatic nerve was tested. Rats were transplanted with autograft nerve or conduit engineered from natural latex membrane, after surgically sectioning the sciatic nerve.
Four or eight weeks post surgery it was observed the functional recovery of gait and the presence of regenerated nerve within the latex conduit and in autotransplanted nerve. Functional recovery was assessed by electromyogram evaluation and walking gait by impression of footprints and computerized treadmill. Functional recovery was correlated with histopathological analysis. Both showed a better performance in animal groups that were transplanted with the latex conduit. Although the quality of footprints did not allow a quantitative comparison, the hind foot of operated side of rats transplanted with latex conduit impressed a footprint with spread toes, while the footprints of autotransplanted nerve group were blurred, sometimes with only heel print well distinguished. In the evaluation of walking gait by treadmill rats that received latex conduit showed results of functional capacity index (FCI) closer to 100, while rats transplanted with autograft nerve had FCI of less than 80. By electrophysiological analysis, rats transplanted with latex conduit showed minor latency and bigger amplitude than rats transplanted with autograft nerve. Therefore, the results of all functional tests used come in proving that the performance of the operated side foot is better in animals that have been transplanted with latex conduits than in animals who have received the autotransplanted nerves. It was also observed that, in animal groups that received latex conduit, the quality of regeneration was also slightly better, with higher density of myelinated nerve fibers, as well as with reduced atrophy and fibrosis of muscles.
Several authors have tested different materials with the same objective of promoting nerve fibers growth. However, the differences between the groups that have received these materials and those submitted to conventional surgery with autograft, were not promising and the functional recovery of damaged human nerves remains a clinical challenge. On the other hand, this work and recent studies analyzing fractions of non-vulcanized latex show the potential of latex conduit in regenerating injured nerves.
Conclusions
Up to now, membrane derived from latex conduit has never been employed as alternative for surgical implant in nerve regeneration. The data reported here showed behavioral and functional recovery in rats implanted with latex conduit for sciatic nerve repair, supporting a complete morphological and physiological regeneration of the nerve. Thus, a conduit engineered from the latex membrane can be considered as a potential conduit for the regeneration of injured peripheral nerves in cases of traumatic damages.
Acknowledgments
The authors are grateful to Antonio Renato Meirelles e Silva (Laboratory of Applied and Experimental Neurology, Department of Neuroscience and Behavior Science, Scholl of Medicine of Ribeirao Preto, University of Sao Paulo) for his assistance with microscope photographs. Technical assistance of Daniel Mazzetto is acknowledged. Marcos Vinícius Muniz Ganga acknowledges the Postgraduate scholarship from CNPq (National Council for Scientific and Tecnological Development) Brazil.
Received: July 11, 2012
Review: September 16, 2012
Accepted: October 18, 2012
Conflict of interest: none
Financial source: FAEPA (Foundation to Support Education, Research and Care)
- 1. Robinson LR. Traumatic injury to peripheral nerves. Muscle Nerve. 2000;23:863-73.
- 2. Battiston B, Geuna S, Ferrero M, Tos P. Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. Microsurgery. 2005;25:258-67.
- 3. Ayhan S, Yavuzer R, Latifoğlu O, Atabay K. Use of the turnover epineurial sheath tube for repair of peripheral nerve gaps. J Reconstr Microsurg. 2000;16:371-8.
- 4. Bian YZ, Wang Y, Aibaidoula G, Chen GQ, Wu Q. Evaluation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials. 2009;30:217-25.
- 5. Zhao Z, Wang Y, Peng J, Ren Z, Zhan S, Liu Y, Zhao B, Zhao Q, Zhang L, Guo Q, Xu W, Lu S. Repair of nerve defect with acellular nerve graft supplemented by bone marrow stromal cells in mice. Microsurgery. 2011;31:388-94.
- 6. Balabanian CA, Coutinho-Netto J, Lamano-Carvalho TL, Lacerda SA, Brentegani LG. Biocompatibility of natural latex implanted into dental alveolus of rats. J Oral Sci. 2006;48:201-5.
- 7. Alves de Sousa LC, Ribeiro de Toledo Piza M, Coutinho-Netto J, Ruiz DB, Schmidt VB. Latex biomembrane: a new method to coat the open cavity in tympanomastoidectomies. Braz J Otorhinolaryngol. 2007;73:331-6.
- 8. Dijkstra JR, Meek MF, Robinson PH, Gramsbergen A. Methods to evaluate functional nerve recovery in adult rats: walking track analysis, video analysis and the withdrawal reflex. J Neurosci Methods. 2000;96:89-96.
- 9. Chang JY, Ho TY, Lee HC, Lai YL, Lu MC, Yao CH, Chen YS. Highly permeable genipin-cross-linked gelatin conduits enhance peripheral nerve regeneration. Artif Organs. 2009;33:1075-85.
- 10. Chen MH, Chen PR, Hsieh ST, Huang JS, Lin FH. An in vivo study of tricalcium phosphate and glutaraldehyde crosslinking gelatin conduits in peripheral nerve repair. J Biomed Mater Res B Appl Biomater. 2006;77:89-97.
- 11. Chiono V, Sartori S, Rechichi A, Tonda-Turo C, Vozzi G, Vozzi F, D'Acunto M, Salvadori C, Dini F, Barsotti G, Carlucci F, Burchielli S, Nicolino S, Audisio C, Perroteau I, Giusti P, Ciardelli G. Poly(ester urethane) guides for peripheral nerve regeneration. Macromol Biosci. 2011;11:245-56.
- 12. Gong Y, Gong L, Gu X, Ding F. Chitooligosaccharides promote peripheral nerve regeneration in a rabbit common peroneal nerve crush injury model. Microsurgery. 2009;29:650-6.
- 13. Haastert-Talini K, Schaper-Rinkel J, Schmitte R, Bastian R, Mühlenhoff M, Schwarzer D, Draeger G, Su Y, Scheper T, Gerardy-Schahn R, Grothe C. In vivo evaluation of polysialic acid as part of tissue-engineered nerve transplants. Tissue Eng Part A. 2010;16:3085-98.
- 14. Hu X, Huang J, Ye Z, Xia L, Li M, Lv B, Shen X, Luo Z. A novel scaffold with longitudinally oriented microchannels promotes peripheral nerve regeneration. Tissue Eng Part A. 2009;15:3297-308.
- 15. Jiang X, Lim SH, Mao HQ, Chew SY. Current applications and future perspectives of artificial nerve conduits. Exp Neurol. 2010;223:86-101.
- 16. Madduri S, Papaloïzos M, Gander B. Trophically and topographically functionalized silk fibroin nerve conduits for guided peripheral nerve regeneration. Biomaterials. 2010;31:2323-34.
- 17. Magnaghi V, Conte V, Procacci P, Pivato G, Cortese P, Cavalli E, Pajardi G, Ranucci E, Fenili F, Manfredi A, Ferruti P. Biological performance of a novel biodegradable polyamidoamine hydrogel as guide for peripheral nerve regeneration. J Biomed Mater Res A. 2011;98:19-30.
- 18. Shin RH, Friedrich PF, Crum BA, Bishop AT, Shin AY. Treatment of a segmental nerve defect in the rat with use of bioabsorbable synthetic nerve conduits: a comparison of commercially available conduits. J Bone Joint Surg Am. 2009;91:2194-204.
- 19. Frade MA, Valverde RV, de Assis RV, Coutinho-Netto J, Foss NT. Chronic phlebopathic cutaneous ulcer: a therapeutic proposal. Int J Dermatol. 2001;40:238-40.
Publication Dates
-
Publication in this collection
29 Nov 2012 -
Date of issue
Dec 2012
History
-
Received
11 July 2012 -
Accepted
18 Oct 2012 -
Reviewed
16 Sept 2012