Abstract
PURPOSE: To investigate the compatibility of a new model of self-expandable tracheal stent in rats. METHODS: A new device of polyurethane covered and non - covered stent was placed in the trachea of Wistar rats. Animals were distributed in two groups: the polyurethane covered and non-covered group. Macroscopic parameters included position within the tracheal lumen, adherence to the mucosa, degree of dilatation, permeability and internal diameter. Microscopic findings evaluated were: incorporation, inflammatory activity, granulation tissue and epithelial revetment injuries. The observation follow-up was six weeks. All parameters were quantified based on determined score values. Incorporation of the stents was evaluated based on the observation if the stent was fixed into the trachea or if it could be removed. Degree of dilatation was performed by external diameter measurements. Granulation tissue was evaluated by measurements of height of the tissue growing into the tracheal lumen. RESULTS: 100% of non-covered stents had total attachment to mucosa and 100% of polyurethane covered type had adherence only. Regarding dilatation, granulation tissue, inflammatory activity and internal diameter measurements, there were no significant differences between the groups. Pathological tracheal wall injuries were present in both groups. CONCLUSION: Both models of stent demonstrated biocompatibility with the trachea. Rats are suitable for an experimental model of tracheal stent study.
Stents; Tracheal Stenosis; Materials Testing; Rats
3 - ORIGINAL ARTICLE
MODELS, BIOLOGICAL
Biocompatibility of a new device of self-expandable covered and non-covered tracheal stent. Comparative study in rats1 1 Research performed at Technological Research Department (TRD), University of Mogi das Cruzes (UMC) and Pathology Laboratory, Heart Institute (INCOR), University of Sao Paulo (USP), Brazil.
Olavo Ribeiro RodriguesI; Hélio MinamotoII; Mauro CanzianIII; Aristides Tadeu CorreiaIV; Fabio Biscegli JateneV
IChairman and Head, Division of General Thoracic Surgery, UMC, Mogi das Cruzes-SP, Brazil. Technical procedures, manuscript writing
IIAssistant Professor, Thoracic Surgeon, Department of Cardio-Pneumology, INCOR, Clinics Hospital, USP, Sao Paulo-SP, Brazil. Conception and scientific content of the study, supervision all phases, critical revision
IIIAssociate Professor, Department of Pathology, INCOR, Clinics Hospital, USP, Sao Paulo-SP, Brazil. Histopathological analysis
IVBiologist, Laboratory of Surgical Anatomy, INCOR, Clinics Hospital, USP, Sao Paulo-SP, Brazil. Acquisition and interpretation of data, statistical analysis
VChairman and Head, Thoracic Surgery Department, INCOR, Clinics Hospital, USP, Sao Paulo-SP, Brazil. Conception and design of the study, supervisor
Correspondence Correspondence: Olavo Ribeiro Rodrigues Rua Conceição Nogueira Ribeiro, 70 8725-070 Mogi das Cruzes - SP Brasil Tel.: (55 11)4796-5312 / 4799-8317 / 99974-3974 olavorr@uol.com.br
ABSTRACT
PURPOSE: To investigate the compatibility of a new model of self-expandable tracheal stent in rats.
METHODS: A new device of polyurethane covered and non - covered stent was placed in the trachea of Wistar rats. Animals were distributed in two groups: the polyurethane covered and non-covered group. Macroscopic parameters included position within the tracheal lumen, adherence to the mucosa, degree of dilatation, permeability and internal diameter. Microscopic findings evaluated were: incorporation, inflammatory activity, granulation tissue and epithelial revetment injuries. The observation follow-up was six weeks. All parameters were quantified based on determined score values. Incorporation of the stents was evaluated based on the observation if the stent was fixed into the trachea or if it could be removed. Degree of dilatation was performed by external diameter measurements. Granulation tissue was evaluated by measurements of height of the tissue growing into the tracheal lumen.
RESULTS: 100% of non-covered stents had total attachment to mucosa and 100% of polyurethane covered type had adherence only. Regarding dilatation, granulation tissue, inflammatory activity and internal diameter measurements, there were no significant differences between the groups. Pathological tracheal wall injuries were present in both groups.
CONCLUSION: Both models of stent demonstrated biocompatibility with the trachea. Rats are suitable for an experimental model of tracheal stent study.
Key words: Stents. Tracheal Stenosis. Materials Testing. Rats.
Introduction
The aim of tracheobronchial stents is to preserve the permeability of the airways. Differently from the conventional tracheotomy tubes, these stents maintain the airflow, what allows preservation of voice, humidification, warming and filtration of inhaled air. They have been used in cases of benign and malignant obstructions, involving the trachea, the carina and the main bronchi. They are inserted under endoscopic control at bronchoscopy.
The benign tracheal stenosis occurs in patients using orotracheal canulas for long-term artificial ventilation in Intensive Care Units and in patients with tracheostomy. The malignant obstructions occur in patients with tracheal primary tumors, advanced lung, esophagus and thyroid neoplasms. Regarding the latter patients, surgical treatment is not always curative and palliative disobstruction of the airways is needed.
Most of the literature on stents is about silicone tubes; self-expandable metal stents have recently been introduced with a simpler placement mechanism in comparison with the silicone models.
In an attempt to group together the advantages of both models, hybrid stents have been developed, such as the self-expanding silicone model and the metal models, totally covered with silicone, polyester, polypropylene and combinations of silicone and polytetrafluoroethylene (PTFE).
In Brazil, there has been an increase in the number of patients with tracheal stenosis who are non-candidates for a surgical treatment, i.e., when surgical reconstruction of the airways is contra-indicated. The tracheobronchial stents have met the demands of this group of patients1. Hence, there is a growing need of tracheobronchial stents.
However, the use of these stent models is limited due to high costs and unavailability in the national market - all of them are imported. This has led to the present experimental study, aiming at the viability of a tracheobronchial stent nationally manufactured.
The objective of this study is to evaluate the macroscopic and histological changings resulting from the placement of a self-expanding nitinol stent covered with polyurethane and an uncovered model in rats.
To reach the proposed objective, an experimental animal model in rats has been designed and tested.
Methods
The tracheal stent was placed in 30 adult Wistar rats, ten months old, male and female, 400g average weight, raised at the Technological Research Department (TRD) of Mogi das Cruzes University (UMC). Evaluation took place after an observation period of 42 days.
Research approved by the Ethics Committee (CEUA) of Mogi das Cruzes University, according to Federal Law nº 11.794, 2008, October 8 (Protocol n.03 from CEMEA/UMC on July 30th 2009).
Fifteen animals received the polyurethane covered stents and the other 15 animals were given the uncovered model.
The stent model and the stent delivery system were proposed, designed and developed by Braile Biomedica (Sao Jose do Rio Preto-SP) in partnership with INCOR and the TRD (Technological Research Department (NPT) of the Mogi das Cruzes University, after a pilot study of 10 animals.
The self-expandable stents were made of nitinol mesh woven from a single strand and covered or not covered with a polyurethane membrane. The dimensions were 18mm in length and ∅ 4 mm external diameter (8FR) for the coated models and ∅ 4 mm (7FR) for the uncoated models, after being released in the tracheal lumen, when fully expanded (Figure 1).
Stent placement procedures, animal observation until euthanasia and evaluation of the anatomical variables were carried out at the TRD of Mogi das Cruzes University from September 2009 to September 2010.
The histological study was done at the Pathology Laboratory at the Heart Institute (HC - Sao Paulo University) in the period from October 2010 to May 2011.
The stents were placed in a cylindrical translucent plastic stent delivery system of 2 mm external diameter; the stent was compressed inside it. An inner catheter enclosed in the stent delivery system completed the device (Figures 2 and 3).
The animals were anesthetized with tiletamine hydroclhoride and zolazepam (Zoletil® 50) (Virbac; Brazil) at a dosage of 20mg/kg intramuscularly.
Sedation was maintained with sulfuric ether and spontaneous ventilation was kept with intermittent oxygen supply by a nasal catheter. A plate inclined at 45 degrees was used to fix the rats in the dorsal decubitus position for the procedure.
The stents were placed through laryngoscopy with the use of a laryngoscope adapted from 12-cm angulated dissection tweezers for visual inspection.
The fiber optic of a pediatric 3mm-diameter rigid bronchoscope was used for illumination, what made the insertion procedure safe and the placement more accurate (Figure 4).
Recovery from anesthesia took place in a warm environment; animals were supplied with an oxygen mixture until full motor recovery was completed and then were accommodated in individual boxes.
The animals were observed daily for a period of 6 weeks and respiratory changings were registered in individual files. Symptoms and signs presented in the period between placement and death or euthanasia were registered and assessed.
Animals having an early death underwent necropsy within 12 hours after death. Deaths occurring in the period < 5 days were considered early. All these animals were kept in a cold chamber until necropsy.
Macroscopic evaluation
The animals that completed the observation period were anesthetized with xylazine (Rompum®) (Bayer, Brazil) and sulfuric ether and then underwent a median cervical thoracic laparotomy. The removal of the visceral block with the trachea and the lungs began with tongue traction and dissection of the muscles of the floor of the mouth and of the pharynx; the esophagus and the lungs were removed together. Next, euthanasia by exsanguinations was performed, with the sectioning of the abdominal aorta.
Macroscopic inspection of the trachea, lungs and esophagus and the register of anatomical parameters followed.
Each macroscopic variable was individually registered and the trachea was measured with a ruler and its diameter with a digital pachymeter.
The observed macroscopic variables and measurements are sees in Chart 1.
Documentation of the measures of each piece removed (tracheal block) followed (Figure 5).
All visceral pieces removed were fixed in 10% formalin solution. Macroscopic and histological evaluation included animals from both groups, submitted to the placement of polyurethane covered and non-covered stents, having survived the six weeks observation period. The animals that died during the experiment were submitted to necropsy and also had their trachea submitted to histological analysis following the same routine. The tracheas were sectioned by transversal cuts at the cricoid cartilage level and separated from the lungs by a transversal cut at the level of the carina
The degree of incorporation or adherence of the stent to the tracheal mucosa was determined by macroscopic evaluation: if the stent could be easily removed from the tracheal lumen, it was considered adhered and non-incorporated.
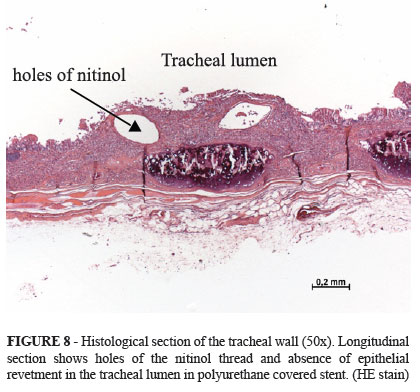
On the other hand, if it was firmly adhered and irremovable from the tracheal lumen, it was considered incorporated, partially or completely, depending on the microscopic evaluation of the epithelial revetment of the tracheal mucosa.
For the microscopic analysis of stent incorporation, the tracheal wall with the stent was opened longitudinally and the nitinol strands were removed one by one, with a pair of tweezers, without damaging the mucous revetment (Figure 6).
The trachea was embedded in paraffin blocks and 5 m histological sections were made at the segments with or without stent of the tracheal wall. The sections were dyed by the HE method and analyzed by a pathologist following the group occultation criterion.
The variables evaluated in the histological analysis are shown in Chart 2.
Statistical analysis
For the statistical analysis of comparison of the qualitative variables (degree of incorporation, granulation tissue score, dilatation score, evaluation of the inflammatory infiltrate and of the parietal involvement) between the two groups, Fisher's exact test was used. For the comparison of parietal revetment, the Likelihood ratio test was used. For the comparison of quantitative variables (stent diameter) between the two groups, the Mann-Whitney test was used.
Results
Thirty animals underwent the placement of a stent - 15 animals in the group of polyurethane covered models and 15 in the group of uncovered models.
Two animals were excluded on the day the stents were being placed - one of them due to death during anesthetic recovery and the other one due to a fault in the stent delivery system as the stent was stuck in the device.
Seven animals died due to early complications. Six animals died of respiratory failure due to pneumonia on the 1st, 2nd, 3rd, 4th and 5th days of the study; one animal died of septicemia because it presented a tracheal rupture and mediastinitis on the 5th day of the study. These seven animals underwent necropsy so that the causa mortis could be elucidated; their trachea was histologically evaluated. During necropsy, it could be observed that these animals had developed pneumonia because of misplacement of the stent and obstruction of the tracheal bifurcation.
Of the seven animals that died of early complications, three had received covered stents and four had received the non-covered model.
Therefore, 21 animals completed the observation period.
At the moment of euthanasia, three animals did not have the stent inside the trachea - one stent was in the esophagus middle third and two were not found.
The trachea of one of the animals was used for cut tests by microtomy with the nitinol strand.
Thus 13 animals were excluded; seventeen animals met the criteria for inclusion and underwent statistical analysis. Ten of them belonged to the covered model group and seven of them to the non-covered group.
Results of macroscopic and histological variables analysis of 17 animals
1) Incorporation and adherence
Incorporation / adherence score: 0 = totally incorporated, 1 = partially incorporated and 2 = adhered without incorporation.
Stent incorporation occurred in 100% of the animals that had received the non-covered model; adherence without incorporation occurred in the animals that had received the polyurethane covered model.
Total incorporation of the non-covered stent with the epithelial revetment can be observed in Figure 7.
Adherence without incorporation in covered stent is demonstrated in Figure 8.
2) Dilatation degree
Dilatation score: 0 = total dilatation and 1 = partial dilatation.
Dilatation, gauged by the measurement of the external diameter, was total in 100% of the non-covered stents cases and occurred in 71.4% of the polyurethane covered stents.
Partial dilatation was observed in 28.6 % of the covered stents, however there was no statistically significant difference between the two groups.
3) Granulation tissue
Granulation tissue score: 0 = absent, 1 = up 1 mm high into the tracheal lumen, 2 = from 1 mm to 2mm and 3 = greater than 2mm.
The presence and the height of the granulation tissue into the tracheal lumen, were measured at the stent extremity.
Granulation tissue ranging from 1mm to 2mm high was observed and it was present in 40% of the non-covered stents and in 42.9 % of the covered models. It was located at the proximal or distal border. The rates were very similar without significant differences in the groups.
4) Inflammation / cellularity
Inflammation / cellularity score: 0 = absent, 1 = prevalence of lymphomononuclear cells, 2 = prevalence of polymorphonuclear cells and 3 = mixed.
Concerning the inflammatory infiltrate, it was more intense in the group of animals with the polyurethane covered stent; however, there were no statistically significant differences (p = 0.603).
In relation to the infiltrate cellularity, it was predominantly lymphomononuclear in the non-covered stents; in the covered stents, the neutrophilic polymorphonuclear infiltrate predominated.
5) Parietal involvement
The extension of changings in the layers of the tracheal wall, named parietal involvement.
Parietal involvement score: 0 = superficial third (mucosa), 1 = superficial and middle third (mucosa and chorion) and 2 = total (all the layers of the involved wall).
It was present in both groups with moderate or total intensity. The total parietal involvement, extending to all the layers of the tracheal wall, was more frequent in the polyurethane covered stents (71.4%) than in the non-covered ones (28.6%). However, these differences were not statistically significant (p = 0.622).
6) Epithelial revetment
The type of histological changes observed in the respiratory epithelium.
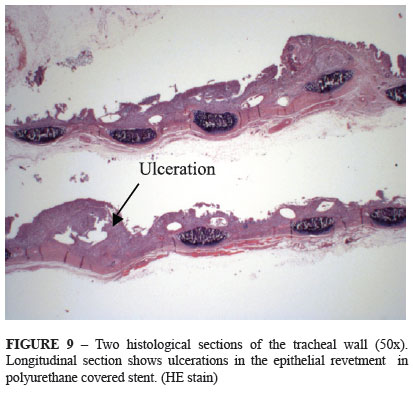
Epithelial revetment score: 0 = epithelial erosion, 1 = ulceration, 2 = regenerative hyperplasia and 3 = squamous metaplasia.
When stratifying the most frequent histological changes observed in the epithelial revetment, it was observed that the polyurethane covered stents presented a high ulceration rate (71.4 %) in relation to the non-covered models (20%) (Figure 9).On the other hand, the epithelial erosion rate was predominant in non-covered stents (60%) while the rate in the covered stents was 14.3%).
There was no significant difference between the two types of stents, but this difference had a marginal statistical significance (p = 0.078).
Discussion
The main outcome of this experimental study was to evaluate the biocompatibility of two models of polyurethane covered and non-covered self-expandable nitinol stents in rats' trachea so that it might be made available for clinical use in human tracheal stenosis.
It also resulted in the development of a new experimental model for the evaluation of tracheal stents in rats.
The majority of experimental studies for testing and development of tracheal stents have been carried out in animal models such as dogs, lambs, pigs, rabbits and cats due to the more adequate gauge and length of the trachea in these animals. However, nowadays many countries prohibit by laws the use of such animals for researches followed by euthanasia.
Experimental models of tracheal stenosis have been also performed by combined methods and reported in the recent literature.
In Brazil,Vaidergorn et al.2 developed a model of extensive and severe tracheal stenosis in dogs with the purpose to test surgical techniques of tracheal reconstructions. An elliptical area of 40% of the tracheal diameter was resected and the stumps were sutured. The authors concluded that the surgical proceeding was effective to promote a model of longitudinal and extensive tracheal stenosis.
Other techniques to establish an animal model of tracheal stenosis have been employed as cauterization of anterior tracheal rings using Nd-YAG laser3; combined bronchoscopic eletrocautery and ethanol injection applied to the trachea4 and tracheal cartilage ring injuries by radiofrequency5. All these animal models were developed in dogs and may be useful in the development of tracheal stenosis stenting
The use of rats as animal model for tracheal stenting are uncommon, perhaps due to the small size of the tracheal lumen observed in this animal.
Rats were chosen to be the animal model for this experiment because they are easily obtained and show resistance and tolerance to endoscopic procedures in the respiratory tract, in spite of the small diameter of their airways. In the present study, ten-month-old animals were chosen because, at this age, Wistar breed weigths 400g on average; average tracheal external diameter is 4.5mm and trachea is 50mm long on average. This choice was adequate because the diameter of the tracheal lumen was compatible with the diameter of the expanding stent.
The two groups were analyzed separately because the aim of the study is to compare the variables observed and the impact on biocompatibility.
Lochbihler et al.6, first reported an experimental study of compatibility of stents for tracheal stabilization in wistar rats. This study demonstrated erosive mucosal injuries in the trachea, as well as distinct polyps of granulation tissue, focal metaplasia of the epithelium and inflammatory infiltrates of the lamina propria.
In a recent study employing a similar model of stent in rabbits' trachea, Faria et al.7 observed that these animals showed low resistance and little tolerance to the aggressions caused by the stents, despite the fact that their airways is larger, longer and has a greater diameter. Exclusion rate in the Faria's experiment was 48% due to early complications.
This study showed a degree of anatomical and histological incorporation in 100% of the animals in the group that received non-covered stents and 100% adherence, without incorporation, in the group with polyurethane-covered stents (p < 0.001). Faria et al.7 also observed that in the polyurethane-covered stents, there was 84% of adherence and only 16% of incorporation to rabbits' trachea in an observation period of 26 days.
Shin et al.8 developed a pilot study in a rat tracheal model to investigate stent-induced granulation tissue. The results showed in the excised specimens tissue hyperplasia through the mesh and all of the stents had become incorporated into the wall of the trachea.They also observed that granulation tissue area, degree of inflammatory cell infiltration were higher in the stenting group.
Tsakayannis et al.9 reported the use of expandable metallic stents for acute tracheal stenosis in growing lamb. With placement of expandable metallic stents, lambs remained symptom-free and gained weight during a two-month follow-up period. Pathological evaluation showed more pronounced granulation tissue in the stented animals when compared with the control group. Authors concluded that expandable metallic stents provide an effective tool in the management of acute stenosis.
Dilatation degree, gauged by the measurement of the external diameter, was total in 100% of the non-covered stents cases and in 71.4% of the covered stents, but values were very close to what was expected, varying no more than one millimeter in the cases with lesser dilatation. This small difference in the degree of dilatation may be attributed to the variation of radial force of the stents covered with a polyurethane thin membrane; or it might be the result of tracheal compliance. In rabbits receiving polyurethane-covered self-expanding nitinol stents, Faria et al.7 observed that dilatation was total in only 54% of the cases.
Regarding granulation tissue, our results were similar to those of Shin et al.8 and Faria et al.7, in which the presence of granulation tissue was identified in 100% and 77% of the cases respectively. In this study, granulation tissue about 2mm high was observed and it did not exceed much. It was present in 40% of the non-covered stents and in 42.9% of the covered ones and located at the proximal or distal border of the stents. These values were similar and without statistical significance between the two groups.
Fraga et al.10 reported an experimental trial of expandable metallic Palmaz stent in the trachea of cats. Stents were placed bronchoscopically in the thoracic trachea of 50 cats.The results of histologic examination indicated a mild inflammatory reaction with granulation tissue in all animals with stents, but in animals with overexpanded stents, the reaction was more severe,with epithelial ulceration, fibrosis and sealed-off perforations in most animals. Ruegemer et al.11 also reported the effect of the same Palmaz expandable metallic stent in the trachea of pigs. Pigs underwent endoscopic midtracheal placement of the expandable Palmaz metallic stent. Tracheal inflammation was evaluated by objective histopathogic criteria. Stent incorporation was evident with significant mucosal ingrowth. Inflammation in the form of non-obstructing granulation tissue was present and no evidence of necrosis or cartilage invasion was evident. Authors concluded that piglet trachea appears to be an excellent model for evaluation of expandable metallic airway stents.
In our study, the frequency of inflammatory infiltrate was intense in the two groups compared. In relation to infiltrate cellularity, it was predominantly lymphomononuclear in the non-covered stents; in the covered models, the neutrophilic polymorphonuclear infiltrate was predominant. It is probable that the presence of polyurethane stimulates an inflammatory reaction of the polymorphonuclear type.
Faria et al.7 study of rabbits' trachea showed the prevalence of acute inflammatory reaction in the segment with the polyurethane-covered stent and less intense inflammatory reaction in the tracheal segments without stent.
In Brazil, Xavier et al.12 developed a modified Dumon stent for tracheal application in an experimental study in dogs. The histopathologic results revealed a well-preserved epithelial basal membrane and mild submucosal inflammatory infiltrate with scattered granulation tissue.The modified silicone stent developed proved to be resistant to mechanical stress and biocompatible in the canine trachea.
In the present study, parietal revetment changes were observed in both groups with stents. They varied from epithelial erosion, deeper ulcerations and regenerative hyperplasia to squamous metaplasia.
Polyurethane-covered stents presented high ulceration rate (71.4%) in relation to the non-covered models (20%). On the other hand, epithelial erosion rate was predominant in the non- covered stents (60%) while the rate was 14.3% in the covered stents. Probably, besides sealing the gaps in the nitinol mesh, the coating of the stent with the polyurethane thin membrane also works as a protection carpet of the mucous epithelium. However, the polyurethane does not prevent ulcerations in the deepest part of the chorion. Faria et al.7 also observed the presence of ulceration in the epithelial revetment of rabbits' trachea in 42% in cases that only polyurethane-covered stents were used.
Liu et al.13 tested a new mesh-type bioresorbable stent and evaluated its safety and biocompatibility in a rabbit trachea model. No animals died during the period of study, but stent migration was noted and secretion accumulation was found. Histological examination showed marked leukocyte infiltration in the submucosa of the stented area. Recently other study was undertaken to evaluate safety and biocompatibility of a novel biodegradable polydioxanone stent in rabbit tracheal model. Macroscopic examination revealed that the tracheal lumen stayed open, however histologic examination showed that tracheal damage score was highest five weeks after stenting, including in-stent necrosis of the epithelium14.
The animal experimental model that was developed and improved was suitable for tracheal stent evaluation and it should be used for this line of research in the future.
Conclusion
The two stent models studied showed biocompatibility in Wistar rats' trachea.
Acknowledgments
The authors are most grateful to Doctor Domingo M. Braile-MD,PhD from Braile Biomedica for his continuous support supplying the tracheal stents.
We thank Mrs. Beatriz Grion for correcting the English version.
Received: August 23, 2012
Review: October 24, 2012
Accepted: November 21, 2012
onflict of interest: none
Financial source: none
- 1. Terra RM, Minamoto H, Jatene FB. Dispositivos intra-traqueais: próteses ou órteses? J Bras Pneumol. 2006;32(6):606-7.
- 2. Vaidergorn J, Fagundes DJ, Machado AL, Ferreira RG, Juliano Y, Novo NF, Gomes PO. Model of extensive and severe tracheal stenosis in dogs. Acta Cir Bras. 2008;23(6):497-500.
- 3. Jung Kwon O, Young Suh G, Pyo Chung M, Kim J, Han J, Kim H. Tracheal stenosis depends on the extent of cartilaginous injury in experimental canine model. Exp Lung Res. 2003;29(6):329-38.
- 4. Lee SS, Shin JH, Woo CW, Hwang JC, Park CS, Kim HJ, Kim EY, Kim TH, Song HY. A new model of tracheal stenosis in dogs using combined bronchoscopic electrocautery and ethanol injection. J Vasc Interv Radiol. 2008;19(5):764-9.
- 5. Chen Z, Luo J, Xu L, Ma R, Zhang N, Cui P. A model of canine tracheal stenosis induced by radiofrequency cauterization. Int J Pediatr Otorhinolaringol. 2012;76(2):183-8.
- 6. Lochbihler H, Hoelzl J, Dietz HG. Tissue compatibility and biodegradation of new absorbable stents for tracheal stabilization: an experimental study. J Pediatr Surg. 1997;32(5):717-20.
- 7. Faria CM, Rodrigues OR, Minamoto H, Cury PM, Costa Neto JM, Braile DM. A new model of a self-expanding tracheal stent made in Brazil: an experimental study in rabbits. J Bras Pneumol. 2012;38(2):214-7.
- 8. Shin JH, Sung KB, Kim EY, Shin DH, Song HY. A rat tracheal model to investigate stent-induced tissue hyperplasia: a pilot study. J Vasc Interv Radiol. 2010;21(12):1878-83.
- 9. Tsakayannis DE, Siddiqui AM, Kozakewich H, Nobuhara KK, Ibla JC, Perry SD, Lillehei CW. The use of expandable metallic stents for acute tracheal stenosis in the growing lamb. J Pediatr Surg. 1998;33(7):1038-41.
- 10. Fraga JC, Filler RM, Forte V, Bahoric A, Smith C. Experimental trial of balloon-expandable,metallic Palmaz stent in the trachea. Arch Otolaryngol Head Neck Surg. 1997;123(5):522-8.
- 11. Ruegemer JL, Perkins JA, Azarow KS, O'Bryant LK, Nielsen RE, Thomas RW. Effect of the Palmaz balloon-expandable metallic stent in the trachea of pigs. Otolaryngol Head Neck Surg. 1999;121(1):92-7.
- 12. Xavier RG, Sanches PR, Macedo Neto AV, Kuhl G, Vearick SB, Michelon MD. Development of a modified Dumon stent for tracheal applications: an experimental study in dogs. J Bras Pneumol. 2008;34(1):21-6.
- 13. Liu KS, Liu YH, Peng YJ, Liu SJ. Experimental absorbable stent permits airway remodeling. J Thorac Cardiovasc Surg. 2011;141(2):463-8.
- 14. Novotny L, Crha M, Rauser P, Hep A, Misik J, Necas A, Vondrys D. Novel biodegradable polydioxanone stents in a rabbit airway model. J Thorac Cardiovasc Surg. 2012;143(2):437-44.
Publication Dates
-
Publication in this collection
18 Jan 2013 -
Date of issue
Jan 2013
History
-
Received
23 Aug 2012 -
Accepted
21 Nov 2012 -
Reviewed
24 Oct 2012