Abstract
PURPOSE: To compare three sterilization methods (autoclave, gamma irradiation and ethylene oxide) over non demineralized lyophilized bone allografts. METHODS: Bone allografts were implanted on paravertebral muscles of 21 rats. After 30 days animals were sacrificed and grafts underwent comparative analysis regarding histomorphometric and macroscopic parameters. RESULTS: Allografts that underwent the three sterilization methods presents similar weight gain, cortical thickness similar to control group, and less fibrosis than the control group. Grafts that underwent sterilization in autoclave presented less presence of multinucleated giant cells, although not statistically significant. There was also no statistically significant difference regarding mineralization on the three groups. CONCLUSION: The three sterilization methods cause similar effects on bone allografts regarding macroscopic and histomorphometric parameters.
Bone and Bones; Transplantation, Homologous; Osteogenesis; Sterilization; Rats
11 - ORIGINAL ARTICLE
MATERIALS TESTING
Comparative study and histomorphometric analysis of bone allografts lyophilized and sterilized by autoclaving, gamma irradiation and ethylene oxide in rats1 1 Research performed at Microsurgery Investigation and Plastic Surgery Laboratory, University of Sao Paulo (USP), Brazil.
Otavio Machado de AlmeidaI; Wanda JorgettiII; Denis OksmanIII; Camilo JorgettiIV; Diógenes Laércio RochaV; Rolf GemperliVI
IMaster, PhD in Oncology, Antonio Prudente Foundation, Sao Paulo-SP, Brazil. Concept and design of the study, acquisition and interpretation of data, supervised all phases of the study, manuscript writing
IIAssociate Professor, Division of Nephrology, USP, Sao Paulo-SP, Brazil. Histopathological examinations
IIIAttending Physician, Hospital Nove de Julho, Sao Paulo-SP, Brazil. Acquisition and interpretation of data, supervised all phases of the study, manuscript writing
IVAttending Physician, Clinics Hospital, USP, Sao Paulo-SP, Brazil. Histopathological examinations
VPhD, Assistant Professor, Division of Plastic Surgery, USP, Sao Paulo-SP, Brazil. Scientific and intellectual content of the study, critical revision
VIPhD, Associate Professor, Division of Plastic Surgery, USP, Sao Paulo-SP, Brazil. Scientific and intellectual content of the study, critical revision
Correspondence
ABSTRACT
PURPOSE: To compare three sterilization methods (autoclave, gamma irradiation and ethylene oxide) over non demineralized lyophilized bone allografts.
METHODS: Bone allografts were implanted on paravertebral muscles of 21 rats. After 30 days animals were sacrificed and grafts underwent comparative analysis regarding histomorphometric and macroscopic parameters.
RESULTS: Allografts that underwent the three sterilization methods presents similar weight gain, cortical thickness similar to control group, and less fibrosis than the control group. Grafts that underwent sterilization in autoclave presented less presence of multinucleated giant cells, although not statistically significant. There was also no statistically significant difference regarding mineralization on the three groups.
CONCLUSION: The three sterilization methods cause similar effects on bone allografts regarding macroscopic and histomorphometric parameters.
Key words: Bone and Bones. Transplantation, Homologous. Osteogenesis. Sterilization. Rats.
Introduction
The difficulties in surgical repair of craniofacial deformities require that new techniques be developed to achieve better functional and esthetic results. Notwithstanding the surgical techniques developed in the sixties1, new procedures for bone grafting are necessary, because of their importance in the restoration of the craniofacial bone structure.
The enhanced knowledge of physiological factors related to the integration of bone grafts has allowed the use of various types of grafts with increasingly consistent results.
The use of bone allografts (from another individual of the same species), not only saves surgery time because of the availability in bone banks, but also decreases the procedure's morbidity. This type of bone graft promotes osteogenesis due to its osteoinductive potential requiring preparation to reduce the antigenic load and the risk for transmission of infectious diseases2-5.
To date there is no consensus regarding the ideal sterilization to ensure preservation of the allograft potential of osteoinduction and ability for integration5-10. The main methods of sterilization of biological materials used today are autoclave, gamma irradiation and ethylene oxide. The most suitable method would seem to be the one that causes less damage to the osteoinductive property.
Thus, the purpose of this experimental work is to compare the effect of these three sterilization methods on lyophilized bone grafts using macroscopic parameters and histomorphometric measurements for the assessment of osteogenesis.
Methods
In the study 42 male, isogenic, adult Wistar rats were used. Initially, 21 rats were sacrificed and within three hours the 42 tibiae were extracted from these animals, and the soft tissue and periosteum were removed. Then bones were severed crosswise in the middle section so as to obtain the proximal half of about 25mm, frozen and then lyophilized for 24 hours and weighed on a precision balance before and after this procedure.
The segments were then divided into two groups. The first, called Group 1 (control) was subdivided into three subgroups:
Subgroup 1a (control of subgroup 2a);
Subgroup 1i (control of subgroup 2i);
Subgroup 1o (control of subgroup 2o).
The second group, called Group 2 was subdivided into three subgroups according to the sterilization method
Subgroup 2a - sterilization method was autoclave (128ºC for 40 minutes);
Subgroup 2i - sterilization method was gamma irradiation (Cobalt -60 to 2.5 mRads);
Subgroup 2o - sterilization method was ethylene oxide (for 6 hours).
After undergoing the three different methods of sterilization the bone segments were hydrated in saline solution and then implanted in the left paravertebral muscles at level of the lumbar region of the 21 recipient rats, and on the right side were implanted the segments of the corresponding group 1 (control).
All animals received intraperitoneal tetracycline (Oxytetracycline Pfizer) 20mg/kg on the 2nd and 28th days of the experiment. The grafts were removed after 30 days, and again weighed on a precision balance and sent for histological analysis. All slides were treated alike and were analyzed under a magnification of 250 times with the same microscope, by the same observer, without identification of the origin. The parameters studied were trabecular volume, fibrosis, cortical thickness, presence of multinucleated giant cells and mineralization.
Measurements were performed on 15 fields in the area of the proximal epiphysis of the tibiae, immediately distal to the epiphyseal growth plate and equidistant from the two cortices.
All data were statistically analyzed. For the variable weight the Kruskal-Wallis test was used. As for the quantitative variables (trabecular volume, cortical thickness and fibrosis) and the semi quantitative (presence of multinucleated giant cells) minimum and maximum values were identified, the median, mean and standard deviation were calculated for each of the subgroups under study. In the comparative analysis of the three types of sterilization utilized, with respect to these variables, the following formula was used to calculate the percentage variation (PV):
The values obtained were submitted to the same statistical test. For analysis of the variable mineralization, contingency tables were employed and the Fischer's t test was used to compare the three types of sterilization.
Results
Macroscopic (weight) and histomorphometric analysis of the segments studied showed the following results:
Comparing the three sterilization methods, regarding the weight of bone allografts, it was found that all segments showed an increase in weight after grafting, regardless of the sterilization method used (Table 1). This increase was found to be similar, with no statistically significant difference between subgroups.
Analysis of the variable trabecular volume, disclosed that sterilized allografts showed higher median values than their controls. The sterilized grafts submitted to gamma irradiation had a lower percentage change in this index than those submitted to the autoclave and ethylene oxide. This difference also was not statistically significant (Table 2).
In the analysis of variable fibrosis, the sterilized allografts showed lesser quantity of fibrosis than their controls, explaining the negative values of percentage variation. Comparing the three sterilization methods, it was found that the largest percentage variation in the median occurred in allografts submitted to the autoclave, but differences were not statistically significant (Table 3).
With regard to cortical thickness, this showed practically no change when the three sterilization methods were compared with the control group, thereby explaining the median percentage variation values around zero (Table 4).
Regarding the presence of multinucleated giant cells, allografts of autoclave presented the lowest values among sterilized allografts, but comparing the percentage change with the controls there was no statistically significant difference (Table 5).
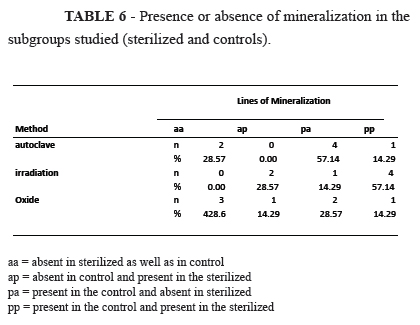
Regarding the variable mineralization, the presence or absence of tetracycline marking was studied, mineralization was found to be more damaged in the subgroup of allografts from the autoclave (Table 6).
The test comparing the three groups regarding the proportion of results that concerning damage to mineralization did not identify a statistically significant difference between the groups (Table 7).
Discussion
Bone is a specialized connective tissue which, besides being the support structure for all other tissues, provides protection for vital organs and bone marrow. It consists of three types of cells (osteoblasts, osteocytes and osteoclasts) and an intercellular material called bone matrix.
The bone matrix is comprised of an inorganic and an organic portion produced by osteoblasts, prior to their developing into osteocytes, the mature cells surrounded by mineralized tissue. Osteoclasts are responsible for bone resorption. Bone is a live and dynamic structure, which is constantly being remodeled.
At this time when organ and tissue transplants have become routine procedures, the bone allografts also began to be employed on a large scale, and currently after blood and skin, the bone is the live human tissue most often transplanted11. This encouraged the development of several studies in an effort to understand what takes place with this structure when it is transplanted and what is the best type of bone graft to be used8,9.
There is consensus regarding two methods for conservation of bone allografts12. Lyophilization, and freezing at - 80ºC12, that both bring about destruction of the bone live cells and reduce their antigenic load.
The three sterilization methods compared here are those most widely used for non-biological and biological materials. Although, all have proven effective for this purpose5-8, however they do cause damage to biological materials, and due to the complexity and variety of factors there is no consensus on which is the best method.
Variations in measurements of weight and volume of the grafts as well as histological studies were possible only through experimental work. Using mathematical equations, the histomorphometric study allows the calculations of volume and surface, by means of information obtained from plane specimens of bone tissue. This enables quantitative evaluation of bone structures and provides data on the mechanisms involved in bone formation and resorption. This method further allows visualizing mineralization zones highlighted by tetracycline deposits providing information about the time related to bone formation and mineralization. For macroscopic examination of the grafts, weight was the only parameter studied.
At the initial stage of integration all bone grafts presented resorption2. Allografts have a longer initial inflammatory response, causing delay in vascularization, with a consequent period of increased resorption and retarded onset of osteogenesis. The weight increase found in these groups is probably due to rehydration in the lyophilized bone segments as well as to invasion by blood vessels and significant presence of an inflammatory process.
If the variable trabecular volume provides the volume occupied by the bone locks, higher values obtained therefore mean lesser resorption. Although allografts submitted to gamma irradiation have a lower percentage variation in relation to the median when compared to allografts submitted to the other two sterilization methods, this difference was not significant.
As for fibrosis, the lower indices found in sterilized allografts in relation to their controls indicates that less cellular activity was present, which also leads to lower bone resorption. Allografts that underwent irradiation displayed higher levels of fibrosis, that even if not significant when compared with other methods explain the lesser weight gain and lower trabecular volume of this subgroup.
Glowacki13 demonstrated that multinucleated giant cells have characteristics similar to those of osteoclasts. Sterilization by autoclave and ethylene oxide induced less formation of these cells than in controls and consequently, lower resorption.
The small variation of the cortical thickness of allograft shows that during the study period of thirty days, the integration process occurs in the cancellous bone and not in the cortical.
The irradiation method caused the least damage to mineralization, yet results were not statistically significant. Mineralization detected in the sterilized allografts confirms the presence of bone neoformation.
Since the allografts under study relied only on their osteoinductive potential for osteogenesis, the 30-day period was considered to be too short because of the retarded osteoinduction. This is described by Chalmers3, who stresses that freeze-dried bone allografts undergo bone neoformation at a later stage, usually after a month.
When analyzing results, no significant differences were found between the sterilization methods, suggesting that they might have caused similar effects to the freeze-dried bone allografts, and therefore justify the variety of options available regarding the sterilization methods. Munting14 in his study reached a similar conclusion.
The results confirmed the findings of several authors about the possibility of clinical use of freeze-dried sterilized bone allografts5,7,9,12,15. Physical changes caused by these processes must be better studied to define other more adequate parameters for the choice of the sterilization method to be utilized 6.
Conclusion
The three sterilization methods studied caused similar effects on bone allografts regarding macroscopic and histomorphometric parameters analyzed.
Correspondece:
Otávio Machado de Almeida
Rua Barata Ribeiro, 490/5º andar
01308-000 São Paulo -SP Brasil
Tel.: (55 11)3255-7599
Received: August 14, 2012
Review: October 15, 2012
Accepted: November 19, 2012
Conflict of interest: none
Financial source: none
- 1. Tessier P. Osteotomies totals de la face. Syndrome de Crouzon, syndrome d'Apert, oxycephaly, scaphocephalies, turricephalies. Ann Chir Plast. 1967;12:273-86.
- 2. Burchardt H. Biology of bone transplantation. Orthop Clin North Am. 1987;18:187-96.
- 3. Chalmers J. Transplantation immunity in bone homografting. J Bone Joint Surg Br. 1959;41:160-79.
- 4. Komender J, Malczewska H, Komendr A. Therapeutic effects of transplantation of lyophilized and radiation-sterilized, allogenic bone. Clin Ortop Relat Res. 1991;272:38-49.
- 5. Katz J. The effects of various cleaning and sterilization processes on allograft bone incorporation. J Long Term Eff Med Implants. 2010;20(4):271-6.
- 6. Zhou Z, Qin T, Yang J, Shen B, Kang P, Peil F. Mechanical strength of cortical allografts with gama radiation versus ethylene oxide sterilization. Acta Orthop Belg. 2011;77(5):670-5.
- 7. Azar FM. Tissue processing: role of secondary sterilization techniques. Clin Sports Med. 2009;28(2):191-201.
- 8. Endres S, Kratz M. Gama irradiation. An effective procedure for bone banks, but does it make sense from an osteobiological perspective? Musculoskelet Neuronal Interact. 2009;9(1):25-35.
- 9. Kim S, Jeon C Kong DS, Park K, Kim JH. Clinical efficacy of radiation-sterilized allografts for sellar reconstruction after transsphenoidal surgery. J Korean Neurosurg Soc. 2011;50(6):503-6.
- 10. Eastlund T. Bacterial infection transmitted by human tissue allograft transplantation. Cell Tissue Bank. 2006;7(3):147-66.
- 11. Hamson KR, Toth JM, Stiehl JB, Lynch KL. Preliminary experience with a novel model assessing in vivo mechanical strength of bone grafts and substitute materials. Calcif Tissue Int. 1995;57:64-8.
- 12. Calvo R, Fiqueroa D, Diaz-Ledezma C, Vaisman A, Figueroa F. Bone allografts and the functions of bone banks. Rev Med Chil. 2011;139(5):660-6.
- 13. Glowacki J, Cox KA. Osteoclastic features of cells that resorb bone implants in rats. Calcif Tissue Int. 1986;39:97-103.
- 14. Munting, E, Wilmart JF, Wijne A, Hennebert P, Delloye C. Effect of sterilization on osteoinduction. Acta Ortop Scand. 1988;59:34-8.
- 15. Zasacki W. The efficacy of application of lyophilized, radiation-sterilized bone-graft in orthopedic surgery. Clin Orthop Relat Res. 1991;72:778-85.
Publication Dates
-
Publication in this collection
18 Jan 2013 -
Date of issue
Jan 2013
History
-
Received
14 Aug 2012 -
Accepted
19 Nov 2012 -
Reviewed
15 Oct 2012