Abstract
PURPOSE: To study the effect of isoflurane (Iso) or propofol (Prop) anesthesia on renal ischemia/reperfusion injury (IRI) during transient hyperglycemia. METHODS: Thirty six rats were randomly assigned into six groups of six animals each: PHS (Sham-Prop=1mg.kg-1.min-1 + Hyperglycemia=2.5g.kg-1 of glucose solution administered intraperitoneally); HIS (Sham-Iso + Hyperglycemia); PHI (Prop + Hyperglycemia + Ischemia); IHI (Iso + Hyperglycemia + Ischemia); PI (Prop + Ischemia), and II (Iso + Ischemia). After 30 minutes of anesthesia induction, right nephrectomy was performed (all animals) and the left renal artery was clamped during 25 minutes (ischemia). The animals were sacrificed after 24 hours and blood collection (to dose creatinine) and left kidney removal were performed for histological analysis, and flow cytometry (FCM): percentage of initial apoptosis (APTi) and viable cells (VC). RESULTS: Serum creatinine (mg/dL) was statistically different in groups PHI (3.60±0.40) and IHI (3.23±1.08), p<0.05. Histological analysis was statistically different in groups PHI (4.0[4.0;5.0]) and IHI (4.5[4.0;5.0]), p<0.05. APTi percentage was statistically different in groups PHI (73.2±7.1), and IHI (48.1±14). VC percentage was statistically different in groups PHI (25.8±6.9) and IHI (38.5±9.2), p<0.05. CONCLUSIONS: Propofol and isoflurane showed the same level of protection against ischemia/reperfusion injury in the normoglycemic groups. Transient hyperglycemia is associated with an increase in IRI.
Kidney; Ischemia; Reperfusion; Hyperglycemia; Propofol; Isoflurane; Rats
1 ORIGINAL ARTICLE
ISCHEMIA-REPERFUSION
Does propofol and isoflurane protect the kidney against ischemia/reperfusion injury during transient hyperglycemia?1 1 Research performed at Experimental Laboratory of Anesthesiology, Botucatu School of Medicine (BSM), Sao Paulo State University (UNESP), Brazil. Part of PhD thesis, Postgraduate Program in Anesthesiology. Tutor: Pedro Thadeu Galvão Vianna.
Antônio Roberto CarrarettoI; Pedro Thadeu Galvão Vianna FilhoII; Yara Marcondes Machado CastigliaIII; Marjorie de Assis GolimIV; Aparecida Vitória Gonçalves de SouzaV; Lídia Raquel de CarvalhoVI; Elenice DeffuneVII; Pedro Thadeu Galvão ViannaVIII
IFellow PhD degree, Postgraduate Program in Anesthesiology, UNESP, Botucatu-SP, Brazil. Main author. Conception, design, intellectual and scientific content of the study
IITrainee, Experimental Laboratory, Department of Anesthesiology, UNESP, Botucatu-SP, Brazil. Acquisition of data
IIIFull Professor, Department of Anesthesiology, UNESP, Botucatu-SP, Brazil. Critical revision
IVBiologist, Department of Medicine, UNESP, Botucatu-SP, Brazil. Acquisition of data
VFellow PhD degree, Postgraduate Program in Anesthesiology, UNESP, Botucatu-SP, Brazil. Acquisition of data
VIAssistant Professor, Department of Statistics, UNESP, Botucatu-SP, Brazil. Statistical analysis
VIIAssistant Professor, Department of Medicine, UNESP, Botucatu-SP, Brazil. Acquisition of data
VIIIFull Professor, Department of Anesthesiology, UNESP, Botucatu-SP, Brazil. Tutor. Supervised all phases of the study, manuscript writing
Correspondence Correspondence: Pedro Thadeu Galvão Vianna Faculdade de Medicina de Botucatu, Departamento de Anestesiologia-UNESP Distrito Rubião Júnior Caixa Postal 530 18618-970 Botucatu - SP Brasil Tel.: (55 14)3811-6222 ptgv@uol.com.br
ABSTRACT
PURPOSE: To study the effect of isoflurane (Iso) or propofol (Prop) anesthesia on renal ischemia/reperfusion injury (IRI) during transient hyperglycemia.
METHODS: Thirty six rats were randomly assigned into six groups of six animals each: PHS (Sham-Prop=1mg.kg-1.min-1 + Hyperglycemia=2.5g.kg-1 of glucose solution administered intraperitoneally); HIS (Sham-Iso + Hyperglycemia); PHI (Prop + Hyperglycemia + Ischemia); IHI (Iso + Hyperglycemia + Ischemia); PI (Prop + Ischemia), and II (Iso + Ischemia). After 30 minutes of anesthesia induction, right nephrectomy was performed (all animals) and the left renal artery was clamped during 25 minutes (ischemia). The animals were sacrificed after 24 hours and blood collection (to dose creatinine) and left kidney removal were performed for histological analysis, and flow cytometry (FCM): percentage of initial apoptosis (APTi) and viable cells (VC).
RESULTS: Serum creatinine (mg/dL) was statistically different in groups PHI (3.60±0.40) and IHI (3.23±1.08), p<0.05. Histological analysis was statistically different in groups PHI (4.0[4.0;5.0]) and IHI (4.5[4.0;5.0]), p<0.05. APTi percentage was statistically different in groups PHI (73.2±7.1), and IHI (48.1±14). VC percentage was statistically different in groups PHI (25.8±6.9) and IHI (38.5±9.2), p<0.05.
CONCLUSIONS: Propofol and isoflurane showed the same level of protection against ischemia/reperfusion injury in the normoglycemic groups. Transient hyperglycemia is associated with an increase in IRI.
Key words: Kidney. Ischemia. Reperfusion. Hyperglycemia. Propofol. Isoflurane. Rats.
Introduction
Ischemia reperfusion injury (IRI) refers to the tissue damage caused by return of blood supply (reperfusion) to tissue after a period of ischemia. Ischemic kidney injury is associated with high morbidity and mortality. Improving the ability of this organ to tolerate ischemic injury would have important implications because ischemic insults are often recurrent in patients1.
Many anesthetics have been shown to be protective against IRI2,3. Knowledge on their potential benefits allows physicians to choose the best anesthetic or combination of anesthetics for their patients. It has been demonstrated that halogenated anesthetics, opioids, and propofol can protect against myocardial IRI4. According to a previous study, isoflurane displays renal protective effects3.
Propofol exerts a number of non-anesthetic effects such as antioxidant, immunomodulatory, analgesic, antiemetic, and neuroprotective effects5. Propofol has been reported to protect cells against apoptosis induced by ischemia/reperfusion (I/R), and that this protection was probably due to a preconditioning effect being, at least in part, mediated by KATP channels6. In addition, propofol attenuated IRI on LLC-PK1 cells when present either one or 24h before initiated I/R, and also during the recovery period, but not when it was added only during ischemia. The early exposure and response of allograft tissue to hyperglycemia may increase the risk of rejection. Acute hyperglycemia may enhance renal ischemic injury, antigen presentation, apoptosis, and increased inflammatory responses in renal transplantation7. Several experimental studies have documented that renal ischemic injury in diabetic animals is worse than similar renal ischemic injury in normoglycemic animals. This increased renal injury seems to be due to exaggerated oxidative stress and to increased inflammatory responses in the kidneys of hyperglycemic animals. However, these data have been obtained using experimental animals with absolute insulin deficiency due to beta-cell destruction8,9. Therefore, it is unclear whether acute hyperglycemia in nondiabetic animals would be equally injurious to renal function10.
In a previous study, hyperglycemia that occurred during renal ischemia-reperfusion resulted in severe functional injury compared with normoglycemia or with hyperglycemia that occurred after reperfusion. Furthermore, the investigated molecular pathways were more profoundly affected by hyperglycemia that occurred before renal ischemia-reperfusion10.
To the best of our knowledge, no study on the protective effect of propofol against renal IRI in the presence of transient hyperglycemia has been conducted. Thus, the purpose of this study was to evaluate the renal protective effects of isoflurane or propofol in the presence of acute hyperglycemia.
Methods
This study was approved by the Institution's Ethics Committee in Animal Research. Thirty six male Wistar rats (250-320g body weight) were used in all experiments. The animals were anesthetized inside an acrylic compartment with an isoflurane/air/oxygen mixture. The trachea was intubated and the lungs were mechanically ventilated (Harvard Rodent Ventilator 683; South Natick, MA, USA) while respiratory rate, end-tidal CO2, and end-tidal isoflurane were monitored. A cervical anterior midline approach was used to cannulate the right jugular vein for the infusion of liquids and drugs. The same method was used to insert a catheter connected to a transducer and recorder (Datex Engstron, Finland) into the left carotid artery for monitoring mean arterial pressure, and to collect arterial blood samples. Rectal temperature was monitored and kept between 37°C and 38°C with the aid of a hot jelly blanket.
The animals were randomly allocated (using randomizing computer software) into six groups, six animals each, to receive anesthesia with isoflurane or propofol with or without hyperglycemia. Group assignments and procedures are summarized in Table 1.
Isoflurane groups (HIS, IHI, and II) were maintained with an end-tidal isoflurane concentration of 1.5%. Propofol groups (PHS, PHI, and PI) were maintained with a propofol infusion of 1mg.kg-1.min-1, with an infusion pump.
Hyperglycemia was induced with an intraperitoneal injection of 2.5g.kg-1 of glucose. The normoglycemic groups received an intraperitoneal (ip) injection of the same amount of saline.
The animals in groups PHS (propofol, hyperglycemia = sham) and IHS (isoflurane, hyperglycemia = sham) were sham-operated (control) and underwent similar operative procedures without occlusion of the left renal artery.
Blood samples for the measurement of serum creatinine and glucose levels were collected after dissection of the left carotid artery, at baseline, before IRI, after IRI, and 24 hours after the end of the experiment.
Both kidneys were exposed through a midline abdomen incision and the right kidney was removed. Following treatment with isoflurane or propofol for 30 minutes, and the mobilization of the left kidney, the renal artery and vein were carefully dissected. Left renal ischemia was induced by placing an atraumatic microvascular clamp on both the renal artery and vein (ischemia). After 25 min of ischemia, the clamp was removed, and reperfusion was confirmed by watching the return of blood flow. At the end of this part of the experiment, the abdominal incision was closed and a blood sample was collected. The animals were then kept in plastic cages and fed standard rat chow and water ad libitum. Twenty-four hours after operation, the animals were anesthetized with isoflurane, blood samples were collected, left kidneys were removed for further analysis and the animals were sacrificed.
The histological analysis of hematoxylin-eosinstained kidney sections was performed by an examiner blinded to the treatment used. The severity of renal tubular damage was graded on a scale from 0 to 5 as follows: grade 0 = no damage, grade 1 (mild damage) = less than 10 % tubular necrosis, grade 2 (mild to moderate damage) = 10 to 25% tubular cell necrosis, grade 3 (moderate to severe damage) = 25 to 50% tubular cell necrosis, grade 4 (severe damage) = 50 to 75%, and grade 5 (severe to very severe) = greater than 75% tubular cell necrosis11. In addition, cells from the left kidney were evaluated for apoptosis by flow cytometry (FCM) as a percentage (%) of initial apoptosis (APTi) and viable cells (VC). Annexin V-FITC was used to determine the percentage of cells undergoing apoptosis, which show translocation of membrane phospholipid phosphatidylserine (PS) from the inner leaflet to the outer leaflet of the plasma membrane, thereby exposing PS to the external environment. Annexin V is a Ca2+ - dependent phospholipid-binding protein that has a high affinity for PS. Propidium iodide (PI) was used to distinguish viable from nonviable cells as the former have intact membranes that exclude PI, whereas membranes of dead or damaged cells are permeable to PI.
Data were expressed as mean ± SEM. Differences among groups were evaluated by analysis of variance and the Kruskal-Wallis test. Non-paired Student t test was used to analyze each variable. Differences were considered significant when p<0.05.
Results
Mean arterial pressure showed no statistically significant difference among groups.
Blood glucose levels (mg/dL) after IRI were greater in PHS (351.8±70.5), IHS (348.3±26.2), IHS (302.5±40.0), and PHI (308.5±37.2) than in groups PI (147.0±31.2), and II (162.7±20.1), p<0.05.
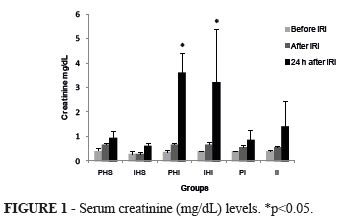
Serum creatinine (mg/dL) levels were similar among groups before and after IRI, but statistically different 24 hours after the experiment in groups PHI (3.60±0.40), and IHI (3.23±1.08) when compared with the other groups: PHS (0.95±0.14), IHS (0.62±0.06), PI (0.88±0.19), and II (1.42±0.5), p<0.05 (Figure 1).
Renal histology analysis revealed that groups PHS (0.0[0.0;1.0]) and IHS (0[0.0;0.0]) statistically differed from groups PHI (4.0[4.0;5.0]), and IHI (4.5[4.0;5.0]). Groups PI (3.0[1.0;3.0]), and II (2.0[1.0;3.0]) showed intermediary values (Figure 2).
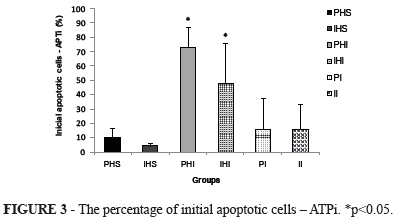
Flow citometry (FCM) showed that APTi (%) in groups PHS (9.9±3.3), IHS (4.1±1.0), PI (15.7±11), and II (15.8±8.6) significantly differed from groups PHI (73.2±7.1), and IHI (48.1±14), p<0.05 (Figure 3).
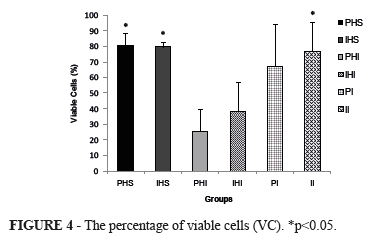
The percentage of viable cells (VC) was statistically higher in groups PHS (80.8±4), IHS (79.9±1.7), and II (77.0±9.2) when compared with groups PHI (25.8±6.9), and IHI (38.5±9.2), p<0.05. In the PI group (67.7±13.6), intermediary values were observed (Figure 4).
Discussion
Clinical studies have documented the adverse effects of chronic hyperglycemia on patient outcomes12,13. Hyperglycemia is viewed as the primary mediator of a cascade of heart damaging events, starting from reactive oxygen species (ROS) formation and leading to myocardial ischemia, inflammation and death of myocytes14. Hyperglycemia acutely increases circulating cytokine concentrations by an oxidative mechanism, and this effect is more pronounced in subjects with impaired glucose tolerance (IGT). This suggests a causal role for hyperglycemia in the immune activation of diabetes. The role of hyperglycemia in inducing renal dysfunction is not completely understood. Several experimental studies have documented that renal ischemic injury in diabetic animals is worse than similar renal ischemic injury in normoglycemic animals10. This increase in renal injury seems to be due to exaggerated oxidative stress and to increased inflammatory responses in the kidneys of hyperglycemic animals. However, these data were generated by investigations using experimental animals that had absolute insulin deficiency due to beta-cell destruction8,9
It is unclear whether acute hyperglycemia in nondiabetic animals would be equally injurious to renal function10. In our study, pre-treatment with glucose produced transient hyperglycemia during the I/R period.
Propofol is known to produce a number of non-anesthetic effects. It stimulates constitutive nitric oxide (NO) production and inhibits inducible NO production. Propofol has also anxiolytic properties, which may be related to several neuromediator systems. Moreover, it has antioxidant, immunomodulatory, analgesic, antiemetic and neuroprotective effects. Furthermore, propofol inhibits both platelet aggregation and intracellular calcium increases in response to thrombin or ADP, and also has direct inhibitory effects on recombinant cardiac sarcolemmal KATP channels. All these beneficial properties are likely to expand the clinical use of propofol5. Propofol might provide protection via different mechanisms. After the onset of ischemia, it could be selected to reduce IRI4. It can attenuate oxidative injury of the pulmonary endothelium as detected by angiotensin-converting enzyme shedding in I/R and H2O2 models of acute lung injury15.
Clinical propofol dosage widely ranges from 0.02 to 0.2 mg.kg-1.min-1. Authors16 used 0.5 mg.kg-1.min-1 as a small dose and 2.0 mg.kg-1.min-1 as a large dose to investigate tissue antioxidant capacity during anesthesia. In this study, a dose of 1 mg.kg-1.min-1 of propofol was used. However, the experimental FDA formula for converting animal doses to human equivalent doses in mg.kg-1 is: human equivalent dose = rat dose x 0.16. Thus, the dose used in our experiment is equivalent to 0.16 mg.kg-1.min-1 of propofol for patients17.
There is increasing evidence of anesthetic agent-induced protection. At present, isoflurane, sevoflurane and morphine appear to be most promising preconditioning-inducing agents4. The proposed mechanisms for this protection include an ischemic preconditioning-like effect, interference in the neutrophil/platelet-endothelium interaction, blockade of Ca2+ overload to the cytosolic space and antioxidant-like effect4. In a similar experimental model, it was showed that in animals receiving isoflurane, IPC had no protective effect. However, isoflurane in association with remifentanil had a beneficial effect on the kidney, as demonstrated by flow cytometry and serum creatinine levels2. High-dose propofol has been reported to attenuate lactate accumulation and edema formation in cerebral ischemia in hyperglycemic rats18.
In an experimental model of aortic reconstructive surgery, propofol anesthesia compared with sevoflurane was associated with less neutrophil infiltration, lower plasma proinflammatory cytokine levels, lower production of oxygen free radicals, less lipid peroxidation, and reduced inducible NO synthase activity. These findings suggest a potential renal protective effect of propofol in this surgical setting19. Erythropoietin is another drug with a potential renal protective effect during ischemia-reperfusion and transiently hyperglycemic20. After renal ischemia and reperfusion, rats anesthetized with isoflurane that were given N-acetylcysteine following anesthetic induction had less variation in serum creatinine 21.
The pretreatment of animals with propofol has led to reduction in the susceptibility to in vitro oxidative stress in five different tissues, demonstrating the drug ability to limit oxidative injury16.
In this study, 24 hours after I/R, serum creatinine in the hyperglycemic-ischemic groups (PHI and IHI) was statistically different from that in the other groups, indicating renal injury in the presence of ischemia and hyperglycemia.
Histological scores were higher in groups PHI and IHI, intermediary in groups PI and II, and lower in the sham groups (PHS and IHS), indicating a higher degree of lesion in the presence of hyperglycemia, without the protection of propofol or isoflurane. FCM showed significant apoptosis in the hyperglycemic-ischemia groups (PHI and IHI). Ischemia with normoglycemia produced intermediary APTi values, with no differences between propofol and isoflurane. The sham groups showed similar values which were lower than those found in the other groups. Viable cell percentage was statistically higher in the sham and normoglycemic groups than in the groups where ischemia was associated with hyperglycemia.
Conclusions
Propofol and isoflurane provided the same level of protection against ischemia reperfusion injury and no protection against renal injury with transient hyperglycemia. Transient hyperglycemia had a potential injury effect on the kidney after an episode of I/R. The control of blood glucose levels may be clinically used for limiting organ dysfunction after periods of tissue ischemia.
Received: October 25, 2012
Review: December 20, 2012
Accepted: January 21, 2013
Conflict of interest: none
Financial source: Sao Paulo Research Foundation (FAPESP)
- 1. Bonventre JV. Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens. 2002;11:43-8.
- 2. Vianna PT, Castiglia YM, Braz JR, Viero RM, Beier S, Vianna Filho PT, Vitoria A, Reinoldes Bizarria Guilherme G, de Assis Golim M, Deffune E. Remifentanil, isoflurane, and preconditioning attenuate renal ischemia/reperfusion injury in rats. Transplant Proc. 2009;41:4080-2.
- 3. Hashiguchi H, Morooka H, Miyoshi H, Matsumoto M, Koji T, Sumikawa K. Isoflurane protects renal function against ischemia and reperfusion through inhibition of protein kinases, JNK and ERK. Anesth Analg. 2005;101:1584-9.
- 4. Kato R, Foex P. Myocardial protection by anesthetic agents against ischemia-reperfusion injury: an update for anesthesiologists. Can J Anaesth. 2002;49:777-91.
- 5. Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, Katsargyris A, Papadimitriou L. Propofol: A review of its non- anaesthetic effects. Eur J Pharmacol. 2009;605:1-8.
- 6. Assad AR, Delou JM, Fonseca LM, Villela NR, Nascimento JH, Vercosa N, Lopes AG, Capella MA. The role of KATP channels on propofol preconditioning in a cellular model of renal ischemia-reperfusion. Anesth Analg. 2009;109:1486-92.
- 7. Ganji MR, Charkhchian M, Hakemi M, Nederi GH, Solymanian T, Saddadi F, Amini M, Najafi I. Association of hyperglycemia on allograft function in the early period after renal transplantation. Transplant Proc. 2007;39:852-4.
- 8. Di Filippo C, Marfella R, Cuzzocrea S, Piegari E, Petronella P, Giugliano D, Rossi F, D'Amico M. Hyperglycemia in streptozotocin-induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion. Diabetes. 2005;54:803-10.
- 9. Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73-80.
- 10. Hirose R, Xu F, Dang K, Liu T, Behrends M, Brakeman PR, Wiener-Kronish J, Niemann CU. Transient hyperglycemia affects the extent of ischemia-reperfusion-induced renal injury in rats. Anesthesiology. 2008;108:402-14.
- 11. Park Y, Hirose R, Dang K, Xu F, Behrends M, Tan V, Roberts JP, Niemann CU. Increased severity of renal ischemia-reperfusion injury with venous clamping compared to arterial clamping in a rat model. Surgery. 2008;143:243-51.
- 12 Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007-21.
- 13. Ouattara A, Lecomte P, Le Manach Y, Landi M, Jacqueminet S, Platonov I, Bonnet N, Riou B, Coriat P. Poor intraoperative blood glucose control is associated with a worsened hospital outcome after cardiac surgery in diabetic patients. Anesthesiology. 2005;103:687-94.
- 14. Di Filippo C, Cuzzocrea S, Rossi F, Marfella R, D'Amico M. Oxidative stress as the leading cause of acute myocardial infarction in diabetics. Cardiovasc Drug Rev. 2006;24:77-87.
- 15. Balyasnikova IV, Visintine DJ, Gunnerson HB, Paisansathan C, Baughman VL, Minshall RD, Danilov SM. Propofol attenuates lung endothelial injury induced by ischemia-reperfusion and oxidative stress. Anesth Analg. 2005;100:929-36.
- 16. Runzer TD, Ansley DM, Godin DV, Chambers GK. Tissue antioxidant capacity during anesthesia: propofol enhances in vivo red cell and tissue antioxidant capacity in a rat model. Anesth Analg. 2002;94:89-93.
- 17. Cui W, Li Y, Li S, Yang W, Jiang J, Han S, Li J. Systemic lidocaine inhibits remifentanil induced hyperalgesia via the inhibition of cPKCgamma membrane translocation in spinal dorsal horn of rats. J Neurosurg Anesthesiol. 2009;21(4):318-25.
- 18. Ishii H, Arai T, Segawa H, Morikawa S, Inubushi T, Fukuda K. Effects of propofol on lactate accumulation and oedema formation in focal cerebral ischaemia in hyperglycaemic rats. Br J Anaesth. 2002;88:412-7.
- 19. Rodriguez-Lopez JM, Sanchez-Conde P, Lozano FS, Nicolas JL, Garcia-Criado FJ, Cascajo C, Muriel C. Laboratory investigation: effects of propofol on the systemic inflammatory response during aortic surgery. Can J Anaesth. 2006;53:701-10.
- 20. Caetano AMM, Vianna Filho PTG, Castiglia YMM, Golim MA, de Souza AVG, Carvalho LR, Deffune E, Oliveira C, Vianna PTG. Erythropoietin attenuates apoptosis after ischemia-reperfusioninduced renal injury in transiently hyperglycemic. Transplant Proc. 2011;43:3618-21.
- 21. Mansano AM, Vianna PTG, Fabris VE, Silva LM, Braz LG, Castiglia YMM, Prevention of renal ischemia/reperfusion injury in rats using acetylcysteine after anesthesia with isoflurane. Acta Cir Bras. 2012;27:340-5.
Publication Dates
-
Publication in this collection
06 Mar 2013 -
Date of issue
Mar 2013
History
-
Received
25 Oct 2012 -
Accepted
21 Jan 2013 -
Reviewed
20 Dec 2012