Abstract
PURPOSE: To evaluate the effects of aroeira (Schinus terebinthifolius) ointment on skin wound healing in rats. METHODS: Adult male rats (n=20) were divided into four groups of five animals each, as follows: G4, G7, G14 and G21, which corresponds to 4th, 7th, 14th and 21th days postoperatively. Each animal were made two incisions on the skin, including the subcutaneous tissue, in the right and left sides of thoracic region, separated by a distance of two inches. The right lesion was treated with base ointment (vaseline, lanolin); the left one was treated with base ointment containing 5% of aroeira oil. At the end of each experimental period the lesions were evaluated for the contraction degree. Then held the collection of fragments that were fixed in 10% formalin and processed for paraffin embedding. In the histological sections (5μm) was evaluated the morphology and quantified the collagen and blood vessels. The data obtained were submitted to ANOVA test complemented by Tukey-Kramer test (p<0.05). RESULTS: The contraction of the lesions was higher in wounds treated with aroeira oil than in controls at 7th and 14th days (p<0.01), whereas in the 21st day all lesions were already completely healed. The morphology showed granulation tissue more developed, with fibroblasts more bulky and collagen fibers more arranged in the experimental group at 4th, 7th and 14th days. The morphometry showed a significant increase in the quantification of collagen fibers in the experimental group at 7th and 14th days (p<0.05). CONCLUSION: The aroeira oil accelerates the healing process of wounds as a macroscopic, morphological and morphometrical analysis.
Wound Healing; Angiogenesis Inducing Agents; Skin; Phytotherapy; Anacardiaceae; Rats
8 ORIGINAL ARTICLE
WOUND HEALING
Effects of aroeira (Schinus terebinthifoliu Raddi) oil on cutaneous wound healing in rats1 1 Research performed at Animal Department of Pharmacy, Federal Rural University of Pernambuco (UFRPE), Brazil. Part of Master degree thesis, Postgraduate Program in Animal Bioscience. Tutor: Joaquim Evêncio-Neto.
Lígia Reis Moura EstevãoI; Fábio de Souza MendonçaII; Liriane Baratella-EvêncioIII; Ricardo Santos SimõesIV; Maria Edna Gomes de BarrosV; Rosa Maria Esteves ArantesVI; Milene Alvarenga RachidVI; Joaquim Evêncio-NetoVII
IFellow Mater degree, Postgraduate Program in Animal Bioscience, Department of Morphology and Animal Physiology, UFRPE, Pernambuco, Brazil. Acquisition and interpretation of data
IIAssociate Professor, Histology Division, Department of Morphology and Physiology, UFRPE, Pernambuco, Brazil. Conception and design of the study, critical revision
IIIAssociate Professor, Histology Division, Department of Morphology, Federal University of Pernambuco (UFPE), Brazil. Conception and design of the study, critical revision
IVFellow PhD degree, Postgraduate Program in Morphology, Department of Morphology and Genetic, Federal University of Sao Paulo (UNIFESP), Brazil. Interpretation of data and manuscript writing
VAssociate Professor, Department of Pharmacology, UFRPE, Pernambuco, Brazil. Analysis and interpretation of pharmacology data
VIAssociate Professor, Pathology Division, Department of General Pathology, Institute for Biological Sciences, Federal University of Minas Gerais (UFMG), Brazil. Histopathological examinations
VIIAssociate Professor, Histology Division, Department of Morphology, UFRPE, Pernambuco, Brazil. Scientific and intellectual content of the study, interpretation of data and critical revision
Correspondence Correspondence: Joaquim Evêncio-Neto Universidade Federal Rural de Pernambuco/DMFA Rua Manoel de Medeiros, s/n 52171-900 Recife PE Brasil evencio@dmfa.ufrpe.br
ABSTRACT
PURPOSE: To evaluate the effects of aroeira (Schinus terebinthifolius) ointment on skin wound healing in rats.
METHODS: Adult male rats (n=20) were divided into four groups of five animals each, as follows: G4, G7, G14 and G21, which corresponds to 4th, 7th, 14th and 21th days postoperatively. Each animal were made two incisions on the skin, including the subcutaneous tissue, in the right and left sides of thoracic region, separated by a distance of two inches. The right lesion was treated with base ointment (vaseline, lanolin); the left one was treated with base ointment containing 5% of aroeira oil. At the end of each experimental period the lesions were evaluated for the contraction degree. Then held the collection of fragments that were fixed in 10% formalin and processed for paraffin embedding. In the histological sections (5μm) was evaluated the morphology and quantified the collagen and blood vessels. The data obtained were submitted to ANOVA test complemented by Tukey-Kramer test (p<0.05).
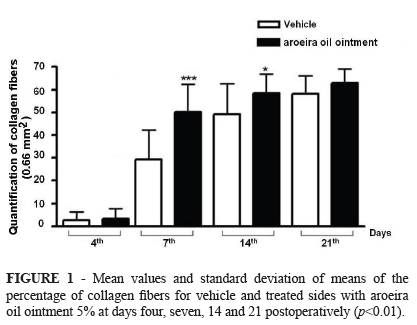
RESULTS: The contraction of the lesions was higher in wounds treated with aroeira oil than in controls at 7th and 14th days (p<0.01), whereas in the 21st day all lesions were already completely healed. The morphology showed granulation tissue more developed, with fibroblasts more bulky and collagen fibers more arranged in the experimental group at 4th, 7th and 14th days. The morphometry showed a significant increase in the quantification of collagen fibers in the experimental group at 7th and 14th days (p<0.05).
CONCLUSION: The aroeira oil accelerates the healing process of wounds as a macroscopic, morphological and morphometrical analysis.
Key words: Wound Healing. Angiogenesis Inducing Agents. Skin. Phytotherapy. Anacardiaceae. Rats.
Introduction
Plants are an unlimited source of potentially active substances and many of them are used to help promote healing and angiogenesis1,2. They must be considered as raw material, the first step in discovering new molecules and developing new phytomedicines1. Although popular knowledge has contributed significantly on understanding the effects of medicinal plants, their active principles, mechanism of action and toxicity are still poorly understood1,2, justifying scientific research to prove their effectiveness.
The aroeira tree (Schinus terebinthifolius Raddi) belongs to the Anacardiaceae family and is widely used in popular medicine possessing high capacity in the production of allelopathic compounds3,4. In Brazil it is found in various vegetations, from Pernambuco to Rio Grande do Sul4 it is popularly known as Brazilian Pepper, Aroeira, Florida Holly, Rose Pepper, and Christmasberry. It is used to treat wounds and ulcers of skin and mucous membranes, against infections of the respiratory system, digestive system, genito-urinary tract, hemoptysis and metrorrhagia3. Aroeira has been the subject of several studies involving the use of extracts of bark, leaves and fruit as a healing promoter5-7 and as an antibacterial and antifungal agent8,9.
Healing is a complex, systemic, physiological process where the body is required to activate, inhibit, and produce a number of cellular and molecular components that are organized in order to contribute to the restoration process and the function of damaged tissues. It is divided into stages; at first the cells are destroyed, characterizing the catabolic phase followed by the formation of new cellular elements, constituting the anabolic phase10.
Under normal conditions, the healing process follows a pattern that can be divided into three specific phases: inflammatory, fibroblastic phase with extracellular matrix deposition and remodeling phase11. The characteristics assumed by the injury during its evolution results of succession or overlapping cellular and tissue events resulting from cell activation by chemical mediators11,12.
In the inflammatory phase, the predominant events are related to blood clotting with thrombous formation and inflammatory process. This phase is characterized by vasoconstriction, platelet aggregation and activation of clotting systems13. The complete repair of tissue depends on the action of leukocytes, which in addition to their immune activities are closely involved in the anabolic and catabolic reactions, in tissue degradation by proteases and production of reactive oxygen and nitrogen in tissue formation and growth factors production11.
The fibroblastic phase and deposition of extracellular matrix is characterized by granulation, contraction and epithelialization of the wound. Beginning approximately four days after injury and according to Rodrigues et al.13, can continue for two weeks. Granulation is the formation of a tissue composed of capillaries, collagen and proteoglycans13. The activation of fibroblasts in this phase is intensified and activated to produce collagen. Thus, fibroplasia is necessary to neovascularization in the region. Capillary formation is a result of angiogenic factors secreted by macrophages and mast cells that stimulate the proliferation of endothelial cells from blood vessels. It is essential at this stage because it allows gas exchange and nutrition of metabolically active cells11. In addition to the direct action of growth factors, particularly VEGF (vascular endothelial growth factor) on the endothelial cells vessels, induction of angiogenesis is also influenced by low oxygen tension that occurs in the center of a wound12,14. Reepithelialization, migration and mitotic division of basal cells at the edges of the wound occurs along with the granulation process15. Finalizing with the process of contraction, this occurs by spontaneous closure of skin wounds and by the action of specialized myofibroblasts13.
The maturation or remodeling stage remains for months or years, in which acute and chronic inflammatory cells gradually decrease and stop angiogenesis and fibroplasia. It is also during this period that we see the balance between synthesis and degradation of collagen, and this remodeling is responsible for increasing the tensile strength of scar tissue11,13.
Based on popular medicine and on the previously known healing action of hydroalcoholic extracts of Schinus terebinthifolius Raddi and the action of its essential oils against bacteria and fungi, also, in order to study the manipulation of its ointment with a percentage of concentration that promotes the tissue repair process, this study was conducted to evaluate the effect of an ointment manufactured with essential oil of Schinus terebinthifolius Raddi leaf 5% in the treatment of cutaneous wound healing in rats.
Methods
The study protocol was approved by the Animal Ethics Committee of the Federal Rural University of Pernambuco (UFRPE) (process nº 23082.014123/2011). At the end of the experiment the animals were euthanized, prepared and discarded according to the requirements of ethical principles for experimental work of the Brazilian College of Animal Experimentation (COBEA), Sao Paulo, Brazil, 1991.
Twenty adult male rats (Rattus norvegicus albinus), weighing 250g, with three months of age were obtained from the Morphology and Physiology Department of Federal Rural University of Pernambuco (UFRPE). The animals were housed in individual boxes with commercial chow (Presence®, Purina) and water ad libitum, maintained at 23-25°C, under 12 hour light/dark cycle, in the animal colony at the Pharmacy Department of Rural Federal University of Pernambuco (UFRPE).
After a week of adaptation to the new environment, the animals were anesthetized with a combination of xylazine (20 mg/Kg) and ketamine (100 mg/Kg), administered intramuscularly12. The animals furry thoracic region was shaved and antisepsis was performed with topical alcoholic chlorhexidine 0.5%. The area was initially marked with the aid of 1.3 cm diameter cylinder. With a surgical blade and blunt scissors, incisions were made in skin and subcutaneous tissue on the right and left sides of the thoracic region, separated by a 2 cm distance. The tissue was dissected and removed leaving adjacent fascia exposed.
Immediately after surgical excision the wounds located on the right antimere, received a daily topical applications of the ointment lanolin and Vaseline base (vehicle) - control group (GCtrl) and the located on the left antimere, received a daily topical application of the ointment containing aroeira Leaf oil at 5% treated group (GTreat). The rats were then divided into four groups of five animals each according to the time of application of the ointment. G4 - four days application; G7 - seven days of application; G14 - 14 days of application and G21 - 21 days of application. Euthanasia was performed by increasing the anesthesia.
Preparation of the extract of Schinus terebinthifolius Raddi
Leaves of the pepper tree (Schinus terebinthifolius Raddi, Anacardiaceae) were collected in the morning of September 2010 on the campus of Federal Rural University of Pernambuco (UFRPE). The plant was identified by comparison with a previously identified specimen and deposited in the Herbarium Vasconcelos Sobrinho UFRPE under the number 49259.
To obtain the essential oil, fresh leaves (200g) were crushed and subjected to hydrodistillation technique in a modified Clevenger apparatus. After two hours of hydrodistillation the oil obtained was separated from water by density difference and excess moisture was removed with anhydrous sodium sulfate (Na2SO4)16. The total oil amount was calculated based on the weight of fresh leaves. The oil was stored in amber glass container, tightly closed kept in freezer, until the experiment, at a temperature of -20 °C. The study of the leaves oil had its chemical profile reported by Silva et al.17, which identified thirty-three components, representing 95.5% of the oil. Among the majority of this oil compounds are p-Cymen-7-ol (22.5%), 9-epi-(E)-cariophyllene (10.1%), carvone (7.5%) and Verbenone (7.4%).
With the oil, an ointment was manipulated with a lanolin-vaselina formulation: lanolin anhydrous - 30%; essential oil of aroeira leaf - 5% VIT; e-oily acetate - 0.5%; solid vaseline qsp 100g.
The formulation was based on a mixture of anhydrous lanolin and vaseline solid and the antioxidant of the essential oil itself (anhydrous lanolin purchased from Pharma Special, solid vaseline and BHT acquired from DEG), as recommended by Gulcan et al.18.
Morphology and morphometry
On 4th, 7th, 14th and 21th days after surgery, the wounds of each group were measured with the aid of a caliper graph (King Tools). To calculate the injured areas the major and minor diameters were observed. From these data the wound area (A) was calculated as A = p.Rr, being R the larger radius and r the smaller radius of the wound.
The calculation of the average degree of contraction (C) was expressed as percentuals, being C = [(A0 Ai)/Ao].100, where A0 is the initial area of mm the wound (day 0) and Ai is the area of the wound in the 4th, 7 th, 14 th and 21 th days postoperatively.
After macroscopic analysis of the respective groups the animals were anesthetized with isoflurane and a fragment was collected for histological analysis was administered to animals from each group (G4, G7, G14 and G21), the wound was dissected with a 0.5 cm margin of healthy skin around the lesion.
Histological analysis
After 24h in 10% formaldehyde, tissue samples were dehydrated in increasing concentrations of ethyl alcohol and diaphanized in xylene. Samples were then processed for paraffin inclusion. For each animal cuts were made in the middle region of the flap Sections longitudinal samples (5µm) were obtained parallel to the greater axis of fragments and stained with hematoxylin-eosin (H.E) and Gomori trichrome for morphological and histometric analysis.
Immunohistochemistry analyses
Two sections (5µm) were dried overnight at 37°C and then during 30 minutes at 60°C. Slides were immersed and dehydrated with xylene and alcohol. Afterwards, they were left in 3% H2O2 for five minutes to prevent endogenous peroxidase activity. Slides were transferred to citrate-buffered solution (pH 6.0) and processed in a microwave (750w) for five minutes twice. Then, slides were submitted to immunohistochemical staining with classic avidin-biotine peroxidase method for VEGF (VEGF -LabVision; 1/100). Immunohistochemical staining was performed with streptavidin-biotine-peroxidase method (UltraVision Polyvalent [rabbit-mouse], LabVision Products) with horseradish peroxidase (HRP) kit. Diaminobenzidin was used as the chromogen.
Six images of each slide was obtained, always immediately below the crust, with the aid of a trinocular biological microscope (NIKON 50i) under 400X magnification and adjusted to a system that captures images. Quantification of newly formed vessels and the collagen content was performed in the center of the lesion in an area of 0.66 mm2 (imaging), with the aid of an image analyzer (Imagelab 2000) in a Windows operational system.
Statistical analysis
The data was evaluated by ANOVA complemented by Tukey-Kramer test (p<0.05). Statistical analysis was performed using Assistat software, version 7.6 beta2.0.
Results
Our results demonstrated an earlier degree of wound contraction in the treated group at day 14 postoperatively (PO). In this period three GTreat wounds had reepithelized and two had epithelia almost complete. In the GCtrl only two wounds were completely healed and three were approaching reepithelization. At day 21 PO all wounds were completely healed with integrate epithelium. Analyzing the averaged percentages of the results, the GTreat wounds contracted more than GCtrl on days 7 and 14 PO, with statistical difference in the latter (Table 1).
Morphological analysis demonstrated a high concentration of polymorphonuclear cells observed in both groups at day four postoperative. Mast cells were visualized both on the side that received the ointment base (GCtrl) and the side that received ointment containing aroeira leaf oil at 5% (GTreat) in the 4th, 7th, 14th and 21st days after the formation of skin wound. However, the control group showed some mast cells scattered through the granulation tissue or in between the newly formed capillaries while the treated group showed multiple aggregates of mast cells in the fibrovascular tissue in all periods.
The concentration of collagen was higher in the Gtreat on days 7 and 14 PO (p<0.007). On the fourth postoperative day the wound showed granulation tissue approaching themselves, with low concentration of collagen fibers in both groups. Morphologically, the collagen fibers of the GTreat were more exuberant and organized when compared to GCtrl (Figures 1 and 2) at all times.
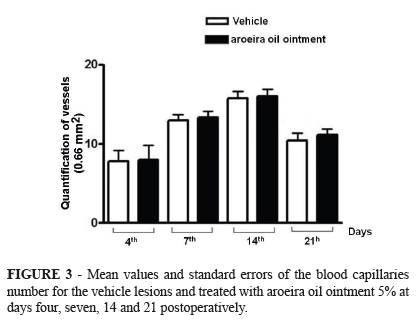
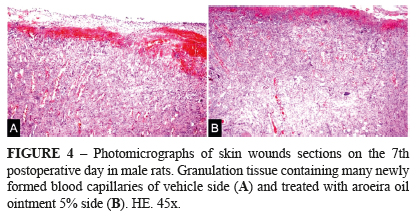
Histometric assessment of neoangiogenesis demonstrated no differences between the mean number of blood vessels in the treated and control groups in different periods. Morphologically, at 4th PO day, the GCtrl had newly formed, dilated and congest capillaries, demonstrating a greater degree of immaturity in relation to GTreat where the tissue had newly formed capillaries of smaller diameter (Figures 3 and 4). In the other periods, the GTrat, with evident neoangiogenesis, had a higher concentration of collagen and the epithelization process occurred earlier.
Discussion
Initially we would mention that extracts of pepper tree (aroeira) are already used in popular medium as cicatrizing agent, however, few works are of a scientific showing effective results. Accordingly our study showed smaller scar area on the 7th and 14th days in wounds treated with ointment containing aroeira oil than in the control group, indicating an early shrinkage. Concerning high concentration of polymorphonuclear cells observed in the fourth postoperative day coincide with the findings of Branco-Neto et al.6 and their work with hydroalcoholic extract of pepper tree. According to Mandelbaum et al.19, polymorphonuclear leukocytes arrive at the time of tissue injury and remain for a period of three to five days, primarily with the function to elimination possible microorganisms by phagocytosis.
According to Ng12, mast cells release inflammatory mediators (TNF-α, IL-1), growth factors (TGF-β1, PDGF) and proteases (chymase and tryptase) in early stages (inflammatory phase) by altering the regulation of vascular permeability and leukocyte infiltration into the wound. They are also able to induce and exacerbate the process of angiogenesis by releasing tumor necrosis factor, tryptase and heparin20. Moreover, mast cells accumulate in the wound edge and participate in the remodeling of collagen21.
Fibroplasia is extremely important in the formation of granulation tissue. With an increase of activated fibroblasts to produce collagen, the extracellular matrix begins to be replaced by a connective tissue stronger and more elastic11. A similar result was observed by Ribas et al.22 where the pepper trees extract increased fibroblast proliferation in the oral mucosa of rats.
The angiogenesis phenomenon is essential to carry oxygen to ischemic or newly formed healing tissues. Stimulation of growth factors and other mediators, endothelial cells of intact vessels inside the margins of the wound migrate toward the injured area, differentiate to form new capillars that sprouts form on the outer side of the vessels. These join the original capillaries and restore blood flow11. In this experiment, the number of newly formed vessels was enough to keep the healing process, aiding the production of collagen by fibroblasts and epithelialization with a superior time on treated lesions.
The Schinus terebinthifolius Raddi is a public domain plant popularly used for the treatment of lesions and ulcers, inflammation and infections3, whose active principles are not fully understood. Morphologically, histological and chemically the leaves and bark of this species are rich in tannins and essential oils, saponins are only in the bark, and the bark is of a different phenolic composition than the leaves23-25.
A research with extracts conducted by Branco-Neto et al.6 a delay in reepithelialization of skin wounds was observed in rats after using the hydroalcoholic extract prepared from the aroeira bark (Schinus terebinthifolius Raddi). Ribas et al.22 demonstrated that Schinus terebinthifolius was effective in the epithelial tissue repair process of ulcers on the oral mucosa of rats. Lucena et al.25 concluded that the use of hydroalcoholic extract of aroeira was favorable to heal rats that were submitted to cystotomy. Copaiba oil was effective in healing of random skin flaps in rats26.
Chemically, essential oils contain mostly terpene substances and eventually phenylpropanoids, together with smaller molecules, such as alcohol, esters, aldehydes and short-chain ketones27. Many of the biological activities of terpenoids, among them antimicrobial, are related to its essential oils28. Terpenes are the main chemicals responsible for the fragrances, culinary and medicinal uses of plants29. According to Lima et al.30 antibacterial and antifungal activity of aqueous extract of S. terebinthifolius, is possibly associated with the presence of certain chemical compounds present in small amounts, such as alkaloids, steroids, and chalconces urundeuvinas.
The aroeira essential oil promoted antimicrobial activity against Escherichia coli, Shigella dysenteriae, Bacillus subtilis and Staphylococcus aureus11 and antifungal activity on Aspergillus niger, A. parasiticus, A flavus, A. oryzae, A. fumigatus, Penicillium digitatum, Trichoderma spp and Helminthosporium oryae9,31 analyzing the antimicrobial activity of aqueous and alcoholic extracts obtained from Schinus terebinthifolius fruits learned that the alcoholic extract showed inhibitory effect on Staphylococcus aureus and Bacillus cereus growth, while the aqueous extract promoted no inhibitory effect on microorganisms growth. This result was due to the significant amount of flavone apigenin, and ellagic acid in the alcoholic extract, which the aqueous extract does not contain.
Most of the oil samples analyzed by Barbosa et al.32 revealed the α-pinene as its major component, especially those collected in India. P-caryophyllene and Cymen were previously reported for its inhibitory effect on microorganisms33. Silva et al.17 observed in vitro a favorable effect of the Schinus terebinthifolius Raddi essential oil on coagulase-positive staphylococci obtained from canine otitis. The antimicrobial activity is usually a result from synergism between various chemical compounds present in the oil, although this may also be due to a particular chemical compound.
Essential oils and extracts may be manufactured as topical dosage forms such as ointments. Semisolid preparations are of soft consistency, for skin and mucous membrane use. Ointments should have plasticity in order to modify its shape with little mechanical effort and easily adapt and adjust to the place where they are being applied. According to Viegas et al.34 the combination of Vaseline and lanolin anhydrous solid results in a hydrophobic, oily and aqueous excipient mixture, promoting at the same time an occlusive effect (vaseline) and endodermal activity (lanolin) in order to penetrate the skin, improving the absorption of the active principles in deeper tissue layers, but without reaching the bloodstream.
This research contributed to assess the healing process aided by the essential oil of aroeira and to develop an ointment with a concentration that promotes the tissue repair process as an alternative treatment of cutaneous wounds. Other studies and further concentrations of this phytomedicine are being developed as to improve the minimum optimal concentration needed for its use on skin wounds. Additional studies are necessary to establish the direct role of this essential oil in the regulatory mechanisms of collagen synthesis and maturation processes and epithelial regeneration mechanisms.
Conclusion
After analysis of results obtained in this experiment we concluded that the ointment containing aroeira oil at 5% was favorable for the tissue repair process of rat skin wounds.
Acknowledgments
To FACEPE for scholarships. To researchers, trainees and employees of the Federal Rural University of Pernambuco (UFRPE) and Federal University of Minas Gerais (UFMG) that participated directly or indirectly in this research.
Received: October 25, 2012
Review: December 20, 2012
Accepted: January 21, 2013
Conflict of interest: none
Financial sources: FACEPE (Pernambuco Research Foundation), FAPEMIG (Minas Gerais Research Foundation) and CNPq (National Council of Technological and Scientific Development).
- 1. Secco RS. Produtos naturais: alternativa segura? Ciênc Cult. (São Paulo). 1990;42(10/12):807-10.
- 2. Maciel MAM, Pinto AC, Veiga Junior VF. Plantas medicinais: a necessidade de estudos multidisciplinares. Química Nova. 2003;5(3):1-32.
- 3. Rocha PM, Rodilla JM, Díez D, Elder H, Guala MS, Silva LA, Pombo EB. Synergistic antibacterial activity of the essential oil of aguaribay (Schinus molle L.). Molecules. 2012;17(10):12023-36.
- 4. Medal JC, Vitorino MD, Habeck DH, Gilmore JL, Pedrosa JH, Souza LP. Host specificity of heteroperreyia hubrichi Malaise (Hemenoptera: Pergidae), a potencial biological control agent of Brasilian Peppertree (Schinus terebinthifolius Raddi). Biological Control. 1999;14:60-5.
- 5. Amorim MMR, Santos LC. Tratamento da vaginose bacteriana com gel vaginal da aroeira (Schinus terebinthifolius Raddi): ensaio clínico randomizado. Rev Bras Ginecol Obstet. 2003;25(2):2-11.
- 6. Branco Neto MLC, Ribas Filho JM, Malafaia O, Oliveira Filho MA, Czeczko NG, Aoki S, Cunha R, Fonseca VR, Teixeira HM, Aguiar LRF. Avaliação do extrato hidroalcoólico de aroeira (Schinus terebinthifolius Raddi) no processo de cicatrização de feridas de pele em ratos. Acta Cir Bras. 2006;21(Supl 6):17-21.
- 7. Ribas MO, Souza MH, Sartoretto J, Lanzoni TA, Noronha L, Acra LA. Efeito da Schinus terebinthifolius Raddi sobre o processo de reparo tecidual das lesões ulceradas induzidas na mucosa bucal do rato. Rev Odonto Ciênc. 2006;21(53):245-52.
- 8. Siddiqui R, Zafar U, Chaudhry SS, Ahmad H. Antimicrobial activity of essencial oils from Schimus terebinthifolius, Cypress sempervireus, Citrus lemon, Ferula assafoetida Part I. Pakistan J Sci Ind Res. 1995;38(9-10):358-61.
- 9. Siddiqui R, Ahmada H, Sultans S, Ehteshamuddin AFM, Shirrem S. Antimicrobial activity of essential oils. Part II. Pakistan J Sci Ind Res. 1996;39(1-4):43-7.
- 10. Pessoa WS, Estevão LR, Simões RS, Barros ME, Mendonça Fde S, Baratella-Evêncio L, Evêncio-Neto J. Effects of angico extract (Anadenanthera colubrina var. cebil) in cutaneous wound healing in rats. Acta Cir Bras. 2012;27(10):655-70.
- 11. Balbino CA, Pereira LM, Curi R. Mecanismos envolvidos na cicatrização: uma revisão. Rev Bras Ciênc Farm. 2005;41(1):27-51.
- 12. NG MFY. The role of mast cells in wound healing. Int Wound J. 2010;7(1):55-61.
- 13. Rodrigues FV, Hochman B, Wood VT, Simões MJ, Juliano Y, Ferreira LM. Effects of lidocaine with epinephrine or with buffer on wound healing in rat skin. Wound Repair Regen. 2011;19(2):223-8.
- 14. Knighton DR, Silver I, Hunt T.K. Regulation of wound-healing angiogenesis effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90:262-70.
- 15. De Nardi AB, Rodaski S, Sousa RS, Baudi DLK, Castro JHT. Secondary cicatrization in dermoepidermal wounds treated with essential fatty acids, vitamins A and E, soy lecithin and polynylpyrrolidone-iodine in dogs. Arch Vet Sci. 2004;9(1):1-16.
- 16. Cheng SY, Xie Y, Feng XL, Huang LF. Study of the volatile constituents in radix flemingiae macrophyllae and a substitute by gas chromatography-mass spectrometry and chemometric methods. Molecules. 2012;17(12):14111-25.
- 17. Silva AB, Silva T, Franco ES, Rabelo AS, Lima ER, Mota RA, Câmara CAG, Pontes-Filho NT, Lima-Filho JV. Antibacterial activity, chemical composition, and cytotoxicity of leaf's essential oil from Brazilian pepper tree (Schinus terebinthifolius, Raddi). Braz J Microbiol. 2010;41(1):158-63.
- 18. Gulcan E, Kuçuk A, Çayci K, Tosun M, Emre H, Koral L, Aktan Y, Avsar U. Topical effects of nebivolol on wounds in diabetic rats. Eur J Pharm Sci. 2012;47(2):451-5.
- 19. Mandelbaum SH, Di Santis EP, Mandelbaum MHS. Cicatrização: conceitos atuais e recursos auxiliares. Parte I. An Bras Dermatol. 2003;78(4):1-15.
- 20. Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5(1):40-6.
- 21. Iba Y, Shibata A, Kato M, Masukawa T. Possible involvement of mast cells in collagen remodeling in the late phase of cutaneous wound healing in mice. Int Immunopharmacol. 2004;4(14):1873-80.
- 22. Ribas MO, Souza MH, Sartoretto J, Lanzoni TA, Noronha L, Acra LA. Efeito da Schinus terebinthifolius Raddi sobre o processo de reparo tecidual das lesões ulceradas induzidas na mucosa bucal do rato. Rev Odonto Ciênc. 2006;21(53): 245-52.
- 23. Bório EBL, Cecy C, Yassumoto Y. Pharmacognostic study of the bark of Schinus terebinthifolius Raddi (Anacardiaceae). Ciênc Cult. (São Paulo). 1973;25(7):631-4.
- 24. Jorge LIF, Markmann BEO. Exame químico e microscópico de Schinus terebinthifolius Raddi (aroeira). Rev Ciênc Farm (São Paulo). 1996;17:139-45.
- 25. Lucena PLH, Ribas Filho JM, Mazza M, Czeczko NG, Diez UA, Correa Neto MA, HENRIQUES GS, SANTOS OJ. Avaliação da ação da aroeira (Schinus terebinthifolius Raddi) na cicatrização de feridas cirúrgicas em bexigas em ratos. Acta Cir Bras. 2006;21(Supl 3):8-15.
- 26. Estevão LRM, Medeiros JP, Scognomillo-Zsabó MVR, Baratella-Evêncio L, Guimarães EC, Câmara CAG, Evêncio-Neto J. Neoangiogênese de retalhos cutâneos em ratos tratados com óleo de copaíba. Pesq Agropec Bras. 2009;44(4):406-12.
- 27. Siane AC, Sampaio ALF, Sousa MC, Henriques MGMO, Ramos MFS. Óleos essenciais. Biotecnol Ciênc Desenvolv. 2000;3(16):38-42.
- 28. Ahameethunisa AR, Hopper W. In vitro antimicrobial activity on clinical microbial strains and antioxidant properties of Artemisia parviflora. Ann Clin Microbiol Antimicrob. 2012;11(1):30.
- 29. Dorman HJD, Deans SG. Antimicrobial agents from plants: activity of plant volative oils. J Appl Microbiol. 2000;88(2):308-16.
- 30. Lima EO, Pereira FO, Lima IO, Trajano VN, Souza EL. Schinus terebinthifolius Raddi: avaliação do espectro de ação antimicrobiana de seu extrato aquoso. Infarma. 2004;16(7-8):83-5.
- 31. Degáspari CH, Waszczynskyj N, Prado MRM. Atividade antimicrobiana de Schinus terebinthifolius Raddi. Ciênc Agrotec. 2005;29(3):617-22.
- 32. Barbosa LCA, Demuner AJ, Clemente AD. Seasonal variation in the composition of volatile oils from Schinus terebinthifolius Raddi. Quím Nova. 2007;30(8):1959-65.
- 33. Cox SD, Mann CM, Markham JL. Interactions between components of the essential oil of Melaleuca alternifolia J Appl Microbiol. 2001;91(3):492-7.
- 34. Viegas TX, Van Winkle LL, Lehman PA, Franz SF, Franz TJ. Evaluation of creams and ointments as suitable formulations for peldesine. Int J Pharm. 2001;219(1-2):73-80.
Publication Dates
-
Publication in this collection
06 Mar 2013 -
Date of issue
Mar 2013
History
-
Received
25 Oct 2012 -
Accepted
21 Jan 2013 -
Reviewed
20 Dec 2012