Abstract
PURPOSE: To investigate immunohistochemical aspects of the myenteric plexus of valves constructed in the colon of rats to verify whether any denervation occurs both at the operative site and in those areas adjacent to the third valve. METHODS: Thirty six male Wistar rats divided into the following three groups were used: Control Group (CG); Amputated Group (AG); Amputated Group with Valves (AGWV). In AG was held in the rectum amputation and the colon was sutured to the skin elaborating the perineal colostomy. In AGWV was held in the rectum amputation. A laparotomy was performed for the manufacture of valves (seromyotomy) in the colon. After this step, the colon was sutured to the skin elaborating the perineal colostomy. The density of the neural elements in the muscular wall as marked specifically using Protein Gene Product (PGP) 9.5 and utilising the proper tools of the KS300 software for measuring the area. From these measurements, a relation and three proportions were drawn and analysed according to the mean of the averages obtained from the measured images. RESULTS: Immunoexpression of PGP 9.5 demonstrated a total absence of neural elements and myenteric plexus at the valve site. The density of the neural elements in the circular muscular layer at sites adjacent to the 3rd valve was lesser, however, was not significantly different. CONCLUSION: The immunohistochemical study of valves constructed in the colon of rats submitted to abdominoperineal amputation and perineal colostomy revealed denervation at the seromyotomy site.
Amputation; Colostomy; Myenteric Plexus; Colorectal Surgery; Immunohistochemistry; Rats
7 - ORIGINAL ARTICLE
ALIMENTARY TRACT
Morphology and immunohistochemistry of the myenteric plexus of valves constructed in the colon of rats submitted to abdominoperineal amputation and perineal colostomy1 1 Research performed at Laboratory of Surgical Technique and Neuroimunopathology, Department of Surgery and General Pathology, School of Medicine, Federal University of Minas Gerais (UFMG), Belo Horizonte-MG, Brazil. Part of PhD degree thesis, Postgraduate Program in Science Applied to Surgery and Ophthalmology. Tutor: Prof. Alcino Lázaro da Silva.
Beatriz DeotiI; Rosa Maria Esteves ArantesII; Camila França CamposIII; Johnny HayckIV; Alcino Lázaro da SilvaV
IPhD, Associate Professor, Department of Surgery, UFMG, Belo Horizonte-MG, Brazil. Intellectual and scientific content of the study, acquisition and interpretation of data, manuscript preparation and writing, critical revision, designed the protocol, involved whit technical procedures, supervised all phases of the study
IIPhD, Researcher 2 CNPq (PQ2), Department of General Pathology, ICB, UFMG, Belo Horizonte-MG, Brazil. Scientific content and design of the study, acquisition and interpretation of data, involved with technical procedures, critical revision
IIIFellow PhD degree, Department of General Pathology, ICB, UFMG, Belo Horizonte-MG, Brazil. Acquisition of data
IVMD, Resident, General Surgery, "Cristiano Machado" Hospital, ICB, UFMG, Belo Horizonte-MG, Brazil. Manuscript preparation
VPhD, Emeritus Professor, Department of Surgery, UFMG, Belo Horizonte-MG, Brazil. Intellectual and scientific content of the study, critical revision
Correspondence Correspondence: Beatriz Deoti Rua Patagônia, 66/203 30320-080 Belo Horizonte - MG Brasil Tel.: (55 31)3273-0491 bardesiro@terra.com.br
ABSTRACT
PURPOSE: To investigate immunohistochemical aspects of the myenteric plexus of valves constructed in the colon of rats to verify whether any denervation occurs both at the operative site and in those areas adjacent to the third valve.
METHODS: Thirty six male Wistar rats divided into the following three groups were used: Control Group (CG); Amputated Group (AG); Amputated Group with Valves (AGWV). In AG was held in the rectum amputation and the colon was sutured to the skin elaborating the perineal colostomy. In AGWV was held in the rectum amputation. A laparotomy was performed for the manufacture of valves (seromyotomy) in the colon. After this step, the colon was sutured to the skin elaborating the perineal colostomy. The density of the neural elements in the muscular wall as marked specifically using Protein Gene Product (PGP) 9.5 and utilising the proper tools of the KS300 software for measuring the area. From these measurements, a relation and three proportions were drawn and analysed according to the mean of the averages obtained from the measured images.
RESULTS: Immunoexpression of PGP 9.5 demonstrated a total absence of neural elements and myenteric plexus at the valve site. The density of the neural elements in the circular muscular layer at sites adjacent to the 3rd valve was lesser, however, was not significantly different.
CONCLUSION: The immunohistochemical study of valves constructed in the colon of rats submitted to abdominoperineal amputation and perineal colostomy revealed denervation at the seromyotomy site.
Key words: Amputation. Colostomy. Myenteric Plexus. Colorectal Surgery. Immunohistochemistry. Rats.
Introduction
Perineal colostomy is a useful technique for replacing the anal function. Reconstruction of neo-anal function following the abdominoperineal amputation of the rectum allows for partial control of intestinal content evacuation. This technique has several notable oncological features: it allows in a block resection of the pelvic tissues and subsequent filling of the pelvic hollow; it avoids abdominal colostomy; and patients have an improved social life, as they are bag-free and can schedule their intestinal hygiene at their convenience, despite their relative incontinence1. Perineal colostomy also avoids alterations to the patient's body image that can be interpreted as disfiguring and necessitate a process of redevelopment2,3.
Seromyotomy, which is conducted to construct surgical valves in the pulled-through colon, consists of a total local section of the serosa layer, including both the external and internal muscular layers of the intestinal wall, to the point of making the protusion of the mucosa evident and creating an invaginating suture of the mucosa in this segment. This procedure involves the section of the myenteric plexus (Auerbach's) with the preservation of the submucosal plexus (Meissner's) and mucosal plexus, thus resulting in localized surgical de-enervation. The histological alterations in the valves constructed at the cellular and neural levels remain unknown4-6.
The objectives are as follows:
1) Reproduce the technique of Seromyotomy in an experimental environment by constructing valves in the colons of rats. 2) Investigate the morphological and immunohistochemical aspects of the myenteric plexus of these valves to verify whether any denervation occurs both at the operative site and in those areas adjacent to the third valve. Furthermore, we are interested in whether alterations occur in the thickness of the muscle layers or the connective tissue in the intestinal wall adjacent to the third valve.
Methods
The study was performed after obtaining approval from the Medical Research Ethics Committee of the institution (CETEA/UFMG).
Thirty six male Wistar rats (Rattus norvergicus albinus) between three and five months, weighing between 230g a 500g were used in experimental model. The rats were divided into the following three groups: 6 specimens in the Control Group (CG), 6 specimens in Amputated Group (AG) and 24 specimens in Amputated Group with Valve (AGWV). The animals were held in fasting conditions (8h), with no rations and free access to water. They were weighed immediately before the anaesthetic procedure and anaesthetised with a mixture of Xylasine (1 mL/kg live weight) and Ketamine (1 mL/kg live weight) in equal parts, via intramuscular injection, on the lateral aspect of the left thigh.
Surgical procedures
Control Group (CG)
Six rats were submitted to laparotomy, a midline incision with 5 cm in length. These rats underwent exploration of the abdominal cavity, injection of 5 mL of 0.9% saline solution, replacement of the intestinal loops at the incision site and abdominal wall closure using PDS (Polydioxanone) 4.0 with continuous surgical suture in the aponeurosis and skin in a craniocaudal direction (xiphoid-pubic).
Amputated Group (AG)
Six rats were subjected to an anal bag-shaped surgical suture (cotton 2.0 sutures with needles) via perineal access and mobilization of the entire rectum and colon to pull them up through to the coccygeal vertebra. Following that step, the anal sphincter structure was resected approximately 1 centimetre from cranial to the anal edge. The descending colon was sutured to the perineal skin with cardinal points (Chromed Catgut 5.0). An extra stitch was placed among the previous four stitches crowning the perineal colostomy.
Amputated Group With Valve (AGWV)
Twenty-four rats were subjected to amputation of the anal sphincter structure via perineal access and construction of 3 valves in the pulled-through colon via abdominal access, followed by perineal colostomy. The perineal operative time followed the same techniques as in the AG, with the colostomy fixed after the sphincters were extracted and valves constructed. A five centimetre midline laparotomy was performed. The arterial arcade and caudal mesenteric artery were preserved. A tweezer was placed by the perineum through the retrorectal area, to access the abdominal cavity, i.e., the dissection of the intestinal segment in the perineal operation was taken to the abdominal cavity. The construction of the valves through a linear section of the seromuscular layer (seromyotomy) was circumferential (360º) and preserving mucosal layer, with preservation of the arcade. The first valve was constructed two cranial centimetres from the anal edge, the second valve was constructed 1 cranial centimetre from the first valve, and the third valve was constructed 1 centimetre from second valve. The raw areas of the seromyotomy were sutured with simple stitches, using mononylon 6.0 thread. The needle was transfixed in the seromuscular layer 4mm to 5 mm from the raw area, and the same steps were repeated on the contralateral border, starting at the mesenteric face and covering the entire circumference in a counterclockwise direction, generating the border union and mucosal invagination (Figure 1). Next, the pull-through of the operated segment was performed, with the resection of the anus approximately 1 centimetre distal to the first valve. Care was taken to maintain the mesocolon in a posterior position. The abdominal wall was closed with polydioxanone (PDS) 4.0 suture. The pulled-through colon was sutured to the perineal skin (Chromed Catgut 5.0) at the four cardinal points, and a fifth extra stitch was also added, thereby completing the perineal colostomy.
On the fortieth post-operative day, the entire large intestine was extracted, and the animals were killed by bloodletting.
Collection, fixation and histological processing of the material
In all three groups, a 9-centimetre segment of the intestine was removed, beginning at the anal edge. The segment was divided into two parts: a 3-cm cranial segment and 6-cm distal segment. The 6-cm distal segment in the AGWV contained the three constructed valves, whereas in the CG and AG, the extracted segment of the intestine corresponded with the area with valves in the AGWV. The colon was opened longitudinally on its antimesenteric face and processed as described elsewhere8. Briefly, the intestine was washed in PBS (phosphate buffer saline) solution and the piece was placed onto filter paper with the serosa side in contact with the paper and immersed in Bouin's fixative solution with 2% glacial acetic acid. The segment was kept submersed as long as necessary for proper former fixation to occur, which permitted the piece to form a roll without compressing the tissue. The proper former fixation time varied between 20 and 30 minutes (Figure 2). Next, the rolls were set in a formaldehyde solution buffered in 4% PBS at a neutral pH until routinely processed for paraffin embedding and microtomy8,9.
The histological cuts were initially stained with haematoxylin and eosin (H&E). Subsequent semi-consecutive sections were obtained for Gomori Trichrome staining and for immunohistochemistry of a generic neural tissue marker, the Protein Gene Peptide 9.5 (PGP 9.5)10,11.
Microscopy and morphometric analysis
The morphometric analysis was planned for the comparative study of the distal and cranial segments of the three experimental groups in accordance with the following parameters:
a. Intestinal wall thicknesses: Internal Muscular (IM); External Muscular (EM); Total Muscular (TM = IM + EM); and serosa (SE) layers (Figure 3).
b. Density of the neural elements in the muscular wall as marked specifically using PGP 9.5 (Figure 4).
c. Connective tissue area present in the intestine wall (Figure 5).
Distal segment
To measure the thickness of the intestinal wall, five images of each slide were photographed at 4x magnification of the distal segment, which included the regions immediately adjacent, distally and cranially, to the third valve in each slide of the AGWV intestines and corresponding region in the CG and AG intestines. Five measurements each of the muscular and serosa layers were obtained from each image. In this segment, the same parameters were evaluated in the distal segment; five images from each slide were selected from a minimum of five and maximum of seven rats.
Cranial segment
Five measurements each of the muscular and serosa layers were made for each image, with a magnification of 4x, which corresponds to 3 centimetres cranial to the third valve of the AGWV and corresponding region in the CG and AG intestines. The thickness measurement was individualised for the IM, EM, TM and SE layers and expressed in µm (Figure 3a and b).
A minimum of four and maximum of six images were selected from each slide, corresponding with four rats from the CG, six from the AG and seven from the AGWV for the distal intestinal segment. At least five images from five to six rats from each the experimental group were also obtained for the cranial segment.
Utilising the proper tools of the KS300 software for measuring the area (binary image in a 10x objective), the internal muscular layer was isolated by masks, and its area was measured in µm2 and denominated the IM Area. The same real image was digitalized, and only the area corresponding with the myenteric plexus marked by PGP was measured in µm2, denominated the Myenteric Plexus Area. From the same real image, only the area corresponding with the intramuscular axons of the internal muscular layer of the enteric neurons marked by the antibody PGP 9.5, the sum of which was obtained in µm2, was denominated the Positive IM PGP Area. From the same real image, the parameter "distance between two points" was activated, which consisted of a line drawn between the circular and longitudinal muscular layers encompassing the field of view of the slide (10x magnification) from one extreme to another, measured in millimetres, denominated the IM Length. The total area was obtained by the sum of the positive PGP area in the internal muscular (IM) plus the myenteric plexus area.
From these measurements, a relation and three proportions were drawn and analysed according to the mean of the averages obtained from the measured images:
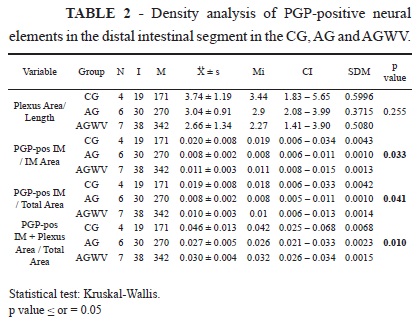
A relation: area of the myenteric plexus (µm2) marked by PGP 9.5 and divided by the length of the examined wall (µm) and the following three proportions: (1) PGP-positive area in the internal muscular area (µm2) divided by the total internal muscular area; (2) PGP-positive area in the internal muscular area (µm2) divided by the total area; and (3) PGP-positive area in the internal muscular area (µm2) added to the myenteric plexus area (µm2) and then divided by the total area (µm2)12. These proportions are shown in Table 2.
Distal and cranial segments were coloured with Gomori Trichrome stain and subjected to a morphometric procedure. In the distal segment, five slides from the CG, six slides from the AG and four slides from the AGWV were selected. Four to eight images were photographed for each slide, at 4x magnification. For the cranial segment, five slides from the CG, five slides from the GA and seven slides from the AGWV were selected, and five to six images were made from each slide using the afore mentioned magnification. The average of the measurements obtained for each group was reported. The results were expressed as a proportion of the area coloured in bluish-green, measured and expressed in square micrometres (µm2) and then divided by the total area of the intestinal wall (area of all layers).
Results
Pre, intra, and post-operative complications are listed in Table 1. The variables considered important and capable of influencing the analytical results of the study in terms of the denervation of the colon after seromyotomy were the number of stitches per valve and punctiform opening of the mucosa membrane. Neither of these variables was significant enough to explain death, with p values greater than the significance level, defined as p = 0.20 (univariate analysis and logistic regression).
Microscopy of the distal intestinal segment
In the CG and AG, the intestinal wall demonstrated habitual continuity of the mucosa, submucosa, muscular and serosa layers (Figure 6). The AG also demonstrated a nonspecific inflammatory reaction restricted to the serosa layer.
In the AGWV, the intestinal wall demonstrated continuity of all layers except in the region where it was possible to identify the presence of the valves by the localised thickening of the serosa and muscular layers, signs of chronic inflammation with the formation of giant cells that phagocytose parts of surgical sutures (Figure 7) and fibrotic repairs (connective-vascular neoformation with collagen deposits). The linear interruption of the muscular layers in a region largely replaced by exuberant vascular connective tissue represents fibrous scarring that mainly extended to the serosa layer and external and internal muscular layers (Figure 8A and B). The submucosal layer, including the muscularis mucosae was found to be preserved (Figure 9).
Nevertheless, despite the intense alterations described in the valve region, a systematic inspection of the adjacent areas demonstrated well-preserved tissue with only minimal deposits of connective tissue that did not result in important structural alterations in the wall. These findings may be observed in Figure 9, which is representative of the intestinal wall between the valves.
The colouration of connective tissue using Gomori Trichrome Stain revealed habitual collagen deposits in several anatomic regions of the wall, which showed no qualitative or quantitative differences between the groups.
The distribution of the myenteric plexus along the intestinal wall sampled in the roll of the regions between the valves remained preserved in terms of the structure and density of the neural elements, as was well demonstrated by PGP 9.5 expression (Figures 10 and 11).
However, at the site of the surgical valves, a concomitant discontinuity in the internal and external muscular layers was observed, in addition to the virtual absence of the elements that constitute the myenteric plexus at these sites. Figure 12 shows the paucity of these filaments in the internal muscular layer at the site immediately adjacent to the valves. This decrease in density was not detected in the intervalve areas where the morphometric analysis was conducted.
Microscopy of the cranial intestinal segment
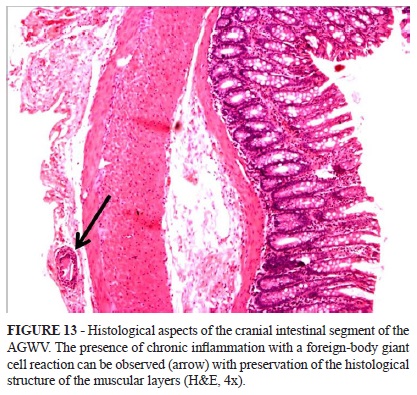
The intestinal rolls in the cranial region reveal well-preserved intestinal layers from a structural point of view. Staining with H&E, Gomori and immunohistochemistry for PGP demonstrated similar findings when comparing the groups. Furthermore, the morphometry of these elements did not reveal any quantitative differences. The serous layers of the representative animals in the AGWV rats can be observed to be discretely enlarged due to a chronic inflammatory process indicative of a giant cell reaction (Figure 13).
Thickness of the distal and cranial intestinal layers
The thicknesses of the internal muscular, external muscular, total muscular and serosa layers of the intestinal wall in the distal region were not statistically significant between the three groups. In the cranial region, the thickness of the specific muscular layers did not differ statistically between the GC, AG and AGWV, whereas the thickness of the serosa layer was significantly different between the groups (p = 0.024). According to the Mann-Whitney test, the serosa layer was significantly larger in the AGWV than the CG.
Gomori Trichrome Stain in the distal and cranial intestinal segments
This colouration reveals the habitual features of collagen deposits evident in the diverse anatomic regions of the wall, although neither qualitative nor quantitative differences were found between the groups.
Density of the neural elements in the distal and cranial intestinal segments
The density of the neural elements of the intestinal wall in the region distal to the third valve was analysed using the Kruskal-Wallis test. Although the differences in density were not statistically significant, the differences in proportions were significant (Tables 2 and 3). One may observe that all of the parameters evaluated for the densities of positive PGP 9.5 elements in this region were significantly greater in the CG than the AG. There was also a significant difference between the CG and AGWV in terms of the density parameter expressed as PGP pos-IM + plexus area / total area, with the CG demonstrating greater values. The intestinal segment cranial to the third valve was analysed in the same manner as described for the distal intestinal segment. The Kruskal-Wallis test was applied. None of the differences in terms of the relation and proportions for the density of the PGP-positive neural elements were statistically significant.
Discussion
The experimental reproduction of the technique for constructing biological surgical valves in the colons of rats subjected to abdominoperineal amputation with surgical reconstruction of the pelvis and exteriorisation of perineal colostomy has proven executable and achieved its objective.
The seromyotomy undertaken in the valve construction results in local denervation4-6,13. In this denervated segment, there is fibrotic replacement without re-enervation up to the 40th post-operative day. There is no cicatricial stenosis. The pathoanatomical analysis did not reveal significant hypertrophy of the intestinal wall in the distal and cranial segments. This absence of hypertrophy represents an intestinal adaptation because the valve, although denervated, is not able to block the functioning of all motility mechanisms. As a result, the valves are likely less effective and uncoordinated, and for this reason, the seromyotomy is not destructive but rather retainer. It is clear that the valve construction of the valve does not result in a disease in the operated segment that may lead to systematic repercussions, i.e., in the descriptive analysis, there was preservation of the histological structure at the intervals between the valves and upstream of the last valve. In the qualitative analysis of the distribution of Auerbach's plexuses along the sampled intestinal wall, the structure and density of its neuronal elements was preserved in the regions between valves. At the valve sites, these plexuses were no longer visible in small microscopic segments of the wall in association with irregularities in the presentation of the muscular layers that lose distinction and present areas of connective tissue deposits. As it is retainer, the valve re-establishes close-to-physiological behaviour in the operated segment.
The thicknesses of the internal and external muscular layers did not significantly vary between groups. However, there was a slight increase in the external muscular thickness in the AGWV. The AG showed smaller values in all of the thickness parameters, indicating that amputation without concurrent construction of the valves not only significantly diminished the transit time but also resulted in some degree of muscular atrophy of the wall.
The creation of the valves leads to changes in peristalsis, with stasis and intestinal distension downstream sum to different degrees, which inhibit and delay intestinal transit. Many reports of this phenomenon can be found in the literature. Dilation is the second morphological parameter of intestinal adaptation6. The increase in intraluminar pressure caused by the accumulation of content in the segment proximal to the surgical injury produces transluminar tension. In this experimental model, dilation occurred in all animals to varying degrees. This distension presented with the formation of segmentation chambers, revealing a gradual intestinal adaptation process towards valve resistance. This adaptation might even enable an increase in the absorption of water, among other local alterations, as direct contact of the mucosa with luminar nutriments and hormones affects the proliferation of cells in a manner similar to what occurs in the human intestine. The valves did not produce intestinal obstruction, and the adaptation took place at different levels. Valve construction leads to alterations of peristalsis, with resulting stasis and intestinal distension upstream at different levels, which inhibits and retards intestinal transit. Many such observations are reported in the literature4-6,14,15.
Biological surgery valves do not obstruct or starve the animals; they are retainers allowing a delay in the intestinal flow and faecal exoneration, re-approximating the evacuation act to the physiological pattern. These valves do not create alterations that stimulate disease, and they provoke denervation only at the valve sites. They generate an intestinal adaption response with the preservation of histological structures in the intestinal segments between and downstream of the valves and from them. Nor do they behave as scar stenosis but rather work as valves and transform the intestinal segment where they are created to storage. The technique is easily executed and has potential importance for clinical applicability.
Biological surgical valves do not become obstructed in animals; they are contentive, which allows for retardation of the intestinal flow, and they function in faecal exoneration, thus restoring the act of evacuation close to its physiological pattern. These valves do not generate alterations that simulate diseases, and they result in denervation exclusively at the creation site. They generate an intestinal adaptive response with preservation of the histological structures in the operated intestinal segment between and upstream of the valves. Furthermore, they do not create cicatricial stenosis but instead function as valves to transform the intestinal segment into a storage segment. This technique is easily performed and has important potential for clinical applications.
To extrapolate the current findings to human beings, we conclude with future perspectives of more studies that consider the systematic analysis of other elements, such as interstitial cells of Cajal, neurotransmitters and the physiology of the operated segment, submucosal plexus of Meissner and colonic mucosa16. Given its certain clinical applicability, this technique may be able to improve patients' quality of life.
Conclusion
The morphological and immunohistochemical valve built in the left colon of rats subjected to abdominoperineal amputation and perineal colostomy revealed denervation site seromyotomy, which contributes to the delay of the intestinal flow.
Acknowledgments
To Prof. Dr. Andy Petroianu, Diogo de Oliveira Lopes Ferreira Santos, Eduardo Barbosa Coelho Neto, Elmo de Paula Campos Junior and Breno Cotta Coelho who provided purely technical help and general support. To Fernando Henrique Pereira for statistical analysis.
Conflict of interest: none
Financial source: Coordination of Improvement for Higher Academic Staff (CAPES)
Received: December 12, 2012
Review: February 14, 2013
Accepted: March 18, 2013
- 1. Lázaro da Silva A. Amputação abdominoperineal com colostomia perineal. Rev Bras Coloproctol. 1991;11(3):105-8.
- 2. Altomare DF, Rinaldi M, Martinelli E, Veglia A, Sallustio P. Perineal colostomy following Miles procedure: from reconstructive surgery to the artificial anal sphincter. Osp Ital Chir. 2000;6:572-7.
- 3. Fernandes RM, Miguir ELB, Donoso TV. Perfil da clientela estomizada residente no município de Ponte Nova, Minas Gerais. Rev Bras Coloproctol. 2011;30(4):385-92.
- 4. Silva Junior A, Lázaro da Silva A, Castro LPF. Histopatologia da seromiotomia dupla e sutura seromuscular no cólon descendente de ratos. Rev Col Bras Cir. 1999;26(6):367-73.
- 5. Veloso SG, Biet R, Rios AM, Leite VHR, Lázaro da Silva A. Eficácia da confecção de válvulas colônicas após ressecção retoanal em ratos. Rev Col Bras Cir. 2001;28(5):356-63.
- 6. Deoti B, Lázaro da Silva A, Oliveira MZ, Dinalli AC. Histological study of the left colon of rats after extra-mucosal seromyotomy (continent valves): evaluation of colonic emptying. Acta Cir Bras. 2008;23(3):230-6.
- 7. Brasil, Lei n. 6.638, de 8 de maio de 1979. Normas para a prática didático-científica da vivissecção de animais e determinação de outras providências. Lex. 1979;43:416.
- 8. Arantes RM, Nogueira AM. Distribution of enteroglucagon and peptide yy-immunoreactive cells in the intestinal mucosa of germ-free and conventional mice. Cell Tissue Res. 1997;290(1):61-9.
- 9. Krammer HJ, Karahan ST, Sigge W, Kuhnel W. Immunohistochemistry of markers of the enteric nervous system in whole-mount preparations of the human colon. Eur J Pediatr Surg. 1994;4:274-8.
- 10. Marvin-Guy L, Lopes LV, Affolter M, Courtet-Compondu MC, Wagnière S, Bergonzelli GE, Fay LB, Kussmann M. Proteomics of the rat gut: analysis of the myenteric plexus-longitudinal muscle preparation. Proteomics. 2005;5:2561-9.
- 11. Burns AJ, Roberts RR, Bornstein JC, Young HM. Development of the enteric nervous system and its role in intestinal motility during fetal and early postnatal stages. Semin Pediatr Surg. 2009;18(4):196-205.
- 12. Karaosmanoglu T, Aygun B, Wade P, Gershon MD. Regional differences in the number of neurons in the myenteric plexus of the guinea pig small intestine and colon: an evaluation of markers used to count neurons. Anat Rec. 1996;244:470-80.
- 13. Rena CL, Lázaro da Silva A, Barra AA, Furtado MCV, Rena LR, Rena LR. Alterações morfométricas da musculatura dos músculos longitudinal e circular de ratos submetidos à criação de piloros no intestino delgado. Rev Col Bras Cir. 2007;34(1):41-7.
- 14. Araújo EJA, Sant'Ana DMG, Molinari SL, Miranda Neto MH. Effect of protein and vitamin B deficiency on the morpho-quantitative aspects of the myenteric plexus of the descending colon of adult rats. Arq Neuropsiquiatr. 2003;61(2A):226-33.
- 15. Schiller WR, Didio LJA, Anderson MC. Production of artificial sphincters. Arch Surg. 1967;95:436-42.
- 16. Huizinga JD, Lammers WJ. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296(1):G1-8.
Publication Dates
-
Publication in this collection
02 Apr 2013 -
Date of issue
Apr 2013
History
-
Received
12 Dec 2012 -
Accepted
18 Mar 2013 -
Reviewed
14 Feb 2013