Abstract
PURPOSE: To investigate the effect of sildenafil citrate (SC) on skeletal muscle ischemia-reperfusion (IR) injury in rats. METHODS: Adult male Wistar rats were randomized into three groups: vehicle-treated control (CTG), sildenafil citrate-treated (SCG), and sham group (SG). CTG and SCG had femoral artery occluded for 6 hours. Saline or 1 mg/kg of SC was given 5.5 hours after occlusion. SG had a similar procedure without artery occlusion. Soleus muscle samples were acquired 4 or 24h after the reperfusion. Immunohistochemistry caspase-3 analysis was used to estimate apoptosis using the apoptotic ratio (computed as positive/negative cells). Wilcoxon rank-sum or Kruskal-Wallis tests were used to assess differences among groups. RESULTS: Eighteen animals were included in the 4h reperfusion groups and 21 animals in the 24h reperfusion groups. The mean apoptotic ratio was 0.18±0.1 for the total cohort; 0.14±0.06 for the 4h reperfusion groups and 0.19±0.08 for the 24h groups (p<0.05). The SCG had lower caspase-3 ratio compared to the control groups at the 24h reperfusion time point (p<0.05). CONCLUSION: Sildenafil citrate administration after the onset of the ischemic injury reduces IR-induced cellular damage in skeletal muscle in this rat hindlimb ischemia model.
Reperfusion Injury; Muscle, Skeletal; Phosphodiesterase; Inhibitors; Caspase 3; Rats
8 - ORIGINAL ARTICLE
ISCHEMIA-REPERFUSION
Sildenafil citrate protects skeletal muscle of ischemia-reperfusion injury. Immunohistochemical study in rat model1 1 Research performed at Operative Technique and Experimental Surgery Division, Department of Surgery, Sao Paulo Federal University (UNIFESP), Brazil. Part of Master degree thesis, Postgraduate Program in Vascular Surgery, UNIFESP. Tutor: Djalma José Fagundes.
Dinani Matoso Fialho de Oliveira ArmstrongI; Anderson da Costa ArmstrongII; Regina Célia Bressan Queiroz FigueiredoIII; Joao Eduardo FlorentinoIV; Paulo Fernandes SaadV; Karen Fox-TalbotVI; Marc Kenneth HalushkaVII; Dan E. BerkowitzVIII; Murched Omar TahaIX; Djalma José FagundesX
IMsc, Assistant Professor, Department of Surgery, Sao Francisco Valley Federal University (UNIVASF), Petrolina-PE, Brazil. Main author. Conception, design, intellectual and scientific content of the study
IIMsc, Preceptor, Department of Cardiology, UNIVASF, Petrolina-PE, Brazil. Statistical analysis and manuscript writing
IIIPhD, Associate Researcher, Department of Microbiology, Research Center Aggeu Magalhaes, Recife-PE, Brazil. Conception and critical revision of the study
IVGraduate student, Pernambuco Federal University (UFPE), Recife-PE, Brazil. Acquisition of data
VPhD, Assistant Professor, Department of Surgery, UNIVASF, Petrolina-PE, Brazil. Critical revision
VIMA, Department of Pathology, Johns Hopkins University, Baltimore-MD, USA. Immunohistochemical processing and interpretation of data
VIIPhD, Associate Professor, Department of Pathology, Johns Hopkins University, Baltimore-MD, USA. Interpretation of immunohistochemical data and manuscript writing
VIIIMB, BCh, Associate Professor, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University, Baltimore-MD, USA. Critical revision
IXPhD, Associate Professor, Operative Technique and Experimental Surgery Division, Department of Surgery, UNIFESP, Sao Paulo-SP, Brazil. Critical revision
XPhD, Associate Professor, Operative Technique and Experimental Surgery Division, Department of Surgery, UNIFESP, Sao Paulo-SP, Brazil. Conception and critical revision of the study
Correspondence Correspondence: Dinani Matoso Fialho de Oliveira Armstrong 720 Rutland Avenue; Room 624, Floor 6, Traylor Bldg Baltimore, MD, 21205, USA Office Phone: 443-287-6199 Mobile Phone: 443-668-2314 darmst13@jhmi.edu
ABSTRACT
PURPOSE: To investigate the effect of sildenafil citrate (SC) on skeletal muscle ischemia-reperfusion (IR) injury in rats.
METHODS: Adult male Wistar rats were randomized into three groups: vehicle-treated control (CTG), sildenafil citrate-treated (SCG), and sham group (SG). CTG and SCG had femoral artery occluded for 6 hours. Saline or 1 mg/kg of SC was given 5.5 hours after occlusion. SG had a similar procedure without artery occlusion. Soleus muscle samples were acquired 4 or 24h after the reperfusion. Immunohistochemistry caspase-3 analysis was used to estimate apoptosis using the apoptotic ratio (computed as positive/negative cells). Wilcoxon rank-sum or Kruskal-Wallis tests were used to assess differences among groups.
RESULTS: Eighteen animals were included in the 4h reperfusion groups and 21 animals in the 24h reperfusion groups. The mean apoptotic ratio was 0.18±0.1 for the total cohort; 0.14±0.06 for the 4h reperfusion groups and 0.19±0.08 for the 24h groups (p<0.05). The SCG had lower caspase-3 ratio compared to the control groups at the 24h reperfusion time point (p<0.05).
CONCLUSION: Sildenafil citrate administration after the onset of the ischemic injury reduces IR-induced cellular damage in skeletal muscle in this rat hindlimb ischemia model.
Key words: Reperfusion Injury. Muscle, Skeletal. Phosphodiesterase 5 Inhibitors. Caspase 3. Rats.
Introduction
Ischemia-reperfusion injury (IRI) in skeletal muscle has complex and multifactorial mechanisms. Reestablishment of blood flow is essential to salvage ischemic tissues, but may also lead to cellular damage beyond that caused by the initial ischemia. Numerous reactive oxygen species (ROS) are brought along when oxygenated blood flow re-enters ischemic tissues during reperfusion, leading to additional tissue injury. Moreover, inflammatory mediators are also generated, aggravating the local cell death1,2.
As mechanisms of cell death, both necrosis and apoptosis can be induced by IRI. Necrosis used to be considered the solo mechanism in ischemic conditions, but current evidence show apoptosis as the major contributor to cell death due to IRI3,4. Quantifying and mapping apoptosis indicates the extension of IRI3. For the quantification of apoptosis, the assessment of caspase-3 expression by immunohistochemistry staining is a validated and standardized method5.
Hindlimb ischemia due to arterial occlusion is a common condition in clinical practice6. Skeletal muscles are the predominant tissue damaged in this situation, with high vulnerability to cell death due to ischemia. Typically, severe injury in skeletal musculature starts after a 2-hours ischemic interval1,7. However, irreversible muscle cell damage seems to start after three hours of ischemia and to be nearly complete after a 6- hours interval8.
Sildenafil citrate (SC) is a phosphodiesterase-5 inhibitor able to increase nitric oxide release, improving endothelial function9. Promising results have been shown for the use of SC in animal models of myocardial IRI, as well as inducing protective effects in the kidney10,11. However, the role of SC as a protective agent during IRI in skeletal muscles is still unknown.
The aim of this study is to determine whether pharmacologic postconditioning with SC is effective to reduce IRI in skeletal muscle, using an experimental model of rodent limb ischemia.
Methods
We included 42 male Wistar albino rats in the study, but three were excluded after the surgical procedure due to research protocol violation. The animals were kept under controlled environmental conditions of temperature, noise, and a cycle of twelve hours for light and dark, with free access to water and adequate chow for the species. The investigational protocol was approved by the Institutional Animal Care Committee, and the research was performed in accordance with the guidelines of the Brazilian legislation for scientific use of animals (Law nº 11.794/08).
Groups and surgical procedure
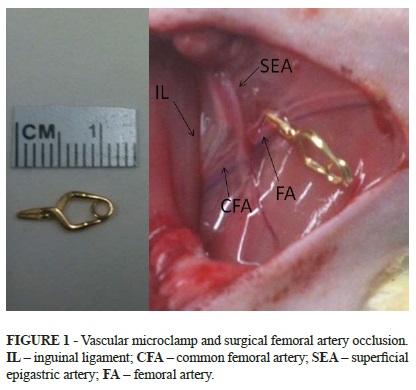
The animals were randomized to one of three groups: 1) vehicle-treated sham group (SG); 2) vehicle-treated control group (CTG); and 3) sildenafil citrate-treated group (SCG). The rats were anesthetised with ketamine hydrochloride (75mg/kg) and xylazine (10mg/kg) intraperitoneally and kept spontaneously breathing in room air. Tramadol hydrochloride (2mg/kg) IM was given immediately before the surgical procedure. For the SCG and CTG, the femoral artery was dissected and then clamped with a vascular microclamp just below the bifurcation of the deep femoral artery (Figure 1). Ischemia was confirmed by the presence of clinical signs of ischemia (cutaneous cyanosis and pallor, reduced temperature compared to the contralateral limb) as well as by the disappearance of arterial pulsatility distal to vascular clamp. In SG, a similar surgical procedure was performed without femoral artery occlusion. The animals were placed in individual cages under heat of red-hot lamps up to the recuperation of the anesthesia and with free access to food and water. The SCG then received 1mg/kg of SC in saline by gavage 5.5h after the onset of ischemia, while the CTG received only saline by gavage at 5.5h. In SG, the animals received saline by gavage 5.5h after the surgical procedure. Six hours after clamping, the blood flow was re-established in the femoral artery by removing the clamp. The reperfusion was confirmed by the disappearance of clinical parameters of ischemia. The animals were placed in individual cages under heat during the reperfusion period. After 4h or 24h (according to group protocol), the soleus muscle was excised and euthanasia was then performed.
Tissue processing and analysis
The soleus muscle was fixed in 10% buffered formalin for 24 hours, dehydrated, and then embedded in paraffin. Sections (6 mm) of the central part of all samples were stained for caspase-3 expression. The immunohistochemical processing was performed using the primary antibody-cleaved anticaspase-3, anti-mouse (DakoTM A/S, Denmark 492), revealing DAB (3, 3-diaminobenzidine) Sigma Co-USAD5632 and obtaining the expression of the caspase-3 staining brown. The quantification of caspase-3 expression was performed in a blinded fashion. Ten images of each animal slide were captured, using an optical microscope at 10x 40x (400x) magnification (NikonTM, Inc.) connected to a workstation. The apoptotic ratio (AR) was determined by quantifying the number of caspase-3 positive myocyte nuclei divided by the number of caspase-3 negative myocyte nuclei in each image (Figure 1). To assess reproducibility, a slide was randomly selected from each animal and independently analyzed by a second reader.
Statistical analysis
Statistical analysis was performed using STATA version 11.2. Difference between groups was tested using the Wilcoxon rank-sum test when two groups were involved or using the Kruskal-Wallis test when more than two groups were examined. The protective effect of sildenafil citrate was assessed by comparing mean values of AR among groups (SG, SCG and CTG) in total and according to the reperfusion time. The reproducibility analysis was performed using intraclass correlation coefficient and Bland-Altman plots. The level of significance for rejecting the null hypothesis was 5% (p<0.05).
Results
A total of 39 animals were included, with the mean weight of 350 ± 20 g. Of those, 18 animals underwent 4h of reperfusion (six animals in each of three groups: SG, CTG and SCG) and 21 animals underwent 24h of reperfusion (seven rats in each of three groups: SG, CTG and CSG).
Figures 2, 3 and 4 show photomicrographs of the immunohistochemical expression characteristics of cleaved caspase-3 in animals from CTG, SCG and SG after 24h of reperfusion.
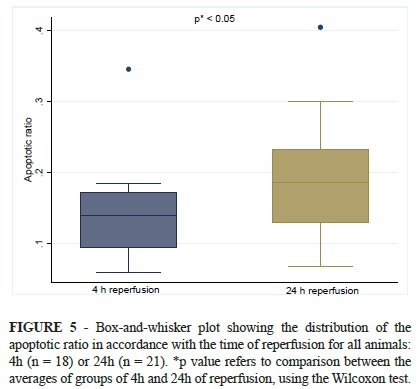
The apoptotic ratio (AR) was expressed by mean AR (± standard deviation). It was 0.18 ± 0.1 for the total sample, being 0.14 ± 0.06 for the animals that underwent 4h of reperfusion and 0.19 ± 0.08 for those after 24h of reperfusion (p<0.05) (Figure 5).
In both 4h and 24h reperfusion groups, the highest AR values were found in the CTG, followed by SCG and SG. Differences among groups were non-significant in animals with 4h of reperfusion, but statistical significance was seen when comparing groups of animals that underwent 24h of reperfusion (Figure 6).
In SG, no statistical significance was found comparing AR values of animals that underwent 4h to those after 24h of reperfusion (p = 0.4). However, animals after 24h of reperfusion had statistically significant higher AR values compared to those that underwent 4h of reperfusion (p = 0.04) in CTG and CSG.
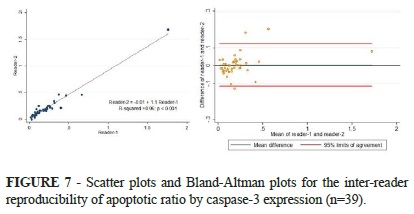
The inter-reader reproducibility for the AR measurement showed a very strong correlation between readers, with intraclass-correlation coefficient of 0.97 (95% confidence interval: 0.96, 0.99). The Bland-Altman plots showed a mean difference of 0.003±0.06 and 95% limits of agreement from -0.12 to 0.12 (Figure 7).
Discussion
To the best of our knowledge, this is the first study assessing the influence of SC in skeletal muscle IRI lesions. SC showed a protective effect for hindlimb skeletal muscle damage due to IRI. These benefitial effects were more pronounced at the late reperfusion (24h) time point, compared to early reperfusion (4h). Additionally, the assessment of apoptotic ratio based on the caspase-3 expression by immunohistochemistry staining had robust precision.
Despite improvements in operative techniques, acute ischemia of the lower limb remains a challenging clinical problem with significant morbidity and mortality. Its incidence may be as high as 13-17 cases per 100.000 people per year and mortality rates may reach 18% in some series12. Due to the high metabolic demand of skeletal muscle, irreversible damage may occur when perfusion occurs even after brief ischemic intervals7,13. In this regard, the soleus muscle is particularly sensitive to ischemia due to its metabolism14. Surgical reperfusion is the established therapy early after the onset of ischemic conditions, but long-term ischemia increases additional tissue damage due to IRI.
Quantitative evaluation of apoptosis by caspase-3 expression using immunohistochemistry staining is well established5. We assessed the precision of the method and found a strong intra-class correlation, with mean difference between two independent readers close to null and narrow limits of agreement (Figure 4). This robust inter-reader reproducibility for the AR strengthens the reliability of our findings.
In our study, a six-hour ischemia ensured a significant injury to the soleus muscle, as showed by the lower AR found in SG when compared to the other groups (Figure 2). Evidence shows that ischemic morphological lesions are present minutes after reperfusion and increase progressively over time, due to an additional inflammatory response. In fact, cell death due to IRI is more evident after 4 hours of reperfusion, reaching the maximum peak at 24 hours14,15. In early reperfusion stages, most of the injury is associated with the acute release of free radicals. In late reperfusion, cell death factors are mostly related to IRI inflammatory pathways, with increasing importance of a neutrophil response.
SC is a PDE5 inhibitor and its mechanism of action involves increased tissue levels of cGMP, resulting in smooth muscle relaxation and increased vasodilation16. SC is effective for the treatment of erectile dysfunction and pulmonary hypertension17,18. Moreover, SC has protective effects over IRI in heart, kidney, liver, and brain19,20. However, the literature is focused on the use of SC before the onset of ischemia, acting in a pharmacological preconditioning. In the clinical setting, the onset of acute ischemia is usually unanticipated, which makes the delivery of a protective agent before the onset of ischemia impossible. We assessed the cytoprotective effect of SC administrated half an hour before the reperfusion, so that they would be present in the blood at the start of the reperfusion period. This nearly simulates the normal human condition when a patient is admitted to an emergency clinic and undergoes a revascularization procedure.
We assessed soleus muscle apoptosis in periods of early reperfusion (4h) and late reperfusion (24h). Animals that received SC had lower AR than those in the control group. Although the full mechanism involved was not explored, our results suggest that SC has a protective effect over IRI of skeletal muscle. In the early reperfusion phase, the mean difference on AR among groups did not show statistical significance. After 24h of reperfusion, AR values were significantly lower for SCG compared to CTG (Figure 3). These findings indicate that SC protects agains skeletal muscle damage, especially in injuries related to the late phase of reperfusion. On the other hand, the results found in a late reperfusion phase may be consequence of the SC action in reducing ROS in the early phase.
Some points to be explored in future studies is the use of parenteral SC, an assessment of its effects when applied before ischemia as a preconditioning pharmacological, the effects of SC in remote organ damage after ischemia of skeletal muscle, as well as investigating the molecular mechanisms by which the SC has the cytoprotective effect in IRI in skeletal muscle. Subsequent studies could confirm these findings and translate the knowledge on the use of SC in IRI in the clinical area, making possible advances that bring therapeutic benefit in future medical practice.
Conclusion
In the model of six hours of ischemia in the rat hind limb, sildenafil citrate significantly reduced the apoptotic ratio in rat soleus muscle after 24 hours of reperfusion.
Conflict of interest: none
Financial source: none
Received: December 12, 2012
Review: February 14, 2013
Accepted: March 12, 2013
- 1. Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10(6):620-30.
- 2. Gillani S, Cao J, Suzuki T, Hak DJ. The effect of ischemia reperfusion injury on skeletal muscle. Injury. 2012;43(6):670-5.
- 3. Wang WZ, Fang XH, Stephenson LL, Khiabani KT, Zamboni WA. Ischemia/reperfusion-induced necrosis and apoptosis in the cells isolated from rat skeletal muscle. J Orthop Res. 2008;26(3):351-6.
- 4. Lopez-Neblina F, Toledo AH, Toledo-Pereyra LH. Molecular biology of apoptosis in ischemia and reperfusion. J Invest Surg. 2005;18(6):335-50.
- 5. Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16(1):3-11.
- 6. Sotoudeh A, Takhtfooladi MA, Jahanshahi A, Asl AH, Takhtfooladi HA, Khansari M. Effect of N-acetylcysteine on lung injury induced by skeletal muscle ischemia-reperfusion. Histopathlogical study in rat model. Acta Cir Bras. 2012;27(2):168-71.
- 7. Henderson PW, Jimenez N, Ruffino J, Sohn AM, Weinstein AL, Krijgh DD, Reiffel AJ, Spector JA. Therapeutic delivery of hydrogen sulfide for salvage of ischemic skeletal muscle after the onset of critical ischemia. J Vasc Surg. 2011;53(3):785-91.
- 8. Wang WZ, Baynosa RC, Zamboni WA. Therapeutic interventions against reperfusion injury in skeletal muscle. J Surg Res. 2011;171(1):175-82.
- 9. Oruc O, Inci K, Aki FT, Zeybek D, Muftuoglu SF, Kilinc K, Ergen A. Sildenafil attenuates renal ischemia reperfusion injury by decreasing leukocyte infiltration. Acta Histochem. 2010;112(4):337-44.
- 10. Kukreja RC, Salloum F, Das A, Ockaili R, Yin C, Bremer YA, Fisher PW, Wittkamp M, Hawkins J, Chou E, Kukreja AK, Wang X, Marwaha VR, Xi L. Pharmacological preconditioning with sildenafil: Basic mechanisms and clinical implications. Vascul Pharmacol. 2005;42(5-6):219-32.
- 11. Lledo-Garcia E, Subira-Rios D, Rodriguez-Martinez D, Dulin E, Alvarez-Fernandez E, Hernandez-Fernandez C, del Canizo-Lopez JF. Sildenafil as a protecting drug for warm ischemic kidney transplants: experimental results. J Urol. 2009;182(3):1222-5.
- 12. Hynes BG, Margey RJ, Ruggiero N, 2nd, Kiernan TJ, Rosenfield K, Jaff MR. Endovascular management of acute limb ischemia. Ann Vasc Surg. 2012;26(1):110-24.
- 13. Adiseshiah M, Round JM, Jones DA. Reperfusion injury in skeletal muscle: a prospective study in patients with acute limb ischaemia and claudicants treated by revascularization. Br J Surg. 1992;79(10):1026-9.
- 14. Brasileiro JL, Fagundes DJ, Miiji LON, Oshima CTF, Teruya R, Marks G, Inouye CM, Santos MA. Isquemia e reperfusão de músculo sóleo de ratos sob ação da pentoxifilina. J Vasc Bras. 2007;6(1):13.
- 15. Carmo-Araujo EM, Dal-Pai-Silva M, Dal-Pai V, Cecchini R, Anjos Ferreira AL. Ischaemia and reperfusion effects on skeletal muscle tissue: morphological and histochemical studies. Int J Exp Pathol. 2007;88(3):147-54.
- 16. Zusman RM, Morales A, Glasser DB, Osterloh IH. Overall cardiovascular profile of sildenafil citrate. Am J Cardiol. 1999;83(5A):35C-44C.
- 17. Corbin JD, Francis SH. Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract. 2002;56(6):453-9.
- 18. Hemnes AR, Champion HC. Sildenafil, a PDE5 inhibitor, in the treatment of pulmonary hypertension. Expert Rev Cardiovasc Ther. 2006;4(3):293-300.
- 19. Zhang RL, Zhang Z, Zhang L, Wang Y, Zhang C, Chopp M. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res. 2006;83(7):1213-9.
- 20. Medeiros PJ, Villarim Neto A, Lima FP, Azevedo IM, Leao LR, Medeiros AC. Effect of sildenafil in renal ischemia/reperfusion injury in rats. Acta Cir Bras. 2010;25(6):490-5.
Publication Dates
-
Publication in this collection
02 Apr 2013 -
Date of issue
Apr 2013
History
-
Received
12 Dec 2012 -
Accepted
12 Mar 2013 -
Reviewed
14 Feb 2013