Abstract
PURPOSE: To investigate whether elastic fiber content in the corpus cavernosum (CC), corpus spongiosum (CS) and tunica albuginea (TA) of the rabbit penis undergoes modifications with age. METHODS: Rabbits were sacrificed, in groups of ten animals each, at 30, 120, 240, and 730 days of age. Histological sections were obtained from the penile middle shaft and were stained with Weigert's resorsin fuchsin. The content of elastic fibers was determined using stereological methods, and was expressed as volume fraction. RESULTS: At 730 days of age, elastic fiber content was increased by 54% (p<0.004), 78% (p<0.004), and 87% (p<0.004) in the TA, CC, and CS, respectively, compared with animals aged 30 days. After 30 days of age, the concentration gradually and significantly increased until 240 days of age. In 730-day old animals, the concentration, compared with the previous age group, was unchanged in the CC and decreased by 20% (p<0.004) in the TA. CONCLUSIONS: Elastic fiber contents in the rabbit penis correlate with properties of penile tissues. Although after one month of age there is a gradual increase in these concentrations, in two-year old animals this trend is interrupted, which suggests that this could be an early alteration due to senescence.
Aging; Elastic Tissue; Penis; Corpus Cavernosum; Rabbits
10 - ORIGINAL ARTICLE
EXPERIMENTAL UROLOGY
Age-related changes in the concentration of elastic fibers in different regions of the rabbit penis11 Research performed at Urogenital Research Unit, State University of Rio de Janeiro (UERJ) and Department of Morphology, Biomedical Center, Fluminense Federal University (UFF), Niteroi-RJ, Brazil.
Marcelo Abidu-FigueiredoI; Waldemar Silva CostaII; Maurício Alves ChagasIII; Francisco José Barcellos SampaioIV; Luiz Eduardo de Macedo CardosoV
IPhD, Associate Professor, Department of Animal Biology, Federal Rural University of Rio de Janeiro (UFRRJ), Rio de Janeiro-RJ, Brazil. Design of the study, histological examinations, stereological measurements, interpretation of data
IIPhD, Associate Professor, Urogenital Research Unit, UERJ, Rio de Janeiro-RJ, Brazil. Conception and design of the study, histological examinations, interpretation of data
IIIPhD, Department of Morphology, Biomedical Center, Fluminense Federal University, Niteroi-RJ, Brazil. Design of the study, histological examinations, interpretation of data
IVPhD, Full Professor, Urogenital Research Unit, UERJ, Rio de Janeiro-RJ, Brazil. Conception and design of the study, interpretation of data, critical revision. VPhD, Associate Professor, Urogenital Research Unit, UERJ, Rio de Janeiro-RJ, Brazil. Conception and design of the study, interpretation of data, statistical analysis, manuscript writing
Correspondence Correspondence: Luiz Eduardo de Macedo Cardoso Unidade de Pesquisa Urogenital-UERJ Avenida 28 de Setembro, 87 Térreo 20551-030 Rio de Janeiro - RJ Brasil Tel.: (55 21)2868-8017 Fax: (55 21)2868-8033luizemcardoso@gmail.com
ABSTRACT
PURPOSE: To investigate whether elastic fiber content in the corpus cavernosum (CC), corpus spongiosum (CS) and tunica albuginea (TA) of the rabbit penis undergoes modifications with age.
METHODS: Rabbits were sacrificed, in groups of ten animals each, at 30, 120, 240, and 730 days of age. Histological sections were obtained from the penile middle shaft and were stained with Weigert's resorsin fuchsin. The content of elastic fibers was determined using stereological methods, and was expressed as volume fraction.
RESULTS: At 730 days of age, elastic fiber content was increased by 54% (p<0.004), 78% (p<0.004), and 87% (p<0.004) in the TA, CC, and CS, respectively, compared with animals aged 30 days. After 30 days of age, the concentration gradually and significantly increased until 240 days of age. In 730-day old animals, the concentration, compared with the previous age group, was unchanged in the CC and decreased by 20% (p<0.004) in the TA.
CONCLUSIONS: Elastic fiber contents in the rabbit penis correlate with properties of penile tissues. Although after one month of age there is a gradual increase in these concentrations, in two-year old animals this trend is interrupted, which suggests that this could be an early alteration due to senescence.
Key words: Aging. Elastic Tissue. Penis. Corpus Cavernosum. Rabbits.
Introduction
Animal models are often used in investigations on penile tissues that are involved in erection and erectile dysfunction, or that are subjected to surgical treatments1. The rabbit is considered to be particularly suitable because, like humans, it has a vascular penis which, in turn, lacks a penile bone as found in rodents2-4. Other similarities with the human penis include the presence of a corpus cavernosum (CC), a ventrally located corpus spongiosum (CS) which surrounds the urethra, and a tunica albuginea (TA) enveloping these erectile structures5.
Erection is normally associated with an intracellular cascade of events that change smooth muscle contractility in penile blood vessels and vascular spaces, thereby modifying blood flow and initiating inflation of the CC6. However, erection also depends on the collagen and elastic fibers of the underlying connective tissue framework, which exerts passive resistance to the expansion of erectile tissues, thereby creating penile turgidity4. Further, elastic fibers provide elastic recoil when the penis returns to a flaccid condition during detumescence7.
Elastic fibers themselves consist of a bundle of fibrillar glycoproteins, such as fibrillins, which are assembled extracellularly and are later embedded with the amorphous elastomeric protein elastin8. Elastic fibers impart viscoelastic properties to tissues and are typically found in structures that, upon application of stretching forces, undergo deformation and then return to the original shape once these forces are removed9. Loss or degradation of elastic fibers can cause significant dysfunctions, such as in degenerative and inflammatory disorders10.
The distribution and structural features of elastic fibers in penile tissues have been investigated in humans11-15 as well as in laboratory and food animals3,16,17. We have previously studied the concentration and distribution of elastic fibers in different regions of the rabbit penis, and the results indicated a close relationship with the known functions of penile tissues3. These results, however, were from young adult animals only, so that there is no data yet on how elastic fibers in the rabbit CC, CS, and TA change with age. In view of the involvement of elastic fibers in penile function and disorders, such data would provide important information on the rabbit reproductive biology and extend the applicability of the penis of this animal as an experimental model. For example, in humans, elastic fibers are altered in different types of erectile dysfunction18,19 and their content is reduced in the TA of elderly individuals18, while similar, age-related data exist for erectile tissues of the rat penis20-22. It should be noted also that correlations between extracellular matrix composition and age are particularly relevant in short-lived animals such as the rabbit. Significant changes can occur in a relatively short period of time, and if they are not taken into account, results from experimental studies might be misleading or even erroneously interpreted.
Although common breeds of the European rabbit (Oryctolagus cuniculus) may have a life expectancy of about five years23, experimental evidences show that metabolic and tissular alterations indicating the onset of senescence occur much earlier. For example, in rabbits aged 30 or less months, age-related degenerative changes have been detected in intervertebral disks24, lens25, and in the serum levels of fertility-associated trace elements26. Notably, changes in sexual behavior, as denoted by increased sexual exhaustion and impaired ejaculation27, as well as hypospermatogenesis28, have been shown to take place at around 20 months of age. Thus, other adverse, senescence-related modifications in the rabbit reproductive system, as in the erectile tissues of the penis, might also occur at these earlier, rather than later ages.
In our previous investigation on the rabbit penis, we showed that the CC of young adult animals has a high concentration of elastic fibers3, which is greater than the values that have been reported for humans14 and young adult rats16. This implies that, in rabbits, these fibrillar proteins play a particularly prominent role during erection and detumescence.
In spite of these facts, it is not known whether elastic fibers in the different regions of the rabbit penis change with age, and whether these fibers would also be susceptible to degenerative alterations due to early senescence, as described above. These issues have been addressed in the present investigation, in which the relative concentration of elastic fibers in the CC, CS, and TA were determined in rabbits aged from one month to two years.
Methods
The present experimental protocol follows the European Commission's Directive 86/609/EEC, and was reviewed and approved by the Ethics Committee for Animal Experimentation of the State University of Rio de Janeiro, Brazil.
Forty newborn rabbits (Oryctolagus cuniculus) of the New Zealand breed were obtained at the Veterinary School of the Federal Rural University of Rio de Janeiro, Brazil, and were randomly assigned to four groups of ten animals each (GI, GII, GIII, GIV). For the present study, the animals were kept under standard conditions, with food and water ad libitum, a 12-hour light:12-hour dark cycle, and environmental temperature control. They were then sacrificed, by pentobarbital overdose, at 30 (GI), 120 (GII), 240 (GIII), and 730 (GIV) days of age.
Tissue samples and histological techniques
Immediately after sacrifice, the whole penis was dissected and a 5 mm transverse segment from the mid shaft was excised and fixed in a 10% formalin solution prepared in phosphate buffered saline. "Ortrip" cleavage of this segment was then performed for stereology. This method consists of three random slice sections, of which the second section is orthogonal to the first one, and the third section is in turn orthogonal to the second one. Thus, isotropically uniform random sections are produced29. The samples were then routinely processed for paraffin embedding, after which 5-µm thick sections were obtained. Elastic fibers were evidenced by staining these sections with Weigert's resorsin fuchsin method, with a previous oxidation by oxone9. During assembly of elastic fibers, the amount of elastin that is embedded in the microfibrillar scaffold may differ. Thus, for a given tissue, there may exist different types of elastic fibers, which vary from those that contain nearly no elastin to those that consist mostly of this protein9. The Weigert-oxone technique as used herein stains all types of elastic fibers irrespective of the amount of elastin9. The specificity of the staining was further confirmed by immunolabeling tissue sections with an anti-elastin antibody (product code ab21610, polyclonal, Abcam, Cambridge, USA), as previously described16.
All tissue sections were photographed under the same conditions and at a final magnification of X400 using a digital camera (Olympus DP71, Tokyo, Japan) directly coupled to the microscope (Olympus BX51, Tokyo, Japan). The captured digital images had a resolution of 2040 X 1536 pixels and were stored in a TIFF file format.
Quantitation of elastic fibers
The relative content of elastic fibers in the different penile tissues was determined as volume fraction (Vv) using stereological methods29. Briefly, the histological images were first loaded into the ImageJ software version 1.46 (NIH, Bethesda, Maryland, USA), after which continuous areas of tissue in the CC, CS, or TA were manually outlined. These areas were defined as reference spaces, and they occupied as much as possible of the image and were of roughly similar sizes. Next, the "Grid_.class" plugin of ImageJ was used to create grids that were digitally superimposed on the captured tissue images. The mesh density of the grids was calculated so that a reference space would contain 200 points. The Vv of elastic fibers was then separately estimated in the different penile regions by point counting and expressed as percent of the reference space29. For each animal, ten sections were selected at intervals of 50 µm, and for each section, five random fields were analyzed.
Statistical analysis
The repeated measurements for each animal were used to calculate one individual mean, which was then used to calculate group means. Comparisons among group means by age or penile region were first carried out by one-way ANOVA. When statistical significance was found, pairwise planned comparisons were done using the Bonferroni method. Numerical results for stereological measurements are given as mean ± standard deviation, and statistical significance was considered when p<0.05.
Results
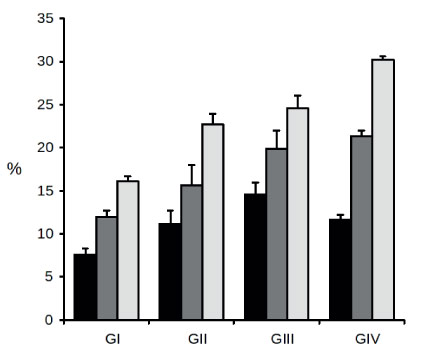
The relative content of elastic fibers in the TA, CC, and CS, in rabbits from 30 to 730 days old, presented an overall pattern of marked increase with age (Figure 1).
- Relative content of elastic fibers in the penis of rabbits at different ages. The amount of elastic fibers was determined as volume fraction (%) separately in the tunica albuginea (black bars), corpus cavernosum (dark gray bars), and corpus spongiosum (light gray bars) from rabbits aged 30 (GI), 120 (GII), 240 (GIII), and 730 (GIV) days. Bars represent mean and standard deviation from ten animals in each group. Refer to Tables 1 and 2 for statistical analysis of these results.
TABLE 1 - Statistical analysis, based on data from Figure 1, for the effect of age on the content of elastic fibers in different regions of the rabbit penis.
- Statistical analysis, based on data from Figure 1, comparing elastic fiber content in different regions of the rabbit penis.
This trend was shown to be significant both by ANOVA for all time points, and by comparing the youngest (GI) versus the eldest (GIV) age group (Table 1).
The amount of elastic fibers was determined as volume fraction separately in the tunica albuginea (TA), corpus cavernosum (CC), and corpus spongiosum (CS) from rabbits aged 30 (GI), 120 (GII), 240 (GIII), and 730 (GIV) days. For each pair of groups, "change" represents the percent change when comparing the mean of the second group against that of the first group, with positive and negative signs denoting an increase and a decrease, respectively. All age groups had ten animals each.
Accordingly, at 730 days of age, the Vv of elastic fibers was significantly increased by 54%, 78%, and 87% in the TA, CC, and CS, respectively, as compared with animals aged 30 days. Examples of these quite noticeable increases in elastic fiber content as a function of age can be seen in tissue sections from the CS of 30- (Figure 2A) and 730-day (Figure 2B) old animals.
FIGURE 2 - Elastic fibers (arrows) in the corpus spongiosum of the penis from 30 (A) and 730-day (B) old rabbits. Representative images of each age group, stained with Weigerts resorsin fuchsin with previous oxidation and photographed at a final magnification of 400X.
However, depending on penile structure, there were small differences in this trend of increasing elastic fiber content as a function of age. Thus, as shown in Figure 1 and Table 1, the contents increased steadily and significantly from 30 to 240 days of age in the TA (7.55 ± 0.73, 11.11 ± 1.62, and 14.55 ± 1.36) and CC (11.92 ± 0.80, 15.63 ± 2.33, and 19.84 ± 2.13). Thereafter, at 730 days of age, elastic fiber content tended to stabilize in the CC, as its Vv value (21.30 ± 0.68) was not significantly different from that of the preceding age group (19.84 ± 2.13), whereas in the TA the content was significantly decreased by 20% (11.67 ± 0.56), also with regard to 240-day old animals (14.55 ± 1.36). In the CS, on the other hand, there was a constant and significant increase from 30 to 730 days of age (16.15 ± 0.52, 22.69 ± 1.29, 24.61 ± 1.47, and 30.25 ± 0.34), as indicated by the pairwise comparisons in Table 1.
It is also noteworthy that, for every age group, the TA and CS consistently had the lowest and the highest concentration of elastic fibers, respectively, whereas the CC had always an intermediate content. These differences among penile regions are clearly noticeable in Figure 1 and they are all statistically significant for the four age groups, as shown by the analysis in Table 2.
The amount of elastic fibers was determined as volume fraction separately in the tunica albuginea (TA), corpus cavernosum (CC), and corpus spongiosum (CS) from rabbits aged 30 (GI), 120 (GII), 240 (GIII), and 730 (GIV) days. For each age group, the three penile regions were statistically analyzed, and the results are shown as p values. All age groups had ten animals each.
Discussion
The results of the present investigation demonstrated that the concentration of elastic fibers in the CC, CS, and TA of the rabbit penis undergoes a steady increase from 30 days of age, when the animals have not yet reached puberty, to the full adult age of eight months (240 days).
Similar growth-related modifications that start before puberty and advance until at least early adulthood or early senescence have been demonstrated in other reproductive organs of the rabbit. For example, even though sexual maturity in New Zealand rabbits is reached at five or six months of age30, testicular morphology continues to steadily change until animals are at least two years old28. The concentration of elastic fibers in the erectile tissues of the rabbit penis follows a similar pattern, as per our results, with ever-increasing values even after puberty. It should be pointed out that this pattern differs from that of humans, in whom the penile concentration of total collagen and total glycosaminglycans, other major components of the penile extracellular matrix, undergo little change in the TA and CC during a physiologically equivalent period31. Also, elastin expression by human skin fibroblasts remains unchanged from the fetal period to adulthood32. In rabbits, therefore, this continuous increase in elastic fiber content up until early in adulthood might be related to the fast onset of sexual maturity, which takes place well before the animal has reached full adult size.
Our results also showed that, in the time window from eight months (240 days) to two years (730 days) of age, elastic fiber content plateaued in the CC and even diminished in the TA. Although in the CS the concentration continued to augment during the time frame of our study, it is a reasonable presumption that elastic fibers would be subjected to similar alterations not much later, based on what we found in the TA and CC. It is noteworthy that these interruptions in the trend of gradual increase are roughly simultaneous with early and age-related degenerative alterations that affect intervertebral disks24, lens25, and fertility-associated trace elements26 of rabbits. But more importantly, the same study that described continuous changes in testicular structure of rabbits until an age of two years, also showed that, at this time point, hypospermatogenesis is already noticeable28. Indeed, impaired sexual behavior and reproduction are detectable in 20-month old rabbits27. Taken together, these published data strongly indicate that early alterations in the organization, function, and physiology of tissues due to senescence are already detectable in rabbits aged from 20 to 24 months. Consequently, our results showing a stabilization or a decrease in the contents of penile elastic fibers, succeeding a period of continuous increase, should likewise be an early alteration due to aging.
Experimental evidences from other animals and humans not only support this contention, but also highlight its implications. For example, in aged rats, degenerative changes have been observed in elastic fibers of the TA, and these could be associated with the lower intracavernosal pressures also found in these aged animals22. In humans, the concentration of elastic fibers in the TA decreases with age18, which is consistent with a reduction in the extensibility33 and elasticity34 of the penis in aged individuals. Although a direct causative role for elastic fibers in erectile dysfunction cannot be established yet, it has been shown nonetheless that their content is decreased in the TA from impotent men15,18. Additionally, in patients with venogenic erectile dysfunction, in whom venous leakage resulting from deficient tension of the TA is thought to preclude normal erection, elastic fibers are diminished or are even undetected in this region of the penis19. It should be noted also that, in erectile dysfunction patients at around 44 years of age, elastic fibers in the CC are fragmented35.
These previously published data, therefore, further support the hypothesis whereby the stabilization and decrease we found in elastic fiber content in the CC and TA, respectively, in the time period spanning from adulthood to early senescence, are the initial phases of an age-related degenerative process that would lead to less and/or altered fibers. Notably, these data also imply that these alterations are likely to be associated with erectile dysfunction. Nevertheless, detailed functional studies would be required to ascertain whether the early changes we observed in the elastic fibers of the rabbit penis would have a negative impact on erection and sexual function.
Our findings, as discussed above, should be important not only for providing information on the reproductive biology of the rabbit, but also because it extends the usefulness and applicability of the rabbit penis as a model for studies on penile function and disorders, including erectile dysfunction. Additionally, for investigations about the effects of aging, and especially of early senescence, on erectile tissues of the rabbit, our data would allow a reduction in the period of animal housing in the laboratory.
The cellular and molecular mechanisms underlying the degenerative structural and functional changes associated with aging are not yet well understood. Intensive research over the past few decades, however, has clearly shown that aging is a process resulting from a complex array of multifactorial events. Accumulation of DNA damage and the ensuing adverse modification of gene expression is widely regarded as the hallmark of cellular aging36. For example, and in agreement with our findings, elastin gene expression by human skin fibroblasts declines in aged individuals as compared with young adults33. But major causes of age-related degenerative changes also involve factors that act extracellularly. In the specific case of elastic fibers, it has been shown that accumulation of matrix metalloproteinase 2, an elastin-degrading enzyme, is greatly increased near broken elastic fibers in the arteries of aged rats37.
Another factor that might play a role in the degenerative changes penile elastic fibers undergo with age is the non-enzymatic glycation of proteins. In a study using human penile samples from autopsy and surgery of non-diabetic individuals, it was found that the concentration of the advanced glycation end-product pentosidine in the CC and TA increases continuously from puberty to 90 years of age or more38. This study also revealed that the amount of pentosidine was greater in the TA compared with the CC. Higher levels of pentosidine have been associated with tissue modifications, including loss of integrity of collagen fibers in bones from aged humans39. Additionally, pentosidine itself can bind to, and modify, aortic elastin40. Therefore, non-enzymatic glycation and/or pentosidine might be yet another factor adversely affecting the organization of penile elastic fibers in two-year old rabbits, especially in the TA.
It was also evident from our results that, for every age group, the TA and CS consistently had the lowest and the highest concentration of elastic fibers, respectively, whereas the CC had always an intermediate content. A low content in the TA makes it less extensible, and this is in line with its main function as a limiter4of cavernosal expansion. As expected, the CC and CS, which undergo inflation and reversible expansion during erection, have the highest concentration of elastic fibers. On the other hand, the comparatively lower content of elastic fibers in the CC should make it less resilient. This is consistent, however, with biomechanical experiments which indicate that, in rabbits, the CC, and not the TA, is the major structure limiting penile expansion4.
Conclusions
The different regions of the rabbit penis contain diverse concentrations of elastic fibers, which reflect the different functional properties of penile tissues. Although after one month of age there is a gradual increase in these concentrations, in two-year old animals this trend is interrupted, which suggests that this could be an early alteration due to senescence.
Received: January 26, 2013
Review: March 25, 2013
Accepted: April 24, 2013
Conflict of interest: none
Financial sources: National Council of Technological and Scientific Development (CNPq), Rio de Janeiro Research Foundation (FAPERJ) and Coordination of Improvement for Higher Academic Staff (CAPES)
- 1. McMurray G, Casey JH, Naylor AM. Animal models in urological disease and sexual dysfunction. Br J Pharmacol. 2006;147(Suppl 2):S62-79.
- 2. Yesilli C, Yaman O, Anafarta K. Effect of experimental hypercholesterolemia on cavernosal structures. Urology. 2001;57:1184-8.
- 3. Maia RS, Babinski MA, Figueiredo MA, Chagas MA, Costa WS, Sampaio FJ. Concentration of elastic system fibers in the corpus cavernosum, corpus spongiosum and tunica albuginea in the rabbit penis. Int J Imp Res. 2006;18:121-5.
- 4. Udelson D. Biomechanics of male erectile function. J R Soc Interface. 2007;4:1031-47.
- 5. Ozgel O, Dursun N, Cengelci A, Ates S. Arterial supply of the penis in the New Zealand rabbit (Oryctolagus cuniculus L.). Anat Histol Embryol. 2003;32:6-8.
- 6. Sáenz de Tejada I, Angulo J, Cellek S, González-Cadavid N, Heaton J, Pickard R, Simonsen U. Physiology of erectile function. J Sex Med. 2004;1:254-65.
- 7. Bossart MI, Spjut HJ, Scott FB. Ultrastructural analysis of human penile corpus cavernosum. Its significance in tumescence and detumescence. Urology. 1980;15:448-56.
- 8. Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today. 2007;81:229-40.
- 9. Montes GS. Structural biology of the fibres of the collagenous and elastic systems. Cell Biol Int. 1996;20:15-27.
- 10. Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med. 2006;8:1-23.
- 11. Hsu GL, Brock G, von Heyden B, Nunes L, Lue TF, Tanagho EA. The distribution of elastic fibrous elements within the human penis. Br J Urol. 1994;73:566-71.
- 12. Bastos AL, Silva EA, Costa WS, Sampaio FJ. The concentration of elastic fibers in the male urethra during human fetal development. BJU Int. 2004;94:620-3.
- 13. Costa WS, Carrerete FB, Horta WG, Sampaio FJ. Comparative analysis of the penis corpora cavernosa in controls and patients with erectile dysfunction. BJU Int. 2006;97:567-9.
- 14. Sattar AA, Wespes E, Schulman CC. Computerized measurement of penile elastic fibres in potent and impotent men. Eur Urol. 1994;25:142-4.
- 15. Iacono F, Barra S, de Rosa G. Microstructural disorders of tunica albuginea in patients affected by impotence. Eur Urol. 1994;26:233-7.
- 16. Pinheiro AC, Costa WS, Cardoso LE, Sampaio FJ. Organization and relative content of smooth muscle cells, collagen and elastic fibers in the corpus cavernosum of rat penis. J Urol. 2000;164:1802-6.
- 17. Babinski MA, deBrito-Gitirana L, Chagas MA, Abidu-Figueiredo M, Costa WS, Sampaio FJ. Immunohistochemical analysis of smooth muscle cells and volumetric density of the elastic system fibers of wild boar (Sus scrofa) penis. Anim Reprod Sci. 2005;86:317-28.
- 18. Akkus E, Carrier S, Baba K, Hsu GL, Padma-Nathan H, Nunes L, Lue TF. Structural alterations in the tunica albuginea of the penis: impact of Peyronie's disease, ageing and impotence. Br J Urol. 1997;79:47-53.
- 19. Shafik A, Shafik I, El Sibai O, Shafik AA. On the pathogenesis of penile venous leakage: role of the tunica albuginea. BMC Urol. 2007;7:14.
- 20. Shen ZJ, Jin XD, Chen ZD, Shi YH. Effect of aging on penile ultrastructure. Asian J Androl. 2001;3:281-4.
- 21. Jiang R, Chen JH, Jin J, Shen W, Li QM. Ultrastructural comparison of penile cavernous tissue between hypertensive and normotensive rats. Int J Impot Res. 2005;17:417-23.
- 22. Calabrò A, Italiano G, Pescatori ES, Marin A, Gaetano O, Abatangelo G, Abatangelo G, Pagano F. Physiological aging and penile erectile function: a study in the rat. Eur Urol. 1996;29:240-4.
- 23. Holst D, Hutzelmeyer H, Kaetzke P, Khaschei M, Rödel HG, Schrutka H. Social rank, fecundity and lifetime reproductive success in wild European rabbits (Oryctolagus cuniculus). Behav Ecol Sociobiol. 2002;51:245-54.
- 24. Leung VY, Hung SC, Li LC, Wu EX, Luk KD, Chan D, Cheung KM. Age-related degeneration of lumbar intervertebral discs in rabbits revealed by deuterium oxide-assisted MRI. Osteoarthritis Cartilage. 2008;16:1312-8.
- 25. Al-Khudari S, Donohue ST, Al-Ghoul WM, Al-Ghoul KJ. Age-related compaction of lens fibers affects the structure and optical properties of rabbit lenses. BMC Ophthalmol. 2007;7:19.
- 26. Fayed AH. Serum and testicular trace element concentration in rabbits at different ages. Biol Trace Elem Res. 2010;134:64-7.
- 27. Villagran C, Navarro J, Fuentes VO. Sexual exhaustion in White New Zealand male rabbits of different ages. Anim Reprod Sci. 2003;76:251-5.
- 28. Tsunenari I, Kast A. Developmental and regressive changes in the testes of the Himalayan rabbit. Lab Anim. 1992;26:167-79.
- 29. Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379-94.
- 30. Macari M, Machado C. Sexual maturity in rabbits defined by the physical and chemical characteristics of the semen. Lab Anim. 1978;12:37-9.
- 31. Bastos AL, Sampaio FJ, Cardoso LE. Compositional changes of collagen and glycosaminoglycans in the tunica albuginea and corpus cavernosum from the human penis during the fetal and postnatal periods. J Urol. 2005;173:1039-43.
- 32. Fazio MJ, Olsen DR, Kuivaniemi H, Chu ML, Davidson JM, Rosenbloom J, Uitto J. Isolation and characterization of human elastin cDNAs, and age-associated variation in elastin gene expression in cultured skin fibroblasts. Lab Invest. 1988;58:270-7.
- 33. Moreira de Goes P, Wespes E, Schulman C. Penile extensibility: to what is it related? J Urol. 1992;148:1432-4.
- 34. Bondil P, Costa P, Daures JP, Louis JF, Navratil H. Clinical study of the longitudinal deformation of the flaccid penis and of its variations with aging. Eur Urol. 1992;21:284-6.
- 35. Shafik A, Ahmed I, El Sibai O, Shafik AA. The hypoactive corpora cavernosa with degenerative erectile dysfunction: a new syndrome. BMC Urol. 2006;6:13.
- 36. Marques FZ, Markus MA, Morris BJ. The molecular basis of longevity, and clinical implications. Maturitas. 2010;65:87-91.
- 37. Li Z, Froehlich J, Galis ZS, Lakatta EG. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension. 1999;33:116-23.
- 38. Jiaan DB, Seftel AD, Fogarty J, Hampel N, Cruz W, Pomerantz J, Zuik M, Monnier VM. Age-related increase in an advanced glycation end product in penile tissue. World J Urol. 1995;13:369-75.
- 39. Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1-7.
- 40. Sakata N, Noma A, Yamamoto Y, Okamoto K, Meng J, Takebayashi S, Nagai R, Horiuchi S. Modification of elastin by pentosidine is associated with the calcification of aortic media in patients with end-stage renal disease. Nephrol Dial Transplant. 2003;18:1601-9.
Publication Dates
-
Publication in this collection
21 May 2013 -
Date of issue
May 2013
History
-
Received
26 Jan 2013 -
Accepted
24 Apr 2013 -
Reviewed
25 Mar 2013