Abstract
PURPOSE: To evaluate the bone repair process in ovariohysterectomized rabbit submitted to an ovarian transplant to stomach that may supplying some quantity of estrogen occurs to improve bone healing. METHODS: In 20 female rabbits three holes of 1, 2 and 3mm diameter in tibial shaft were made and after that all animals received OHE through a ventral incision and they were randomly divided into two groups of ten rabbits each. In group one, animals received one of their self-ovaries that transplanted on serosal layer of stomach and group two did not receive treatment. Animals were kept during bone healing for a period of 45 days and radiological, biochemical, biomechanical and histopathological evaluation. RESULTS: The tibial defects in group one healed completely after 45 days and had more callous than second group. There is significant difference between two groups after operation in 21, 28 and 35 days about estrogen, progesterone and phosphatase Alkaline. The maximum forces in group one, were significantly higher than that for the group two. CONCLUSION:Ovarian transplantation prevents the effects of ovariohysterectomized on bone healing of rabbit tibia, suggesting that unilateral transplanted ovaries can substitute for the action of ovaries on the skeleton in ovariohysterectomized animals.
Hysterectomy; Ovary; Organ Transplantation; Rabbits
2 ORIGINAL ARTICLE
WOUND HEALING
Experimental ovarian transplantation on stomach for bone repair in ovariohysterectomized rabbits1 1 Research performed at Department of Surgery, Faculty of Specialized Veterinary Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Gholam Reza AbediI; Amir SotoudehII; Ali BazzazanIII; Amin GanjaiIV
IAssociate Professor, Department of Surgery, Faculty of Specialized Veterinary Sciences, Science and Research Branch, Islamic Azad University,
Tehran, Iran. Design, supervised all phases of the study
IIAssistant Professor, Faculty of Veterinary Science, Kahnooj Branch, Islamic Azad University, Kerman, Iran. Analysis and interpretation of data,
manuscript writing
IIIAssistant Professor, Faculty of Veterinary Science, Garmsar Branch, Islamic Azad University, Semnan, Iran. Technical procedures
IVAssistant Professor, Faculty of Experimental Science, Kahnooj Branch, Islamic Azad University, Kerman, Iran. Analysis and interpretation of data
Correspondence Correspondence: Amir Sotoudeh Islamic Azad University Kahnooj Branch Kahnooj, Iran Phone: 00989121768066 Fax: 00983495230203 dramirsotoudeh@kahnoojiau.ac.ir
ABSTRACT
PURPOSE: To evaluate the bone repair process in ovariohysterectomized rabbit submitted to an ovarian transplant to stomach that may supplying some quantity of estrogen occurs to improve bone healing.
METHODS: In 20 female rabbits three holes of 1, 2 and 3mm diameter in tibial shaft were made and after that all animals received OHE through a ventral incision and they were randomly divided into two groups of ten rabbits each. In group one, animals received one of their self-ovaries that transplanted on serosal layer of stomach and group two did not receive treatment. Animals were kept during bone healing for a period of 45 days and radiological, biochemical, biomechanical and histopathological evaluation.
RESULTS: The tibial defects in group one healed completely after 45 days and had more callous than second group. There is significant difference between two groups after operation in 21, 28 and 35 days about estrogen, progesterone and phosphatase Alkaline. The maximum forces in group one, were significantly higher than that for the group two.
CONCLUSION:Ovarian transplantation prevents the effects of ovariohysterectomized on bone healing of rabbit tibia, suggesting that unilateral transplanted ovaries can substitute for the action of ovaries on the skeleton in ovariohysterectomized animals.
Key words:Hysterectomy. Ovary. Organ Transplantation. Rabbits.
Introduction
Considering that the removal of the ovaries can prevent complications such as breast tumors, and also asked the owner's to prevent pregnancy and oestrus animals comes1, the ovary is the major source of estrogen that is involved in the bone remodeling process, being responsible for the balance between resorption and bone formation2.
The effects of estrogen deficiency lead to reduced bone matrix, osteoblastic activity and loss of calcium and phosphate deposition in bone3,4. Hormone replacement therapy can prevent unintended bone healing delay; but the drugs have certain side effects with long-term use5 such as increasing risk of gallbladder disease and breast cancer6.
After ovariectomy, the absorption of bone prevails over osteogenesis and the fracture healing is poor in quality7,8. This might reflect a failure to reproduce certain aspects of gonadal function. Auto transplantation of an ovary to stomach wall, which drained exclusively by the portal vein may prevents this complication. Circulating estradiol levels are inadequate to initiate estrus but may be sufficient to prevent the effects of estrogen deficiency after OHE.
The aim of this study was to evaluate the bone repair process in ovariohysterectomized rabbit submitted to an ovarian transplant to stomach that may supplying some quantity of estrogen occurs to improve bone healing.
Methods
The experimental protocol used was reviewed and approved by Kahnooj Institutional Animal Care and Use Committee; 20 female rabbits of New Zealand white strain were used in the present study. All animals had free access to food and water and were individually housed in a 12 hours light-dark cycle.
All animals were anesthetized by intramuscular injection of xylazine (5 mg/Kg of body weight) and ketamine hydrochloride (30 mg/Kg of body weight) and under sterile conditions 5 cm incision was made on the proximal-anterior part of right tibia. The periosteum was elevated with a periosteal elevator and retained by a selfretaining retractor then three holes of 1, 2 and 3mm diameter in tibial shaft were made with a hand drill. The holes pass through the both cortex. Physiological saline solution was used during drilling to prevent overheating. After that all animals received OHE through a ventral incision and they were randomly divided into two groups of ten rabbits each.
In group I; animals received one of their self-ovaries that transplanted on serosal layer of stomach body without vascular anastomoses9. Group II did not receive treatment.
Antibiotic prophylaxis with cefonicid sodium (100 mg/kg) was administered before and during the 4 days following surgery to minimize any complications. For pain prophylaxis during the initial 48 h postoperatively four injections of carprofen (4 mg/kg) were administrated.
Animals were kept during bone healing for a period of 45 days. Process of holes filling with callus formation was evaluated on radiographs taken on days zero, 15 and 30 and 45. Radiographs in the lateral views were taken of all tibias in the Faxitron Cabinet X-ray System (43855A, Hewlett-Packard, IL, USA). A high-resolution film and 40 KV/6 min radiation was used. The description and evaluation of the fracture healing was performed in a blinded manner for all the test groups.
Radiographic Evaluation was scored as follows: 0, no callus formation; 1, filling 1mm holes; 2, filling 2mm holes; 3, filling 3mm holes with callus formation.
Blood samples were taken from the marginal veil of rabbits. Estrogen, progesterone, and alkaline phosphatase were analyzed on days zero (before surgery), seven, 14, 21, 28, 35 and 45 days.
The rabbits were euthanized at 45 days post operation; each tibia was placed on the breaking test. Before testing, the specimens were thawed and all musculature was carefully removed so that the bony structures were not damaged. The tibias were continuously moistened with isotonic saline solution. For biomechanical analysis we used the bending test machine (7100L, Gotech, Thaiwan). Maximum load is a widely used parameter for mechanical evaluation, and represents the maximum force that a material can sustain before failure10.
The measuring range was from 2 to 200 Newton (N) at a relative accuracy of 0.2% at 0.4% nominal force. A primary force of 1N to fix the tibia on the device was chosen. The software (Test Xpert, Gotech, Thaiwan) continuously recorded the force. The investigation was manually stopped by the first linear displacement visibly to prevent damage or fracture of the callus formation. The software program indicated the maximum load. This procedure was performed in a blinded manner with regard to the test groups.
For histopathological analysis, all specimens were fixed with 10% neutral buffered formaldehyde (pH 7.2) for 72 h and decalcified with 15% neural EDTA for three weeks. Each bone defect area was sagittally sectioned perpendicular to the defect and dehydrated in a series of concentrations of alcohol (80% up to absolute). The samples were embedded in paraffin and sectioned at 5 μm thickness. The sections were stained with hematoxylin and eosin. Assessment was performed by a blinded assessor according Table 1.
Results
No sign of inflammation was seen in all animals.
The tibial defects of all ten rabbits in group I healed completely after 45 days.
In relation to constitute callous in both groups, there are meaningful variations in 30 and 45 day and group I had more callous than group II (Figure 1) (Table 2).
There is significant difference between two groups after operation in 21, 28 and 35 days about estrogen and in 21th day to the end of the research period about progesterone. About phosphatase Alkaline, there are meaningful differences between two groups in 14, 21, 28 and 35 days (Table 3).
To prevent damage of the callus formation, the maximum forces were calculated when assessor can see the first linear displacement of samples. The maximum forces in group I (182.80 ± 15.38 N) were significantly higher than that for the group II (134 ± 14.85 N) (Table 4).
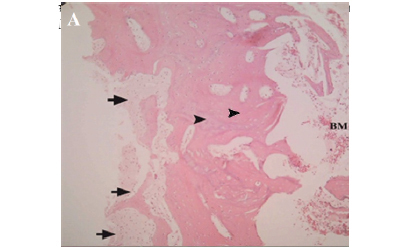
Pathological evaluations showed that the osseous trabecula became thinner and approximately disrupted in group II, whereas in group I it became massive, thicker and closer to normal (Figure 2A) and haversian systems had been formatted in new bone (Figure 2B). In group II the outer cortex had not been formatted completely (Figure 3A) and connective tissue was observed in the outer cortex and the bone healing parameters were significantly lower than group I (Table 5).
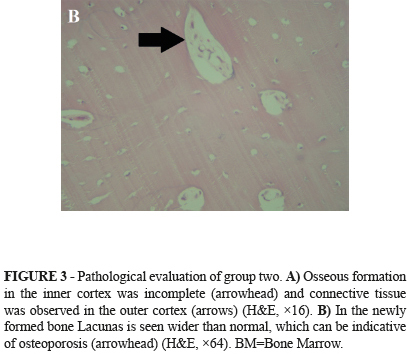
In the newly formed bone Lacunas is seen wider than normal, which can be indicative of osteoporosis (Figure 3B). Transplanted ovary and stomach both seem healthy and no inflammatory reaction was seen.
Discussion
Ovariohysterectomy is the safest and most effective treatment for pyometra11,12 and it is a radical treatment for postoestrus metritis when it recurs following failure of medical treatment and also that is, sterilisation, as many owners complain of the manifestations of heat with vulvular discharge, as well as the problems associated with repeated mattings13 and finally, ovariohysterectomy is a tool used for combating pet overpopulation14.
The bone remodeling function is always in balance and depends on systemic and local factors. Amongst systemic factors, estrogen is probably the most important hormone responsible for the maintenance of normal bone turnover15, after elimination of ovary, the absorption of bone prevails over osteogenesis and bone loss occurs as soon as 1 month after OHE7.
Xu et al.16 reported a reduction in callus and bone mineral density in femoral fracture healing after Ovariectomy in rats, also osteoporotic changes occur and healing of fractures was poor quality. Similarly in this study in group II after 45 days in newly formed bone the lacunas were much wider than normal which could be indicative of osteoporosis.
In a study ovariectomized rabbits were examined with a combination of estradiol and progesterone for a period of 42 days. Results indicated that estradiol and progesterone prevent bone loss by either inhibited reducing the number of osteotrabecula and bone callus18.
In research transplanted allogeneic ovaries secreted estrogen at normal levels. Furthermore, bone loss was prevented to a certain extent19. In our study, group II showed significantly reduction of estrogen whereas in group I this reduction was very low, especially on 21 to 35 days after transplantation. At the end of 45 days, estrogen was less than normal, as the day zero, but it was significantly higher to compare the group II.
Tobias et al.20 investigated whether renal capsular or subcutaneous ovarian transplants prevent the effects of ovariectomy on histomorphometric indices of tibiae and concluded that ovarian transplantation largely prevents the effects of ovariectomy such as reduction in cancellous bone volume. We investigated unilateral ovarian auto transplantation on serosal layer of stomach to prevent the effects of ovarian hormones elimination on bone healing after OHE. Circulating estradiol levels after transplantation of the ovary to the portal vein drainage area, which is partially metabolized by the liver, are inadequate to initiate estrus but may be sufficient to supplying some quantity of estrogen to improve bone healing on ovariohysterectomized animals. Our results showed that ovarian transplantation led to a better remodeling of regenerated bone as measured by radiologic, histologic and biomechanical analysis and the process of bone repair in the ovarian transplanted group was relatively better than the control group.
Compression testing is recommended for testing the mechanical properties of bone21. Qiao et al.7 demonstrated that on 50 days after ovariectomy in rats, tibia fracture healing and biomechanical strength is 24% and 22% lesser than normal rats. We used load presser on tibia and biomechanics results showed that the strength of specimens in group I is higher than the group II, so ovarian transplantation could be effective to bone strength.
The transplanted ovary tissues under the renal capsules of mice were accepted without using immunosuppressants19, similarly in the present study any signs of rejection or inflammatory was not seen.
Gallagher et al.17 compared the effects of administering estradiol to osteopenic ovariectomized rats with those of ovarian transplantation. Both groups largely restored indices of estrogenic exposure. Animals receiving ovarian transplants also showed a small increase in serum progesterone.
In this study progesterone were significantly different between two groups on 21 to 45 days.
Alkaline phosphatase had increased in both groups after day zero, which would be specifically due to bone damage, but it was significantly different between groups on 14 to 35 days, this reason was not clear.
Conclusion
The ovarian transplantation prevents the effects of ovariohysterectomized on bone healing of rabbit tibia, suggesting that unilateral transplanted ovaries can substitute for the action of ovaries on the skeleton in OHE animals.
Received: February 20, 2013
Review: April 18, 2013
Accepted: May 17, 2013
Conflict of interest: none
Financial source: Islamic Azad University
- 1. Bencharif D, Amirat L, Garand A, Tainturier D. Ovariohysterectomy in the bitch. Obstet Gynecol Int. 2010;2010:542693.
- 2. Belsito KR, Vester BM, Keel T. Impact of ovariohysterectomy and food intake on body composition, physical activity, and adipose gene expression in cats. J Anim Sci. 2009;2:594-602.
- 3. Chow J, Tobias JH, Colston KW. Estrogen maintains trabecular bone volume in rats not only by suppression of bone resorption but also by stimulation of bone formation. J Clin Invest. 1992;1:74-8.
- 4. Malluche HH, Faugere MC, Rush M. Osteoblastic insufficiency is responsible for maintenance of osteopenia after loss of ovarian function in experimental beagle dogs. Endocrinology. 1986;6:2649-54.
- 5. Tezval M, Biblis M, Sehmisch S, Schmelz U, Kolios L, Rack T, Stuermer KM, Stuermer EK. Improvement of femoral bone quality after low-magnitude, high-frequency mechanical stimulation in the ovariectomized rat as an osteopenia model. Calcif Tissue Int. 2011;88:3340.
- 6. Ottesen B, Pedersen AT. Physiological effects of ovarian hormones: clinical aspects and compliance. Eur Heart J. 1996;17:20-6.
- 7. Qiao L, Xu KH, Liu HW. Effects of ovariectomy on fracture healing in female rats. Sichuan Da Xue Xue Bao Yi Xue Ban. 2005;36(1):108-11.
- 8. Yong H, Cheng Y. The impacts of sex hormones on histomorphometric and histological appearances of bone in ovariectomized rats. Zhonghua Fu Chan Ke Za Zhi.1999;34(2):90-3.
- 9. Petroianu A, Alberti LR, Vasconcellos LS. Allogeneic ovarian orthotopic transplantation in rabbits without a vascular pedicle: morphological, endocrinologic, and natural pregnancy assessment. Transplant Proc. 2006;9:3092-3.
- 10. Baofeng L, Zhi Y, Bei C, Guolin M, Qingshui Y, Jian L. Characterization of a rabbit osteoporosis model induced by ovariectomy and glucocorticoid. Acta Orthop. 2010;81(3):396-401.
- 11. Slatter D. Textbook of small animal surgery. 3rd ed. Philadelphia: Elsevier; 2003.
- 12. Pelander L, Hagman R, Haggstrom J. Concentrations of cardiac Troponin I before and after ovariohysterectomy in 46 female dogs with pyometra. Acta Vet Scand. 2008;50:35.
- 13. Concannon PW. Meyers VN. Current and proposed methods for contraception and termination of pregnancy in dogs and cats. J Am Vet Med Assoc. 1991;7:1214-25.
- 14. Ekici H, Sontas BH, Toydemiri TSF. Effect of prepubertal ovariohysterectomy on bone mineral mineral density and bone mineral content in puppies. Acta Vet Hung. 2005;53(4):469-78.
- 15. Raisz LG. Physiology and pathophysiology of bone remodeling. Clin Chem. 1999;8:1353-8.
- 16. Xu SW, Yu R, Hao GF. Early period of fracture healing in ovariectomized rats. Chin J Traumatol. 2003;6(3):160-6.
- 17. Gallagher AC, Chambers TJ, Obias JH. Distinct effects of ovarian transplantation and exogenous 17 beta-oestradiol on cancellous bone of osteopenic ovariectomized rats. Eur J Endocrinol. 1995;133(4):483-8.
- 18. Gallagher JC, Kable WT, Goldgar D. Effect of Progestind therapy on cortical and trabecular bone: comparison with estrogen. Am J Med. 1991;90:171-8.
- 19. Feng W, Cui Y, Song C. Prevention of osteoporosis and hypogonadism by allogeneic ovarian transplantation in conjunction with intra-bone marrow-bone marrow transplantation. Transplantation. 2007;11:1459-66.
- 20. Tobias JH, Chambers TJ, Gallagher A. The effects of ovarian transplantation on bone loss in ovariectomized rats. J Endocrinol. 1994;1:187-92.
- 21. Adinoff AD, Hollister JR. Steroid-induced fractures and bone loss in patients with asthma. N Engl J Med. 1983;5:265-8.
Publication Dates
-
Publication in this collection
05 June 2013 -
Date of issue
June 2013
History
-
Received
20 Feb 2013 -
Accepted
17 May 2013 -
Reviewed
18 Apr 2013