Abstract
PURPOSE: To evaluate the effect of bioflavonoid ternatin (TRT) on rat liver regeneration and oxidative stress after 70% partial hepatectomy (PH). METHODS: Thirty six young male Wistar rats were randomly assigned to two groups of 18 animals each - control (G1) and experimental (G2) - and were submitted to PH under inhalatory diethylether anesthesia. G1 rats received daily intraperitoneal (ip) injections of saline (NaCl 0.9% solution) 0.1 mL/kg for 14 days; G2 animals received daily ip injections of TRT 0.1% 1.0mg/kg for 14 days. At 36h (T1), 168h (T2) and 336h (T3) post-PH timepoints, a subgroup of six rats in each group was chosen in a randomized way to complementary hepatectomy (CH) and blood samples haversting. Collected material was saved for laboratory analysis (total bilirubin (TB), D-Glucose, glutathione (GSH) and thiobarbituric acid reactive substances (TBARS) and assessment of liver regeneration. RESULTS: TRT induced a significant decrease in liver and plasma GSH concentrations; liver regeneration process was not affected. TRT promoted a significant decrease in blood glucose levels 168h after partial hepatectomy compared with controls. TB levels remained unchanged. CONCLUSION: Intraperitoneal bioflavonoid ternatin injection in partially hepatectomized rats induces a decrease in oxidative stress and a significant hypoglycemic state, but does not promote any change in the evolution of liver regeneration.
Hepatectomy; Oxidative Stress; Liver Regeneration; Flavonoids; Rats
6 - ORIGINAL ARTICLE
EFFECTS OF DRUGS
Effects of bioflavonoid ternatin on liver regeneration and oxidative stress in rats1 1 Research performed at Experimental Surgery Laboratory (LABCEX), Faculty of Medicine, Federal University of Ceara (UFC), Fortaleza-CE, Brazil. Part of PhD degree thesis, Postgraduate Program in Surgery, UFC. Tutor: Prof. Paulo Roberto Leitão Vasconcelos.
José Ulisses de Souza MeloI; Radamés Bezerra MeloII; Jefferson Menezes Viana SantosIII; Manoel Messias Campos JúniorIII; Sérgio Botelho GuimarãesIV; Paulo Roberto Leitão VasconcelosV
IPhD, Associate Professor, Department of Surgery, UFC, Ceara, Brazil. Conception and design of the study, technical procedures, acquisition of data
IIFellow Master degree, Postgraduate Program in Surgery, Department of Surgery, UFC, Ceara, Brazil. Technical procedures, acquisition of data
IIIGraduate student, UFC, Ceara, Brazil. Animal care, application and maintenance of inhalatory anesthesia, post-operative animal care
IVPhD, Associate Professor, Department of Surgery. Head, LABCEX, UFC, Ceara, Brazil. Manuscript writing and critical revision
VPhD, Associate Professor. Coordinator, Postgraduate Program in Surgery, Department of Surgery, UFC, Ceara, Brazil. Conception, design, intellectual and scientific content of the study, final approval of manuscript
Correspondence Correspondence: Prof. Paulo Roberto Leitão de Vasconcelos Rua Professor Costa Mendes, 1608/ 3º andar 60430-140 Fortaleza CE Brasil Tel.: (55 85)3366-8083 Fax: (55 85)3366-8064 paulo.vasconcelos@ufc.br
ABSTRACT
PURPOSE: To evaluate the effect of bioflavonoid ternatin (TRT) on rat liver regeneration and oxidative stress after 70% partial hepatectomy (PH).
METHODS: Thirty six young male Wistar rats were randomly assigned to two groups of 18 animals each control (G1) and experimental (G2) and were submitted to PH under inhalatory diethylether anesthesia. G1 rats received daily intraperitoneal (ip) injections of saline (NaCl 0.9% solution) 0.1 mL/kg for 14 days; G2 animals received daily ip injections of TRT 0.1% 1.0mg/kg for 14 days. At 36h (T1), 168h (T2) and 336h (T3) post-PH timepoints, a subgroup of six rats in each group was chosen in a randomized way to complementary hepatectomy (CH) and blood samples haversting. Collected material was saved for laboratory analysis (total bilirubin (TB), D-Glucose, glutathione (GSH) and thiobarbituric acid reactive substances (TBARS) and assessment of liver regeneration.
RESULTS: TRT induced a significant decrease in liver and plasma GSH concentrations; liver regeneration process was not affected. TRT promoted a significant decrease in blood glucose levels 168h after partial hepatectomy compared with controls. TB levels remained unchanged.
CONCLUSION: Intraperitoneal bioflavonoid ternatin injection in partially hepatectomized rats induces a decrease in oxidative stress and a significant hypoglycemic state, but does not promote any change in the evolution of liver regeneration.
Key words: Hepatectomy. Oxidative Stress. Liver Regeneration. Flavonoids. Rats.
Introduction
The liver exhibits a remarkable regenerative capacity after tissue damage, including partial hepatectomy (PH)¹. Following removal of the two major lobes of the rat liver (70% partial hepatectomy, Higgins-Anderson partial hepatectomy), the remaining minor lobes rapidly undergo an essentially hyperplastic response to repopulate loss of tissue and cells² attaining its original size by three to 14 days2-4.
Reactive oxygen species (ROS), antioxidant substances and lipid peroxidation have been implicated in control mechanisms of cellular growth and proliferation5-7. The administration of exogenous antioxidants such as a-tocopherol (vitamin E) and reduced gluthatione (GSH) retards liver regeneration7,8. Moreover, several studies have reported the genesis and/or formation of free radicals as an important factor on the liver regeneration phenomenon, necessary to its natural outcome6,9.
Flavonoids are claimed to have protective effects against free radicals induced lipid peroxidation of living cell membranes10. Ternatin (TRT), 4'.5-dihydroxy-3.3',7.8-tetramethoxyflavone, a natural antioxidant, was evaluated under the classical experimental model of partial hepatectomy due to Higgins and Anderson¹ to assess its effects on rat liver regeneration and oxidative stress.
TRT was isolated from the flowering tops of Egletes viscosa L. (Asteraceae)11, popularly known as "macela da terra", which is a small empirical medicinal herb that grows abundantly in northeast of Brazil and others areas of South America. Previous studies have demonstrated anti-inflammatory, antianaphylactic, antihrombotic and antihepatotoxic properties of TRT12-14; these pharmacological effects have been related to its free radical scavenging and antioxidant properties15.
Methods
All procedures were approved by the Commission of Ethics on Animal Research (now Committee of Ethics on the Use of Animals (CEUA), Federal University of Ceara, protocol 14/06 on August 11, 2006. Surgical procedures and animal handling were conducted in accordance with the Brazilian Federal Law No. 11794 of October 8, 2008 (http://www.planalto.gov.br/ccivil_03/_Ato2007-2010/2008/Lei/L11794.htm). Rats were raised under controlled conditions, housed in polypropylene cages at temperature of 23+4ºC on a light schedule of 12h light/dark cycle and fed regular rat chow and potable water ad libitum. Thirty six young (70+10 days) male Wistar rats weighting 100-235g were included in the study and randomly assigned to two groups (n=18) control (G1) and experiment (G2). All rats were submitted to the classical Higgins-Anderson 70% partial hepatectomy¹ (PH). G1 rats received daily intraperitoneal (ip) injections of saline (NaCl 0.9% solution) 0.1 mL/kg for 14 days; G2 animals received daily ip injections of TRT 0.1% 1.0mg/kg for 14 days. At 36h (T1), 168h (T2) and 336h (T3) post-PH, a subgroup of six rats in each group was chosen in a randomized way to complementary hepatectomy (CH) and blood samples haversting. All surgical procedures were performed under inhalatory diethylether anesthesia. Collected material was stored for laboratory analysis
Thiobarbituric acid reactive substances (TBARS), glutathione (GSH), glycemia and total bilirubin (TB) were assayed. Tissue samples were snap-frozen in liquid nitrogen and stored in glass tubes at -70ºC until subsequent preparation and analysis of liver tissue homogenate. Plasma samples were obtained from heparinized blood after 10 minutes of refrigerated centrifugation (4.000 rotations/minute) and were stored as well.
Chemicals and drugs
Saline (NaCl 0.9%) was obtained from Química Farmacêutica Gaspar Viana, Brazil. TRT was isolated from dried flower buds of E. viscosa and its chemical identification was confirmed by Prof. E. R .Silveira of the UFC. The voucher specimen (#16327) is deposited in the Herbarium Prisco Viana (UFC). TRT 100mg was dissolved in 3% dimethylsulfoxide (DMSO) 100mL in order to achieve a 0.1% TRT solution. DMSO was purchased from Cromato Produtos Químicos Ltda, Brazil. All other chemicals were of analytical grade.
Biochemical determinations
The amount of GSH was determined from a standard curve simultaneously obtained under the same conditions with various concentrations of GSH. Liver regeneration was estimated by weighting the rat liver residual lobes. TB content was valuated following Meites modification of Malloy and Evelyn procedure16. D-glucose was determined according to the Slein´s method17.
Lipid peroxidation, a measure of free radical damage, was assayed by measuring malondialdehyde (MDA) as thiobarbituric acid-reactive substances (TBARS) levels using the thiobarbituric acid method18. In brief, H3PO4 (1%, 3ml) and aqueous TBA solution (0.6%, 3 mL) were added to the 10% homogenate (0.5 ml). The assay medium was shaken and heated on a boiling-water bath for 45 min. After cooling, 4 ml of n-butanol was added and the mixture shaken. After separation of the n-butanol layer by centrifugation at 1200 g for 15 min its optical density was determined in a spectrophotometer (Beckman DU 640 B) with 535 and 520nm as absorption wavelengths, respectively. The difference between the results of the two optical density determinations was taken as the TBA value and the amount of malondialdehyde (MDA) in the testis was calculated, comparing with MDA standards and expressed as µmol MDA per gram of wet tissue. GSH levels were estimated by the method of Sedlak and Lindsay19 which is based on the reaction between thiol groups and 5-5'-dithiobis-(2-nitrobenzoic acid) to give a compound that absorbs light at 412 nm. The amount of GSH was determined from a standard curve simultaneously obtained under the same conditions with various concentrations of GSH.
Statistical analysis
GraphPad Prism 4.0 (GraphPad Software, San Diego, California, USA, www.graphpad.com) was used for computation and statistical analysis. To ensure the appropriateness of parametric testing, all data were examined for normality, using Kolmorogov-Smirnov test (with Dalal-Wilkinson-Lilliefor P Value). Non-parametric data were analyzed using ANOVA (Kurskal-Wallis/Dunn) tests. Values of p<0.05 were accepted as statistically significant.
Results
No animal died during the experiment.
Liver regeneration
Figure 1 depicts the evolution of residual liver lobes in each subgroup throughout the experiment. There was a significant increase in liver weight at T3 timepoint in both groups.
Total billirubin
Although there was an apparent decrease of TB concentrations in both groups (G1 / G2), no significant differences were demonstrated, as illustrated in Figure 2.
D-Glucose
D-Glucose levels decreased significantly (p<0.01) in TRT-treated rats in T2 timepoint compared with control T2 (Figure 3).
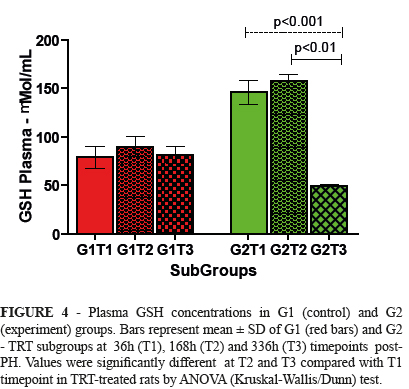
Plasma GSH
Plasma GSH concentrations decreased significantly in TRT treated rats, 336h post-PH (T3) compared with T1 (p<0.001) and T2 (p<0.05) (Figure 4).
Liver GSH
Liver GSH concentrations decreased significantly (p<0.01) in TRT treated rats, 168h post-PH (Figure 5).
Plasma TBARS
Plasma TBARS values were not different in G1 and G2 rats (Figure 6).
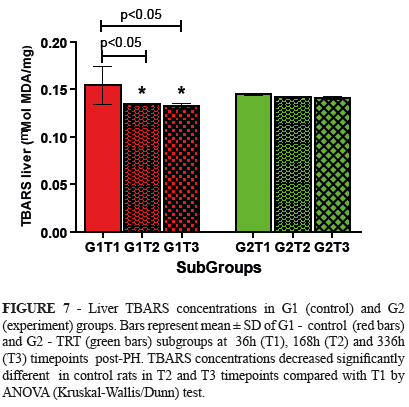
Liver TBARS
Liver TBARS values decreased in T2 and T3 subgroups compared with T1 in control rats. No differences were found in TRT-treated rats (Figure 7).
Discussion
The rat was chosen for this study because it is the most studied animal in hepatic regeneration research20. Male young animals were used whereas older rats may compromise the regenerative process21. Besides, steroids present in greater quantity in females may influence hepatic regeneration22. The maximum period of sample collecting (14 days) was chosen based on previous studies that show that liver regeneration in rats subjected to HP is completed in two weeks1,20.
Considering that TB concentrations did not show any significant change at any time we can perceive that TRT administration had no effects on TB levels. The absence of significant differences in the weight of the remnant liver in G1 and G2 groups suggests that TRT administration does not interfere in a significant way with the natural post-HP rat liver regeneration.
Some antioxidants have different effects on liver regeneration. In fact, the administration of omega-3 polyunsaturated fatty acids (PUFA), inhibits the liver regeneration23 and the administration of a-tocopherol (vitamin E) and GSH retards liver regeneration evolution7,8.
Partial hepatectomy is related to the formation of free radicals24-26. Indeed, many studies have shown an increased production of free radicals production measured by malonyldialdehyde in liver mitochondria following partial hepatectomy24-26. Decreased liver and plasma GSH observed in G1 and G2 rats could be related to the utilization of GSH in order to attenuate the oxidative stress generated by the partial hepatectomy.
The lipid peroxidation inhibitory effects of several flavonoids such as luteolin, apigenin, galangin, gardenin D, (+)catechin27, quercitin28, rutin (quercetin-3-rhamnosyl glucoside)29 ant TRT30 have been previously reported. In the present study, liver TBARS values decreased in T2 and T3 subgroups compared with T1 in control rats. This could be explained by the reduction of GSH concentration in G1 rats. The absence of reduction of TBARS concentration in rats subjected to HP and treated with TRT suggests that the antiperoxidative effect of TRT was not observed here.
In the present study, blood glucose concentrations decreased significantly in TRT-treated group. Studies have demonstrated that insulin levels decrease31,32 while glucagon levels increase33 in partially hepatectomized rats. These changes may reflect part of homeostatic mechanism to maintain glucose levels within normal limits. Moreover, antioxidants improve insulin action, due in part by the protection of b-cells from free radicals injuries32.
Conclusions
Bioflavonoid ternatin administration induces an inhibitory effect on oxidative stress but does not change the liver regeneration evolution. Moreover, TRT promotes a significant hypoglycemic state in TRT-treated rats.
Acknowledgement
The authors thank PhD João Aragão Ximenes Filho for his valuable help with statistical analysis.
Received: February 11, 2013
Review: April 12, 2013
Accepted: May 14, 2013
Conflict of interest: none
Financial source: none
- 1. Higgins GM, Anderson RM. Experimental pathology of the liver: I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186-202.
- 2. Michalopoulos G. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990;4:176-87.
- 3. Steer CJ. Liver regeneration. FASEB J. 1995;9:1396-2000.
- 4. Hockings PD, Roberts T, Campbell SP, Reid DG, Greenhill RW, Polley SR, Nelson P, Bertram TA, Kramer K. Longitudinal magnetic resonance imaging quantification of rat liver regeneration after partial hepatectomy. Toxicol Pathol. 2002;30(5):606-10.
- 5. Van Norden CJ. Effects of n-3 and n-6 polyunsaturated fatty acid-enriched diets on lipid metabolism in periportal and pericentral compartments of female rat liver lobules and the consequences for cell proliferation after partial hepatectomy. J Lipid Res. 1995;36(8):1708-20.
- 6. Nakatani T, Inouye M, Mirochnitchenko O. Over expression of antioxidants enzymes in transgenic mice decreases cellular ploidy during liver regeneration. Exp Cell Res. 1997;236(1):137-46.
- 7. Holocec M, Skopec F, Sprongl L. Influence of buthionine, s-adenosylmethionine and glutathione on liver regeneration following partial hepatectomy. Arzneimittelforschung. 2000;50(12);1093-8.
- 8. Trejo-Solis C, Sanchez VC, Fraustro AA, Sevilla LS, Ruiz CG, Munoz RH. Inhibitory effect of vitamin E administration on the progression of liver regeneration induced by partial hepatectomy in rats. Lab Invest. 2003;83(11):1669-79.
- 9. Kurir TT, Markotić A, Katalinić V, Bozanić D, Cikes V, Zemunik T, Modun D, Rincić J, Boraska V, Bota B, Salamunić I, Radić S. Effect of hyperbaric oxygenation on the regeneration of the liver after partial hepatectomy in rats. Braz J Med Biol Res. 2004;37(8):1231-7.
- 10. Czaplińska M, Czepas J, Gwoździński K. Structure, antioxidative and anticancer properties of flavonoids. Postepy Biochem. 2012;58(3):235-44.
- 11. Lima MAS, Silveira ER, Marques MSL, Santos RH, Gambardela MPT. Biologically active flavonoids and terpenoids from Egletes viscosa. Phytochemistry. 1996;41;217-23.
- 12. Souza MF, Rao VSN, Silveira ER. Antianaphylactic and anti-inflammatory effects of ternatin, a flavonoid isolated from Egletes viscosa Less. Braz J Med Biol Res. 1992;25:1029-32.
- 13. Rao VSN, Figueiredo EG, Melo CL, Viana GSB, Menezes DB, Matos MSF, Silveira ER. Protective effect of ternatin, a flavonoid isolated from Egletes viscose Less., in experimental liver injury. Pharmacology. 1994;48:392-97.
- 14. Souza MF, Cunha GMA, Fontenele JB, Rao VSN, Silveira ER. Antithrombotic activity of ternatin, a tetramethoxy flavone from Egletes viscosa Less. Phytother Res. 1994;8:478-81.
- 15. Souza MF, Rao VSN, Silveira ER. Inhibition of lipid peroxidation by ternatin, a tetrametoxyflavone from Egletes viscosa L. Phytomedicine. 1997;4:25-9.
- 16. Malloy HT, Evelyn KA. The determination of bilirubin with the photoelectric colorimeter. J Biol Chem. 1937;119:481-90.
- 17. Slein MW. Determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Verlag Chemie, Weinheim/Academic Press; 1963. p.117-23.
- 18. Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271-8.
- 19. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25(1):192-205.
- 20. Ramalho FS, Ramalho LNZ, Zucoloto S, Castro e Silva Jr O. Regeneração hepática: algumas indefinições num universo de incertezas. Acta Cir Bras. 1993;8:177-89.
- 21. Bucher NL, Swaffield MN, Ditroia JF. The influence of age upon the incorporation of thymidine-2-c14 into the DNA of regenerating rat liver. Cancer Res. 1964;24:509-12.
- 22. Francavilla A, Gavaler JS, Makowka L, Barone M, Mazzaferro V, Ambrosino G, Iwatsuki S, Guglielmi FW, Dileo A, Balestrazzi A, Van Thiel DH, Starzi TE. Estradiol and testosterone levels in patients undergoing partial hepatectomy. A possible signal for hepatic regeneration? Dig Dis Sci. 1989;34(6):818-22.
- 23. Melo JUS, Santos JMV, Kimura OS, Campos Junior MM, Melo RB, Vasconcelos PRL. Effects of fatty acids on liver regeneration in rats. Rev Bras Cir. 2010;37(5):351-7.
- 24. Aguilar-Delfin I, Lopez-Barrera F, Hernandez-Munoz R. Selective enhancement of lipid peroxidation in plasma membrane in two experimental models of liver regeneration: partial hepatectomy and acute CC14 administration. Hepatology. 1996;24:657-62.
- 25. Guerrieri F, Vendemiale G, Grattagliano I, Cocco T, Pellecchia G, Altomare E. Mitochondrial oxidative alterations following partial hepatectomy. Free Radic Biol Med. 1999;26:34-41.
- 26. Hernandez-Munoz R, Sanchez-Sevilla L, Martinez-Gomez A, Dent MA. Changes in mitochondrial adenine nucleotides and in permeability transition in two models of rat liver regeneration. Hepatology. 2003;37:842-51.
- 27. Cholbi MR, Paya M, Alcaraz MJ. Inhibitory effects of phenolic compounds on CCl4-induced microsomal lipid peroxidation. Experientia. 1991;47(2):195-9.
- 28. Kanter M. Protective effect of quercetin on liver damage induced by biliary obstruction in rats. J Mol Histol. 2010;41(6):395-402.
- 29. Korkmaz A, Kolankaya D. Protective effect of rutin on the ischemia/ reperfusion induced damage in rat kidney. J Surg Res. 2010;164(2):309-15.
- 30. Guimarães SB, Santos JM, Aragão AA, Kimura OS, Silveira ER, Vasconcelos PR. Ternatin pretreatment attenuates testicular injury induced by torsion/detorsion in Wistar rats. Acta Cir Bras. 2011;26(4):325-8.
- 31. Facchini FS, Humphreys MH, Nascimento CA, Abbasi F, Reaven GM. Relation between insulin resistance and plasma concentrations of lipid hydroperoxides, carotenoids, and tocopherols. Am J Clin Nutr. 2000;72(3):776-9.
- 32. Koksal C, Bozkurt AK, Cangel U, Ustundag N, Konukoglu D, Musellim B, Sayin AG. Attenuation of ischemic/reperfusion injury by n-acetylcysteine in a rat hind limb Model. J Surg Res. 2003;111(2):236-9.
- 33. Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276(5309):60-6.
Publication Dates
-
Publication in this collection
04 June 2013 -
Date of issue
June 2013
History
-
Received
11 Feb 2013 -
Accepted
14 May 2013 -
Reviewed
12 Apr 2013