Abstract
PURPOSE: To investigate nephrocalcinosis due to hyperoxaluria induced by two different inducing agents in rats. METHODS: Forty Sprague-Dawley male rats were randomly distributed into four groups: Group1 (Clinical control, n = 10); Group 2 (0.5% Ethylene Glycol + Vitamin D3, n = 10); Group 3 (1.25% Ethylene Glycol, n = 10); and Group 4 (5%Hydroxy L-proline, n = 10). Five animals from each group were euthanized after one week of follow-up (M1 Moment) and the remaining, after four weeks (M2 Moment). All animals underwent 24h urine dosages of calcium, oxalate, uric acid, citrate and serum creatinine. Histology and histomorphometric analyses were performed using Image J program in the hematoxylin-eosin stains. Calcium deposits in the renal parenchyma were quantified by PIXE technique (Proton Induced X-Ray Emission). RESULTS: 24h urinary parameters did not show any significant variations after 28 days of experiment except by hyperoxaluria that was significantly higher in Group 3. Histomorphometric analyses showed a significantly higher nephrocalcinosis in Group 2 (p<0.01). The calcium deposits in the renal parenchyma were 10 and 100 times higher in Group 2 in comparison to other groups in the M1 and M2 moments, respectively. CONCLUSION: The Group 2 (vitamin D3+Ethylene Glycol 0.5%) was the best model to induce nephrocalcinosis in rats after 28 days.
Nephrocalcinosis; Lithiasis; Hyperoxaluria; Rats
4 ORIGINAL ARTICLE
MODELS, BIOLOGICAL
Nephrocalcinosis induced by hyperoxaluria in rats1 1 Research performed at Urology Experimental Laboratory, Botucatu School of Medicine, São Paulo State University (UNESP), Botucatu-SP, Brazil. Part of Master degree thesis, Postgraduate Program in Bases General Surgery, Botucatu School of Medicine, UNESP. Tutor: João Luiz Amaro.
Natália Baraldi CunhaI; Paulo Roberto KawanoII; Carlos Roberto PadovaniIII; Flávio de Oliveira LimaIV; Suene BernardesV; Eloá Siqueira MagalhãesVI; Carmen Regina Petean AmaroVII; João Luiz AmaroVIII
IFellow Master degree, Postgraduate Program in Bases General Surgery, Botucatu School of Medicine, São Paulo State University UNESP, Botucatu-SP, Brazil. Design, intellectual and scientific content of the study; acquisition of data, technical procedures
IIPhD, Assistant Professor, Department of Urology, Botucatu School of Medicine, São Paulo State University UNESP, Botucatu-SP, Brazil. Scientific content of the study, anesthesia, technical procedures, critical revision
IIIPhD, Full Professor, Department of Biostatistics, Botucatu Institute of Biosciences, São Paulo State University UNESP, Botucatu-SP, Brazil. Biostatistical analysis
IVPhD, Assistant Professor, Department of Pathology, Botucatu School of Medicine, São Paulo State University UNESP, Botucatu-SP, Brazil. Acquisition and interpretation of data, histological analysis
VFellow PhD degree, Postgraduate Program Applied Physics, Institute of Physics, University of Sao Paulo (USP), Brazil. Acquisition of data, technical procedures of calcium in the renal parenchyma
VIGraduate student, scientific initiation project, IC FAPESP, Botucatu Institute of Biosciences, São Paulo State University UNESP, Botucatu-SP, Brazil. Acquisition of data, technical procedures
VIIPhD, Department of Urology, Botucatu School of Medicine, São Paulo State University UNESP, Botucatu-SP, Brazil. Critical revision
VIIIPhD, Full Professor, Department of Urology, Botucatu School of Medicine, São Paulo State University UNESP, Botucatu-SP, Brazil. Conception, design, intellectual and scientific content of the study; critical revision, final approval of manuscript
Correspondence Correspondence: Paulo Roberto Kawano Departamento de Urologia, UNESP Distrito de Rubião Jr., s/n 18618-970 Botucatu SP Brasil Tel.: (55 14)3880-1569 kawano@fmb.unesp.br
ABSTRACT
PURPOSE: To investigate nephrocalcinosis due to hyperoxaluria induced by two different inducing agents in rats.
METHODS: Forty Sprague-Dawley male rats were randomly distributed into four groups: Group1 (Clinical control, n = 10); Group 2 (0.5% Ethylene Glycol + Vitamin D3, n = 10); Group 3 (1.25% Ethylene Glycol, n = 10); and Group 4 (5%Hydroxy L-proline, n = 10). Five animals from each group were euthanized after one week of follow-up (M1 Moment) and the remaining, after four weeks (M2 Moment). All animals underwent 24h urine dosages of calcium, oxalate, uric acid, citrate and serum creatinine. Histology and histomorphometric analyses were performed using Image J program in the hematoxylin-eosin stains. Calcium deposits in the renal parenchyma were quantified by PIXE technique (Proton Induced X-Ray Emission).
RESULTS: 24h urinary parameters did not show any significant variations after 28 days of experiment except by hyperoxaluria that was significantly higher in Group 3. Histomorphometric analyses showed a significantly higher nephrocalcinosis in Group 2 (p<0.01). The calcium deposits in the renal parenchyma were 10 and 100 times higher in Group 2 in comparison to other groups in the M1 and M2 moments, respectively.
CONCLUSION: The Group 2 (vitamin D3+Ethylene Glycol 0.5%) was the best model to induce nephrocalcinosis in rats after 28 days.
Key words: Nephrocalcinosis. Lithiasis. Hyperoxaluria. Rats.
Introduction
Calcium oxalate (CaOx) is the most important metabolic component involved in urolithiasis1. Different authors have reported that two thirds of calculi have oxalate (Ox) in its composition. This condition can be also influenced by genetic alterations (primary hyperoxaluria) or environmental factors (secondary hyperoxaluria)2. As calcium oxalate is slightly soluble, increasing in urinary oxalate concentration (hyperoxaluria) can lead to CaOx supersaturation, resulting in crystals deposition on the renal parenchyma (nephrocalcinosis) and in the collector system (nephrolithiasis)3.
Different authors used hyperoxaluria to study CaOx deposition in kidney of rats4,5. According to these models, renal deposition of crystals occurs mainly intra-tubular or in the renal parenchyma. This process causes damages and progressive inflammation that, when associated to tubular obstruction, may lead to decreased renal function and end-stage renal failure5,6.
Acute or chronic hyperoxaluria may be induced in rats by the administration of different induction agents. According to Khan et al.8, ingestion of ethylene glycol (EG 1.25%)7 or Hydroxy-L-Proline (HLP 5%) may produce chronic hyperoxaluria. However, the use of EG in concentrations higher than 0.75% may cause renal lesion and damage to multiple organs9,10. Therefore, some authors have preferred the hydroxy-L-proline (HLP) administration, which is a physiological precursor of oxalate8. In the attempt to minimize their toxicity, these inducing agents can be associated to potentiating substances, such as vitamin D3, which can haste CaOx precipitation in renal parenchyma11.
According to the literature, these inducing agents of nephrocalcinosis (EG and HLP) may be used in different concentrations and periods of time, causing variations in the intensity and severity of the renal parenchyma calcification. However, it is not clear what is the best agent or the optimal concentration necessary to obtain a stable non-nephrotoxic model of CaOx crystallization. Therefore, the aim of this study was to evaluate the intensity of nephrocalcinosis caused by different inducing agents.
Methods
The study was previously approved by the Ethics Committee on Animal Experiments, from São Paulo State University, Botucatu. School of Medicine
A total of 40 Sprague-Dawley adult male rats weighing 200 to 300 g were randomly distributed into four groups with ten animals (Groups 1,2,3,4) which were placed in metabolic cages and maintained under controlled lighting and temperature.
Group 1 was clinical control and no surgical intervention was performed; in Group 2, animals received, ad libitum, 0.5% ethylene glycol (EG) diluted in water and 0.5µM of D3vitamin dissolved in one ml of vegetable oil, administered by gavage once a day. Rats of the Groups 3 and 4 received 1.25% ethylene glycol (EG) and 5% hydroxy L-proline (HLP), respectively, diluted in their water supply, which was offered ad libitum. The total consumption of water and food was individually measured daily.
The study was divided into three periods. The initial moment (M0) was considered the beginning of the experiment. After one week (M1), five animals of each group were euthanized; and the remaining, sacrificed after four weeks (M2 Moment).
During the study period, urinary volume was individually measured daily, and 24 hours urine was collected weekly for analysis of calcium, oxalate, creatinine, uric acid and citrate. Before freezing of 24-h urine sample, the volume and pH were measured.
Immediately before the sacrifice (M1 and M2), the animals were sedated and, after asepsis, a sample of urine was collected by bladder puncture and sent for microbiological culture. At this moment, about five milliliters of blood were collected by cardiac puncture for biochemical dosage of creatinine. After euthanasia with a lethal dose of sodium pentobarbital, the kidneys were harvested by classical laparotomy. The right kidneys were prepared for histological analysis and quantification of nephrocalcinosis, while the left ones, were reserved for determination of calcium dosages in the renal parenchyma.
Histomorphometric and histological analysis
The kidneys were fixed in formalin and embedded in paraffin. The blocks were sectioned (5µm) and stained with hematoxylin and eosin (H.E). For each sample, five random fields were selected and photographed under x40 magnification by a digital camera coupled to a polarized optical microscope. The images were analyzed using a grid with a hundred points created by the plug-in of Image J® program (Figure 1), and the final result expressed in percentage.
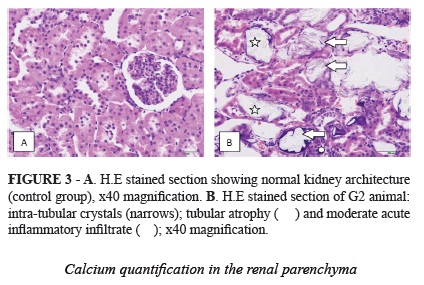
The histopathological analysis was performed by the same pathologist in a blind way that classified the intensity of histological parameters in mild, moderate and severe. The following parameters were studied: tubular atrophy, inflammatory process and stroma extravasation. The crystals of CaOx in the renal parenchyma were analyzed and counted in five fields for each sample under 40x magnifications and expressed as the number of renal tubules with crystals.
Calcium quantification in the renal parenchyma
After two days in the incubator at 60°C for dehydration, the left kidneys were powdered using a crusher at 1070rpm for five minutes. The renal parenchyma, now lyophilized, was sent to the lab for calcium (Ca) dosage by PIXE technique (Proton Induced X-Ray Emission).
In this process, the powdered renal tissue was converted into a homogeneous solution at a ratio of 0.1g of tissue, 90µg of Gallium (Ga) and 1.2 ml of pure nitric acid. The Ga is an absent element in the sample, and was used as standard for measurement of Ca, considering the peaks of the Ga spectrum and calcium element. The samples were aconditioned in the PIXE cam (Pelletron-tandem electrostatic accelerator; 5SDH manufactured by National Electrostatic Corporation, USA), and irradiated by ten minutes to obtain the Ca concentration.
Statistical analysis
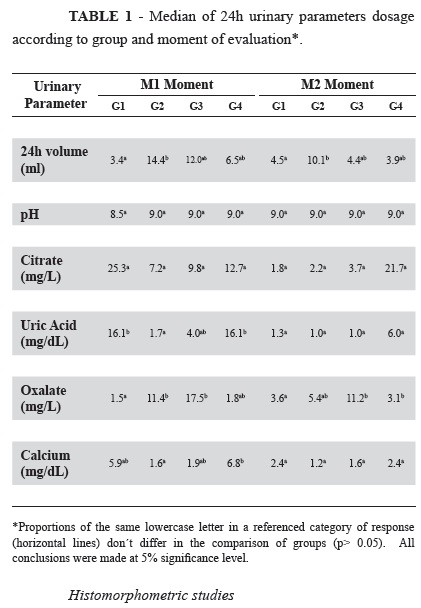
The statistical analysis was performed using the Goodman test for contrast among binomials populations12. In the study of quantitative variables and moments, was used the analysis of variance in a non-parametric model for two-factor model, complemented by the Dunn test13. In the tables, lowercase letters were used to indicate statistical significance in the comparisons among groups. Proportions of the same lowercase letter in a referenced category of response do not differ in the comparison of the groups (p>0.05). All conclusions were made at 5% significance level.
Results
No animal died during the study period and all of them demonstrated a satisfactory increasing of corporal weight. There was no statistical difference in the diary fluid ingestion among different groups and microbiological urine culture was negative. At M1, serum creatinine was significantly higher only in G2 animals when compared to the control group (0.8 x 0.5mg/dl; p<0.05). However, after 28 days of experiment (M2), all animals had normal levels of creatinine (median of 0.6mg/dl)
Urinary volume (UV) was significantly higher in G2 when compared to control group in both moments.
Urinary pH was alkaline and remained stable in both moments. Urinary citrate did not show statistical difference considering different groups and moments.
At M1, uric acid dosage was significantly lower in G2 compared to control group. There was not statistical among other groups.
At M1, oxalate was significantly higher in G2 and G3 animals when compared to the control group. However, at M2, only G3 presented persistently high dosage of urinary oxalate in relation to control group.
Urinary calcium dosage in different groups didn't show significant statistical results compared to control group, considering different moments of evaluation.
All these results are shown in Table 1.
Histomorphometric studies
At M1, computer analysis didn't find statistical difference in the presence of calcifications in the renal parenchyma considering different groups. However, after 28 days of induction with 0.5% EG+ vitamin D3, G2 animals presented a high grade of calcifications, with average of 15 crystals/animal, counted by plug-in grid of image J software. No calcifications were observed in the other groups (Figure 2).
However, histological evaluation by optical microscopy demonstrated the presence of calcifications in G2 at M1, with a median of 8 intra-tubular crystals/animal. After four weeks of induction, this median increased to 58. As in the computerized analysis, calcifications were not observed for the remaining groups in both moments of evaluation (p<0.01).
In G2, tubular atrophy was observed in 60% and 100% of the animals at M1 and M2, respectively (Figure 2). None tubular alterations were observed in other groups.
At M1, a mild acute inflammatory process was observed in 80% of the rats in G2 (0.5% EG + vitamin D3). After 28 days of experiment, the inflammatory process was classified as "moderate" by the pathologist in 25% of these animals (Figure 3). No inflammatory process was identified in the other groups.
Calcium quantification in the renal parenchyma
The calcium dosages in the renal parenchyma by PIXE technique were significantly higher in G2 when compared to the other groups in both moments (Table 2).
Discussion
Urinary volume (UV) is described as an important factor in the pathogenesis of nephrolithiasis because a low urine output may increase the concentration of lithogenic solutes and, consequently, contribute to increase the formation of kidney stones14. Paradoxically, we observed a higher UV in G2 when compared to the control group (G1) in both moments. This fact suggests that this parameter is not influenced the CaOx precipitation in our study, as reported in different studies15,16.
Urinary pH was alkaline (average pH=9.0), with no significant variance in different moments, suggesting that this urinary biochemical parameter did not affect crystals formation. This observation is accordance with other studies that found that the CaOx solubility is not influenced by pH variation17,18.
Citrate is accepted as an effective inhibitor of urinary calcium precipitation because it increases the calcium solubility, thus reducing the ionic Ca activity and supersaturation of CaOx19. Another important property of citrate is the ability to prevent the nucleation and aggregation of crystals20. In this study, urinary citrate levels did not influenced nephrocalcinosis formation.
Hyperuricosuria can be considered the major risk factor for the development of urinary uric acid calculi and may also be involved in calcium oxalate lithiasis21. Although we observed a significantly higher excretion of urinary uric acid in groups G1 and G4 in comparison to G2 at M1, it was not observed tubular or parenchymal calcifications in these groups.
At the M1 moment, there was a significant increase in the urinary oxalate levels in groups G2 and G3 when compared to the other groups, demonstrating that the ethyleno glycol is an adequate model of hyperoxaluria. However, after 28 days, only the group G3 (1.25%EG) maintained a significant hyperoxaluria compared to the other groups. Nevertheless, CaOx deposition was observed only in G2 in the different moments, worsening after 28 days. This fact could explain the decrease of urinary oxalate observed in this group22.
Urinary calcium was significantly higher in G1 (control group) and G4 (HLP) after seven days, however this fact did not result in a higher CaOx deposition in renal parenchyma. After 28 days, no statistical difference was observed among groups, demonstrating that there was no correlation between hypercalciuria and nephrocalcinosis.
Although serum creatinine is not considered an ideal biomarker of glomerular filtration rate (GFR), it can be used to estimate overall renal function in the absence of more sensitive markers23. Some authors have reported a correlation between decreased renal function and urolithiasis due to urinary obstruction and damage of the renal parenchyma24,25. In this study, we observed significantly higher levels of serum creatinine in G2 when compared to G1 (control group) and G4 (HLP) after seven days. However, when the animals were followed for 28 days, there was no statistical difference in serum creatinine in the different groups. Considering that the prolonged period of exposure to different inducing agents could cause deterioration in the renal function25, normal levels of creatinine at M2 suggests that the initial statistical difference observed in M1 could be considered a biological variation.
Some authors have reported that CaOx crystals are formed in the renal tubules and are carried to the interstitial space, causing inflammatory reaction and morphological alterations in the renal architecture5. In our study, the histopathological analysis showed the predominance of acute inflammation, epithelial atrophy and stromal extravasation in animals of G2, which presented important nephrocalcinosis.
Renal tubular calcifications were significantly higher in G2 animals in booth moments (M1 and M2) compared to the other groups. At M2, computer analysis (Image J software) showed a clear predominance of CaOx crystals in Group 2, with a median of 15 crystals per field against zero in the other groups (p<0.05). This finding was confirmed by the microscopic analysis performed by the pathologist demonstrating a positive correlation between two different methods.
Histological findings concerning calcification were supported by the calcium dosages in the renal parenchyma by PIXE technique. This method showed a higher concentration of Ca in the renal parenchyma of G2 animals when compared to the other groups in both moments, which were about 10 and 100 times higher than the values observed for the others groups at M1 and M2, respectively.
Conclusion
Ethylene Glycol 0.5% supplemented with Vitamin D3 (Group G2) was the best model to induce nephrocalcinosis in rats after 28 days.
Acknowledgment
To Professor Manfredo Harri Tabanacniks for technical assistance of LAMFI (Laboratório de Materiais e Feixes Iônicos), Institute of Physics, USP.
Received: March 12, 2013
Review: May 14, 2013
Accepted: June 11, 2013
Conflict of interest: none
Financial source: Sao Paulo Research Foundation (FAPESP - 2011/11699-0)
- 1. Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest. 2005;115:2598-608
- 2. Duncan SH, Richardson AJ, Kaul P, Holmes RP, Allison MJ, Stewart CS. Oxalobacter formigenes and its potential role in human health. Appl Environ Microbiol. 2002;68:3841-7.
- 3. Serra A, Correia M. Nefrocalcinose medular humana. Rev Port Nefrol Hipert. 2004;18:15-32.
- 4. Mandel NS, Henderson JD Jr, Hung LY, Wille DF, Wiessner JH. A porcine model of calcium oxalate kidney stone disease. J Urol. 2004;171:1301-3.
- 5. Khan SR. Nephrocalcinosis in animal models with and without Stones. Urol Res. 2010;38:42938.
- 6. Toblli JE, Ferder L, Stella I, De Cavanaugh, Angerosa M, Inserra F. Effects of angiotensin II subtype 1 receptor blockade by losartan on tubulointerstitial lesions caused by hyperoxaluria. J Urol. 2002;168:1550-5.
- 7. Khan SR, Glenton PA. Experimental induction of calcium oxalate nephrolithiasis in mice. J Urol. 2010;184:1189-96.
- 8. Khan SR, Glenton PA, Byer KJ. Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-L-proline. Kidney Int. 2006;70:914-23.
- 9. Eder AF, McGrath CM, Dowdy YG, Tomaszewski JE, Rosenberg FM, Wilson RB, Wolf BA, Shaw LM. Ethylene glycol poisoning: toxicokinetic and analytical factors affecting laboratory diagnosis. Clin Chem. 1998;44:16877.
- 10. Green ML, Hatch M, Freel RW. Ethylene glycol induces hyperoxaluria without metabolic acidosis in rats. Am J Physiol Renal Physiol. 2005;289:53643.
- 11. de Water R, Boevé ER, van Miert PP, Deng G, Cao LC, Stijnen T, de Bruijn WC, Schroder FH. Experimental nephrolithiasis in rats: the effect of ethylene glycol and vitamin D3 on the induction of renal calcium oxalate crystals. Scann Microsc. 1996;10:591601.
- 12. Goodman LA. Simultaneous confidence intervals for contrasts among multinominal populations. Ann Math Stat. 1964;35:716-25.
- 13. Zar JH. Biostatistical analysis. 5ed. New Jersey: Prentice-Hall; 2009.
- 14. Hong YH, Dublin N, Razack AH, Mohd MA, Husain R. Urinary metabolic evaluation of stone formers - a Malaysian perspective. Urology. 2012;80:529-34.
- 15. Heilberg IP. Update on dietary recommendations and medical treatment of renal stone disease. Nephrol Dial Transplant. 2000;15:117-23.
- 16. Taylor EN, Curhan GC. Diet and fluid prescription in stone disease. Kidney Int. 2006;70:835-9.
- 17. Copelovitch L. Urolithiasis in children: medical approach. Pediatr Clin North Am. 2012;59:881-96.
- 18. Otocka A, Jabłońska J, Głowińska-Olszewska B, Porowski T, Bossowski A. Metabolic acidosis in children with newly diagnosed type 1 diabetes and risk factors of urolithiasis. Pediatr Endocrinol Diabetes Metab. 2012;18:101-6.
- 19. Soygur T, Akbay A, Kupeli S. Effect of potassium citrate therapy on stone recurrence and residual fragments after shockwave lithotripsy in lower caliceal calcium oxalate urolithiasis: a randomized controlled trial. J Endourol. 2002;16:149-52.
- 20. Chow K, Dixon J, Gilpin S, Kavanagh JP, Rao PN. Citrate inhibits growth of residual fragments in an in vitro model of calcium oxalate renal stones. Kidney Int. 2004;65:1724-30.
- 21. Siener R. Impact of dietary habits on stone incidence. Urol Res. 2006;34:131-3.
- 22. Khandrika L, Koul S, Meacham RB, Koul HK. Kidney injury molecule-1 is up-regulated in renal epithelial cells in response to oxalate in vitro and in renal tissues in response to hyperoxaluria in vivo. PLoS One. 2012;7:1-7.
- 23. Dalton RN. Creatinina sérica e taxa de filtração glomerular: percepção e realidade. J Bras Patol Med Lab. 2011;47:8-11.
- 24. Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Morgan C, Samuel S, Klarenbach SW, Curhan GC, Tonelli M. Kidney stones and kidney function loss: a cohort study. BMJ. 2012;345:e5287.
- 25. Jagannath N, Chikkannasetty S, Govindadas D, Devasankaraiah G. Study of antiurolithiatic activity of Asparagus racemosus on albino rats. Indian J Pharmacol. 2012;44:576-9.
Correspondence:
Publication Dates
-
Publication in this collection
01 July 2013 -
Date of issue
July 2013
History
-
Received
12 Mar 2013 -
Accepted
11 June 2013 -
Reviewed
14 May 2013