Abstract
PURPOSE:To design an animal model of ischemia-reperfusion (I/R) in kidneys and evaluate the role that predetermined ranges of local hypothermia plays on markers of stress-oxydative as well as on histologic sections. METHODS: Twenty eight male rats Wistar, under general anesthesia, undergone right nephrectomy (G0, control group) followed by left kidney ischemia during 40 min. Four temperatures groups were designed, with seven animals randomized for each group: normothermic (G1, ±37ºC), mild hypothermia (G2, 26ºC), moderate hypothermia (G3, 15ºC) and deep hypothermia (G4, 4ºC). Left kidney temperature was assessed with an intraparenchymal probe. Left nephrectomy was performed after 240 min of reperfusion. After I/R a blood sample was obtained for f2-IP. Half of each kidney was sent to pathological evaluation and half to analyze CAT, SOD, TBARS, NO3, NO2. RESULTS:Histopathology showed that all kidneys under I/R were significantly more injured than the G0 (p<0.001). TBARS had increased levels in all I/R groups compared with the G0 (p<0.001). CAT had a significant difference (p<0.03) between G1 and G4. Finally, no difference was found on SOD, NO3, NO2 nor on f2-IP. CONCLUSION: This model of I/R was efficient to produce oxidative-stress in the kidney, showing that 4ºC offered significant decrease in free radicals production, although tissue protection was not observed.
Hypothermia; Ischemia; Reperfusion; Kidney; Oxidative Stress; Lipid Peroxidation; Rats
3 - ORIGINAL ARTICLE
MODELS, BIOLOGICAL
Oxidative stress evaluation of ischemia and reperfusion in kidneys under various degrees of hypothermia in rats1 1 Research performed at Laboratory of Physiology and Experimental Pathology, Experimental Research Center (CPE), Porto Alegre Clinics Hospital (HCPA), Rio Grande do Sul Federal University (UFRGS), Porto Alegre-RS, Brazil.
Emanuel Burck dos SantosI; Walter José KoffII; Tomaz de Jesus Maria Grezzana FilhoIII; Samanta Daiana De RossiIV; Lisiane TreisIV; Silvia Regina BonaV; Karla Laís PêgasVI; Betina KatzVII; Fabíola Schons MeyerVIII; Norma Anair Possa MarroniIX; Carlos Otávio CorsoX
IMSc, PhD, Physician, Division of Urology, Porto Alegre Clinics Hospital, Brazil. Conception and design of the study, surgical procedures, acquisition, interpretation and analysis of data, manuscript writing
IIPhD, Full Professor, Department of Surgery, School of Medicine, Rio Grande do Sul Federal University (UFRGS), Brazil. Conception of the study, critical revision
IIIMSc, PhD, Physician, Division of Surgery, Porto Alegre Clinics Hospital, Brazil. Conception of the study, technical procedures
IVUndergraduate student, School of Medicine, UFRGS, Rio Grande do Sul, Brazil. Acquisition of data, technical procedures
VMSc, Fellow PhD degree, Postgraduate Program in Genetics and Applied Toxicology, Brazilian Lutheran University (ULBRA). Biologist, Department of Physiology, School of Medicine, UFRGS, Rio Grande do Sul, Brazil. Acquisition and interpretation of data, technical procedures
VIMSc, Physician, Department of Pathology, Porto Alegre Clinics Hospital, Brazil. Histopathological examinations, acquisition of data
VIIMD, Resident, Department of Pathology, Porto Alegre Clinics Hospital, Brazil. Histopathological examinations, acquisition of data
VIIIMSc, Veterinarian, Experimental Research Center (CPE), Porto Alegre Clinics Hospital, Brazil. Anesthesia, technical procedures
IXMSc, PhD, Full Professor, Department of Physiology. Chief of the Laboratory of Experimental Research in Hepatology, UFRGS, Rio Grande do Sul, Brazil. Acquisition and interpretation of data, technical procedures
XMSc, PhD, Associate Professor, Department of Surgery, School of Medicine, UFRGS, Rio Grande do Sul, Brazil. Conception and design of the study, interpretation of data, manuscript writing, critical revision
Correspondence Correspondence: Emanuel Burck dos Santos Hospital de Clínicas de Porto Alegre Serviço de Urologia Rua Ramiro Barcelos, 2350 90035-903 Porto Alegre RS Brasil emanuelburck@yahoo.com
ABSTRACT
PURPOSE:To design an animal model of ischemia-reperfusion (I/R) in kidneys and evaluate the role that predetermined ranges of local hypothermia plays on markers of stress-oxydative as well as on histologic sections.
METHODS: Twenty eight male rats Wistar, under general anesthesia, undergone right nephrectomy (G0, control group) followed by left kidney ischemia during 40 min. Four temperatures groups were designed, with seven animals randomized for each group: normothermic (G1, ±37oC), mild hypothermia (G2, 26oC), moderate hypothermia (G3, 15oC) and deep hypothermia (G4, 4oC). Left kidney temperature was assessed with an intraparenchymal probe. Left nephrectomy was performed after 240 min of reperfusion. After I/R a blood sample was obtained for f2-IP. Half of each kidney was sent to pathological evaluation and half to analyze CAT, SOD, TBARS, NO3, NO2.
RESULTS:Histopathology showed that all kidneys under I/R were significantly more injured than the G0 (p<0.001). TBARS had increased levels in all I/R groups compared with the G0 (p<0.001). CAT had a significant difference (p<0.03) between G1 and G4. Finally, no difference was found on SOD, NO3, NO2 nor on f2-IP.
CONCLUSION: This model of I/R was efficient to produce oxidative-stress in the kidney, showing that 4ºC offered significant decrease in free radicals production, although tissue protection was not observed.
Key words: Hypothermia. Ischemia. Reperfusion. Kidney. Oxidative Stress. Lipid Peroxidation. Rats.
Introduction
In transplants is widely accepted that hypothermia is protective against injury caused by ischemia and reperfusion (I/R)1. Even though the preconized temperature to obtain preservation is around 4ºC, there are evidences that much higher temperatures, 26ºC or even 34ºC, are enough to achieve liver protection without necessity of deeper hypothermia2,3. It is known that is easier to obtain mild or moderate cooling, and that deep hypothermia is more associated with thermal injury of tissue. Also, it is important to mention that slight hypothermia has a lesser interference in the systemic temperature4. Although the demonstration that mild hypothermia is sufficient to offer liver protection against the deleterious actions of I/R, there are no studies concerning the kidney2,3.
The main target regarding the I/R renal damage is the proximal convoluted tubule, with relative glomerular preservation5,6. The intensity of necrosis is related to the ischemic time. The recovery of acute tubular necrosis starts in two days from the I/R5. The longer the cold storage is, the greater the changes on sodium-potassium adenosine triphosphate (Na/K-ATP) and in the proteins cytoskeleton, such as ezrin. All these events cause time-dependent lipid membranes injury, disorganizing the nephron tubular function during the cold storage, contributing with the damage that occurs during rewarming7. Cold storage induces cell death due to the appearance of iron-dependent reactive oxygen species, with apoptosis in the rewarming period. Even without ischemia, the lesion induced by hypothermia occurs regardless the kind of preservation solution used8.
Lipid peroxidation is a consequence from the reaction between free radicals and lipid membranes of the cells and mitochondria9. It is known that hypothermia is associated to protection against I/R injury1, however there is not any experimental systematic study in vivo that demonstrate what range of temperature protects kidney, although this information has already been available for the liver2,3. That gap of information is important because is relevant know how much cooling is necessary to achieve renal protection without causing hypothermic damage8,10.
Methods
In order to evaluate the role played by free radicals, indirect markers are usually searched1114, because it is difficult to perform straight assessment of such unstable reactive species15. The antioxidant activity is measured through enzymes as superoxide dismutase (SOD) and catalase (CAT). Besides the enzymes, the lipid peroxidation may be evaluated through thiobarbituric acid reactive species (TBARS) determination. SOD, CAT and TBARS are evaluated from tissues samples. To systemic assessment, f2-isoprostanes (F2IP) from plasma can be useful to observe lipid peroxidation. Nitrites (NO2) and nitrates (NO3) from tissue are useful to evaluate nitric oxide (NO) metabolism16. Qualitative analyze of renal tissue is obtained from histopathological examination5.
Animals, anesthesia and surgical procedure
The study received approbation of the Animal Research Ethical Committee (Research and Post-Graduation Group, Porto Alegre Clinics Hospital) according to the Good Animal Practice guidelines (Guide for the Care and Use of Laboratory Animals, National Institute of Health, Bethesda, US, 2011).
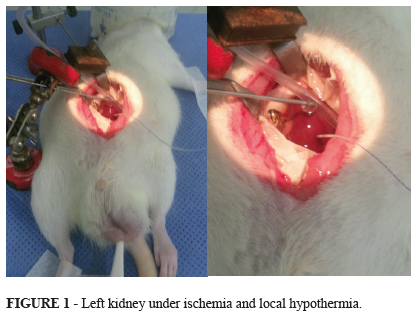
Twenty eight males Wistar adult rats, weighting between 240g and 487g, were kept in cages with free access to water and feed. The cages were acclimatized, including light and noise control. The cages with the animals were kept in the Animal Experimentation Center (Porto Alegre Clinics Hospital). The rats were weighted and underwent general anesthesia with intra-peritoneal administration of ketamine (75mg/Kg) and xylazine (10mg/Kg). Rear paw reflexes were tested to ensure that full general anesthesia was achieved. The animal was put in a warm surgical table (37ºC) and oxygen (1 L/min) was administrated through campanula. Before abdominal incision, bupivacaine 0.5% was injected in the abdominal wall to ensure pain control during and after the procedure. A longitudinal median incision was made, and surgical retractors were applied. Right nephrectomy was performed. Half of the right kidney was sent in formalin to histopathological examination, and the other half in liquid nitrogen to -80ºC freezer. Afterwards the left kidney had his vessels clamped for 40 minutes (ischemia). The left kidney was isolated from the rest of the abdominal cavity through the application of a specially designed device made of latex and polystyrene. The left kidney target-temperature was achieved through superfusion of iced saline solution and kept during all the time of ischemia (40 minutes) (Figure 1). After this time, the renal pedicle was unclamped (start of the warm reperfusion) and abdominal wall was closed. The animal was moved to a warmed new cage, with water, but without food. After 240 minutes of reperfusion, the rat was moved from the cage to the surgical table. New anesthesia was done with the same doses of ketamine and xylazine, but this time the way of administration adopted was intra-muscular instead of intra-peritoneal to avoid the previous violation of the peritoneal cavity. The abdominal wall sutures were removed and left nephrectomy was done. The same procedures applied to the right kidney were made for the left one. Blood sample was collected through heart puncture. Cardiectomy was performed to ensure the animal death still under anesthesia.
Adjustment of left kidney temperature
During the 40 minutes of ischemia, the cortical left kidney temperature was assessed with an intra-parenchymal probe connected to a specific thermometer (BAT 12, IITC Life Science, W.Hills, CA, US). The microprobe was inserted short before of the placement of the vascular clamp. To avoid systemic hypothermia (groups G2, G3 and G4) the left kidney was carefully placed on a specially designed concaved-shaped polystyrene well and a vacuum device was connected inside the well to aspirate the solution used to cool down the kidney. To keep the normal systemic temperature, the animal was warmed with blankets. The surgical table was electrically warmed. For systemic temperature measurement it was applied a common electronic rectal thermometer (Termomed 1.0, ref. 29832, Incoterm, Brazil)2,1719.
Experimental protocol
The effects of I/R (40min/240min) on markers of lipid peroxidation were evaluated in four groups: Group 1, I/R without cooling (G1, 37ºC); group 2, I/R with mild renal hypothermia (G2, 26ºC); group 3, I/R with moderate renal hypothermia (G3, 15ºC); group 4, deep renal hypothermia (G4, 4ºC). For statistical purposes, the right kidneys of all animals in all groups were considered as the group 0 (G0, control group, without I/R). In each group there were seven rats, i.e., a total of 28 animal providing control kidneys for the experiment.
Assessment of the lipid peroxidation markers
For SOD it was employed a method based on inhibition of the adrenochrome through the autoxidation of adrenaline by the SOD20. The change on the absorbance spectrophotometric of the adrenaline has indicated SOD. The CAT activity was assessed by the velocity of peroxide decomposition in water and oxygen through the peroxide absorbance measured by spectrometry. For TBARS it was made a measure of the malondialdehyde (MDA) absorbance by spectrometer on the presence of thiobarbituric acid. The quantification of proteins was obtained by the Lowry method. For plasmatic F2-IP it was performed an evaluation through the ALX-850-205-KI01 8-iso Prostaglandin F2-alpha EIA kit (F2-isoprostanos; Enzo Life Sciences International, Inc., US). Nitrate reductase associated with NADPH cofactor, TrisBuffer, glucose-6-phosphate and also glucose-6-phosphate-dehydrogenase were used to measure NO2 and NO3.
Histopathology
Half of each kidney was fixed in formalin, included in paraffin, cut in slides and colored with hematoxylin and eosin. The criteria employed to classify the findings were an adaptation of the work of Jablonski et al.5. According to these authors, there are four grades of lesion: 1. Mitosis and necrosis of individual cells; 2. Necrosis of all cells in adjacent proximal convoluted tubules, with survival of surrounding tubules; 3. Necrosis confined to the distal third of the proximal convoluted tubule with a band of necrosis extending across the inner cortex; 4. Necrosis affecting all three segments of the proximal convoluted tubule. We created the grade zero to designate normal findings.
Statistical analysis
Data are presented as means ± standard deviation. For the variables with normal distribution it was used one-way ANOVA. Bonferroni post-test was employed to analyze the differences among the means. The non-parametric test Kruskall-Wallis was used to the histology findings. p<0.05 were considered significant.
Results
Histopathology
There was no evidence of any kind of lesion in control group (right kidney, G0, without I/R), but all remaining groups showed variable levels of damage, regardless if under hypothermia (G2, G3, G4) or not (G1). Examining the three hypothermic groups no difference was found among them, taken into account the grade of lesions (1 to 4). The average grade of injury on G0 was zero. On the remaining groups, the respective means and standard deviations found were: G1 2.57±1.13; G2 1.71±1.25; G3 2±1.15; G4 2.57±1.13. The difference between the G0 and all ischemic groups (G1 to G4) had a p value = 0.005 (G0xG2) and p<0.001 (G0xG1; G0xG3; G0xG4) (Figure 2).
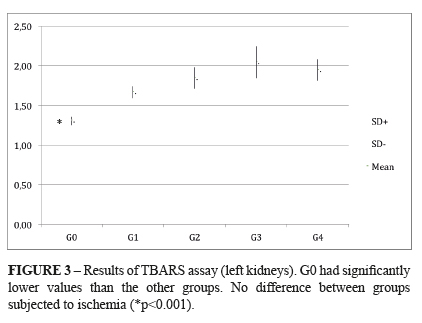
TBARS (thiobarbituric acid reactive species): It was found a statistically significant difference between the control group (G0) and all the I/R groups (G1-G4), regardless the renal target-temperature. The respective means and standard deviation were: G0 1.31±0.05; G1 1.67±0.07; G2 1.85±0.13; G3 2.05±0.20; G4 1.95±0.13. The units of the above means were expressed in nmol/mg of protein. P < 0.004 G0 x I/R groups. (Figure 3)
CAT (catalase)
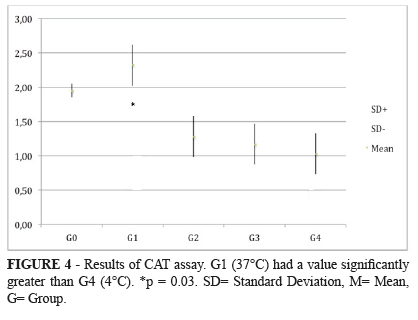
It was found a statistically significant difference between the most hypothermic group (G4, 4ºC) and the normothermic group (G1, 37ºC). The mean found to catalase was expressed in pmol/mg of protein. Means and standard deviations observed were: (G1= 2.32±0.30) x (G4= 1.03±0.30), with a p = 0.03 (G1xG4); (G0= 1.95±0.01), with a p = 0.08 (G0xG4). The remaining comparisons among the groups had no difference with statistic significance. (G0 = 1.954 → G1 = 2.323* → G2 = 1.282 → G3 = 1.179 → G4 = 1.038* pmol/mg of protein). *p = 0.03 (Figure 4).
SOD (superoxide dismutase)
The results showed no difference among groups. The respective means and standard deviations observed were: G0 15.6±0.43; G1 17.4±1.42; G2 18.3±1.45; G3 16.9±0.81; G4 15.7±0.95. The units of the above means were expressed in U SOD/mg of protein.
NO2 and NO3 (nitrites and nitrates)
No significant difference was found among the five groups for NO2 as well as NO3. The means and standard deviations observed for NO2 and NO3 respectively were: G0 0.83±0.00 and 2.07±0.17; G1 0.07±0.00 and 2.11±0.28; G2 0.08±0.00 and 2.89±0.25; G3 0.79±0.00 and 2.36±0.32; G4 0.07±0.01 and 2.054±0.270. The units of the above means were expressed in μmol/L.
Protein
No significant difference was found among the five groups. The respective means and standard deviations observed were: G0 6.65±0.07; G1 6.07±0.28; G2 6.09±0.37; G3 6.84±0.26; G4 6.99±0.23. The units of the above means were expressed in mg/mL.
F2IP (F2-Isoprostanes)
It was measured on plasma instead of the renal tissue. No significant difference was found among the four experimental groups. The respective means and standard deviation observed were: G1 205±65.7; G2 195±32.6; G3 203±92.2; G4 189±71.6. The units of the above means were expressed in pg/mL.
Discussion
The results presented above show interesting aspects about what happened on the adult Wistar rats' kidneys after 40 minutes of ischemia followed by 240 minutes of reperfusion. According to Jablonski et al.5, 30 minutes of warm ischemia are enough to induce changes on the proximal convoluted tubules from rats´ kidneys. After 60 minutes of warm ischemia there is severe tubular necrosis. As expected, normal findings were found in all animals. Nonetheless, all animals underwent ischemia, regardless the target-temperature, showed changes when compared to the controls. Although any statistical significance had not occurred between the I/R normothermic group (G1) and the I/R hypothermic groups (G2-G4), it was observed a trend to worse lesions in the extremes temperature groups: G1 (37ºC) and G4 (4ºC). Nevertheless, those findings did not achieve statistical significance, maybe due to the short time of reperfusion (acute experiment). The outcomes derived from TBARS analyses matched with the histopathologic results, since the TBARS had higher averages in all I/R groups compared to the control group. As well as the tissue analyses, evaluation of TBARS revealed significant difference between the control group and the remaining groups. However the temperature had not interfered with the TBARS results. The significantly lesser value of TBARS when I/R was not present is an evidence that free radicals production happened on the presence of I/R, because TBARS is a very sensible marker of lipid peroxidation of the cell membrane3. The most interesting outcome, however, came from the assessment of CAT, which is a quite important enzyme. This protein converts peroxide in water, leading to its inactivation1116. On the presence of warm ischemia followed by reperfusion, the CAT usually rises in order to protect against the lipid peroxidation. When a protection factor is present concomitantly with I/R, as hypothermia, for instance, CAT cannot undergo an increment even in the presence of I/R, because the addition of cooling might dispense the antioxidant effect of CAT. Our data demonstrate that CAT has risen in G1 (warm I/R) in relation to G0 (control without I/R), and then CAT began to progressively fall soon as the temperature has been becoming lower. Therefore, the control group showed the basal level of CAT when I/R was not present. When warm ischemia was induced, CAT has increased. Nevertheless, on the presence of intense hypothermia (G4, 4ºC) CAT has significantly decreased compared with the normothermic ischemic group (G1), with a p<0.03. These findings occurred due to the protection against lipid peroxidation offered by deep hypothermia, suggesting that the need of antioxidant´s CAT proprieties during the period of I/R were reduced. According to the results obtained for CAT, the target-temperature should be 4ºC in order to obtain antioxidant effect. However, this statement did not have correspondence on histological examination (in four hours of reperfusion), maybe due to direct thermal damage against the renal tissue. Besides, there were no differences among the hypothermic groups relative to the CAT activity. SOD had no statistically significant difference among the groups. Interestingly the SOD had the onset of its decreasing on G3 (15ºC) and the value went down until reach on G4 (4ºC) with almost the same value found on G0 (control). For NO2, NO3 (renal tissue) and F2IP (plasma), no significant difference was detected among the groups. Concluding, the results obtained suggest that the experimental model here presented was adequate to show the occurrence of histological changes derived from I/R compared with controls. Moreover, this model was able to increase the release of TBARS when lipid peroxidation was present (under I/R). Another important conclusion is that the increased catalase during profound hypothermia (4ºC) suggests that this level of temperature is associated with higher antioxidative effect, although this temperature may cause direct thermal damage.
Conclusion
This model of ischemia-reperfusion was efficient to produce oxidative-stress in the kidney, showing that 4ºC offered significant decrease in free radicals production, although tissue protection was not observed.
Acknowledgements
To Marta Cioato, nurse from the Experimental Research Center (CPE); Vânia Naomi Hirakata, statistical counselor from the Group of Research and Post-Graduation; Department of Pathology and the Laboratory of Physiology and Experimental Hepatology.
Received: April 10, 2013
Review: June 12, 2013
Accepted: July 15, 2013
Conflict of interest: none
Financial sources: Incentive to Events and Research Fund-Porto Alegre Clinics Hospital (FIPE-HCPA).
- 1. Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45(4):6736.
- 2. Biberthaler P, Luchting B, Massberg S, Teusper D, Langer S, Leiderer R, Messmer K, Krombach F. The influence of organ temperature on hepatic ischemia-reperfusion injury: a systematic analysis. Transplantation. 2001;72(9):148690.
- 3. Behrends M, Hirose R, Serkova NJ, Coatney JL, Bedolli M, Yardi J, Park YH,Niemann CU. Mild hypothermia reduces the inflammatory response and hepatic ischemia/reperfusion injury in rats. Liver Int. 2006;26(6):73441.
- 4. Tveita T, Johansen K, Lien AH, Myklebust R, Lindal S. Morphologic changes in tubular cells from in situ kidneys following experimental hypothermia and rewarming. APMIS. 2005;113(1):1320.
- 5. Jablonski P, Howden BO, Rae DA, Birrel CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35(3):198204.
- 6. Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17(6):150320.
- 7. Mangino MJ, Tian T, Ametani M, Lindell S, Southard JH. Cytoskeletal involvement in hypothermic renal preservation injury. Transplantation. 2008;85(3):42736.
- 8. Bartels-Stringer M, Kramers C, Wetzels JF, Russel FG, Groot HD, Rauen U. Hypothermia causes a marked injury to rat proximal tubular cells that is aggravated by all currently used preservation solutions. Cryobiology. 2003;47(1):8291.
- 9. McCord JM. The superoxide free radical: its biochemistry and pathophysiology. Surgery. 1983;94(3):4124.
- 10. Ahmad N, Hostert L, Pratt JR, Billar KJ, Potts DJ, Lodge JP. A pathophysiologic study of the kidney tubule to optimize organ preservation solutions. Kidney Int. 2004;66(1):7790.
- 11. Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142(2):23155.
- 12. Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab. 2007;9(6):81339.
- 13. Mayne ST. Antioxidant nutrients and chronic disease: use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr. 2003;133 Suppl 3, 933S40S.
- 14. Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53(6):184156.
- 15. Halliwell B, Gutteridge J. Free radicals in biology and medicine. 4ed. New York: Oxford University Press, USA; 2007.
- 16. Bulkley GB. Free radical-mediated reperfusion injury: a selective review. Br J Cancer Suppl. 1987:8:6673.
- 17. Grezzana Filho TJ, Mendonça TB, Gabiatti G, Rodrigues G, Marroni NA, Treis L, De Rossi SD, Corso CO. Topical hepatic hypothermia plus ischemic preconditioning: analysis of bile flow and ischemic injuries after initial reperfusion in rats. Acta Cir Bras. 2011;26(3):194201.
- 18. Grezzana Filho TJ, Mendonça TB, Gabiatti G, Kruel CDP, Corso CO. Topic liver hypothermia and ischemic preconditioning: a new model of ischemia and reperfusion in rats. Acta Cir Bras. 2009;24(4):2626.
- 19. Grezzana Filho TJ, Corso CO, Zanotelli ML, Marroni CA, Brandão AB, Schlindwein E, Leipnitz I, Meine MH, Fleck A Jr, Hoppen R, Kiss G, Cantisani GP. Liver glutathione depletion after preservation and reperfusion in human liver transplantation. Acta Cir Bras. 2006;21(4):2239.
- 20. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):31705.
Publication Dates
-
Publication in this collection
26 July 2013 -
Date of issue
Aug 2013
History
-
Received
10 Apr 2013 -
Accepted
15 July 2013 -
Reviewed
12 June 2013