Abstract
PURPOSE: To investigate and compare the biocompatibility of two types of Ferrara intracorneal ring segment: with and without chondroitin sulfate coating by clinical and histopathological evaluation. METHODS: A randomized experimental study was carried out on thirty right-eye corneas from 30 Norfolk albino rabbits allocated into two experimental groups: Group G1 - implanted with Ferrara intracorneal ring segment without coating (FICRS) and Group G2 - implanted with Ferrara intracorneal ring segment with chondroitin sulfate coating (FICRS-CS). Left eyes formed the control group. Clinical parameters analyzed were: presence of edema, vascularization, infection and ring extrusion one, 30, and 60 days after surgery. Histopathological parameters analyzed were: number of corneal epithelial layers over and adjacent to the ring, presence of spongiosis, hydropic degeneration, basement membrane thinning, inflammatory cells, neovascularization and pseudocapsule formation. RESULTS: At clinical examination 60 days after implant, edema, vascularization and extrusion were observed respectively in 20%, 26.7%, 6.7% of FICRS corneas and in 6.7%, 6.7%, and 0% of FICRS-CS corneas. Histopathological evaluation showed epithelial-layer reduction from 5 (5;6) to 3 (3;3) with FICRS and from 5 (5;5) to 4 (3;5) with FICRS-CS in the region over the ring. Epithelial spongiosis, hydropic degeneration, and basement membrane thinning were present in 69.2%, 53.8%, and 69.2% of FICRS and in 73.3%, 73.3%, and 46.7% with FICRS-CS, respectively. Vascularization was present in 38.5% of FICRS and 13.3% with FICRS-CS, inflammatory cells in 75% of FICRS and 33.3% with FICRS-CS, and pseudocapsule in 66.7% of FICRS and 93.3% with FICRS-CS. Giant cells occurred only in the FICRS-CS group (20%). CONCLUSION: Ferrara intracorneal rings coated with chondroitin sulfate (FICRS-CS) caused lower frequency of clinical and histopathological alterations than Ferrara intracorneal rings without the coating (FICRS), demonstrating higher biocompatibility of the FICRS-CS.
Prosthesis Implantation; Corneal Neovascularization; Chondroitin Sulfates; Cornea; Rabbits
acb
2 - ORIGINAL ARTICLE
MODELS, BIOLOGICAL
Biocompatibility of Ferrara intracorneal ring segment with and without chondroitin sulfate coating. Clinical and histopathological evaluation in rabbits1 1 Research performed at Experimental Laboratory, Department of Ophthalmology, Otolaryngology and Head and Neck Surgery, Faculty of Medicine of Botucatu (FMB), Sao Paulo State University (UNESP), Brazil. Part of PhD degree thesis, Postgraduate Program in Surgery. Tutor: Prof. Maria Rosa Bet de Moraes Silva.
Eduardo AndreghettiI; Mitsuo HashimotoII; Maria Aparecida Custódio DominguesIII; Vitor Andrigheti Coronado AntunesII; Paulo de Souza SegundoIV; Maria Rosa Bet de Moraes SilvaV
IFellow PhD degree, Postgraduate Program in Surgery, Faculty of Medicine of Botucatu (FMB), Sao Paulo State University (UNESP), Botucatu-SP, Brazil. Technical procedures, acquisition and interpretation of data, manuscript writing
IIFellow PhD degree, Postgraduate Program in Surgery, FMB, UNESP, Botucatu-SP, Brazil. Technical procedures, acquisition of data
IIIPhD, Assistant Professor, Department of Pathology, FMB, UNESP, Botucatu-SP, Brazil. Histopathological examinations
IVResident, Division of Ophthalmology, Department of Ophthalmology, Otolaryngology and Head and Neck Surgery, FMB, UNESP, Botucatu-SP, Brazil. Technical procedures
VPhD, Associate Professor, Division of Ophthalmology, Department of Ophthalmology, Otolaryngology and Head and Neck Surgery, FMB, UNESP, Botucatu-SP, Brazil. Conception and design of the study, manuscript writing, critical revision
Correspondence Correspondence: Profa. Maria Rosa Bet de Moraes Silva Departamento de Oftalmologia, Otorrinolaringologia e CCP Faculdade de Medicina de Botucatu - UNESP Distrito de Rubião Júnior, s/n 18618-970 Botucatu - SP Brasil rosabet@fmb.unesp.br
ABSTRACT
PURPOSE: To investigate and compare the biocompatibility of two types of Ferrara intracorneal ring segment: with and without chondroitin sulfate coating by clinical and histopathological evaluation.
METHODS: A randomized experimental study was carried out on thirty right-eye corneas from 30 Norfolk albino rabbits allocated into two experimental groups: Group G1 - implanted with Ferrara intracorneal ring segment without coating (FICRS) and Group G2 - implanted with Ferrara intracorneal ring segment with chondroitin sulfate coating (FICRS-CS). Left eyes formed the control group. Clinical parameters analyzed were: presence of edema, vascularization, infection and ring extrusion one, 30, and 60 days after surgery. Histopathological parameters analyzed were: number of corneal epithelial layers over and adjacent to the ring, presence of spongiosis, hydropic degeneration, basement membrane thinning, inflammatory cells, neovascularization and pseudocapsule formation.
RESULTS: At clinical examination 60 days after implant, edema, vascularization and extrusion were observed respectively in 20%, 26.7%, 6.7% of FICRS corneas and in 6.7%, 6.7%, and 0% of FICRS-CS corneas. Histopathological evaluation showed epithelial-layer reduction from 5 (5;6) to 3 (3;3) with FICRS and from 5 (5;5) to 4 (3;5) with FICRS-CS in the region over the ring. Epithelial spongiosis, hydropic degeneration, and basement membrane thinning were present in 69.2%, 53.8%, and 69.2% of FICRS and in 73.3%, 73.3%, and 46.7% with FICRS-CS, respectively. Vascularization was present in 38.5% of FICRS and 13.3% with FICRS-CS, inflammatory cells in 75% of FICRS and 33.3% with FICRS-CS, and pseudocapsule in 66.7% of FICRS and 93.3% with FICRS-CS. Giant cells occurred only in the FICRS-CS group (20%).
CONCLUSION: Ferrara intracorneal rings coated with chondroitin sulfate (FICRS-CS) caused lower frequency of clinical and histopathological alterations than Ferrara intracorneal rings without the coating (FICRS), demonstrating higher biocompatibility of the FICRS-CS.
Key words: Prosthesis Implantation. Corneal Neovascularization. Chondroitin Sulfates. Cornea. Rabbits
Introduction
Highly irregular astigmatism caused by diseases that deform the cornea preclude vision correction with the available optical resources. Keratoconus, laser-assisted in situ keratomileusis (LASIK), and penetrating or laminar keratoplasty are some of the main causes of corneal deformity1-3.
Keratoconus is a generally bilateral non-inflammatory progressive corneal ectasia that occurs in all races. Its prevalence is approximately 3.34% in first generation relatives and varies from 0.05% to 0.23% in the general population4. The disease begins in puberty and the refractive error is generally corrected with hard contact lenses, but 21.6% to 44% of cases require penetrating keratoplasty to restore vision5. Intracorneal rings are an alternative to delay potentially aggressive keratoplasty, or in patients intolerant to contact lens6.
Iatrogenic corneal ectasia is the most feared post-LASIK complication, yet little is known about its incidence and prevalence. Pallikaris et al.7 reported this complication in 0.66% of eyes submitted to LASIK in a study involving 2873 patients submitted to the procedure between May 1995 and November 1999. LASIK is performed in many countries. In the USA, the estimated 8 million persons who have been submitted to LASIK could potentially lead to a large number of iatrogenic ectasias8.
Corneal transplants are performed all over the world. APABO9 estimates that in 2004 there were 8400 cornea transplants in Brazil and 46841 in the USA, whereas the post-transplant incidence of high degree astigmatism varied from 10% to 20%10,11.
In the last 20 years, studies of intracorneal ring implants have shown promising evolution in relation to corneal ectasia stabilization, corneal apex regularization, improvement in contact lens adaptation, and improved visual acuity in keratoconus and post-LASIK corneal ectasia as well as in post-transplant astigmatism12-14. More recently the association of such implants with corneal cross-linking has shown good results15.
Implants of polymethylmethacrylate (PMMA) rings have been described as a simple, reversible, easy-to-learn method suitable for outpatients which allows the normal cornea structure to be preserved without removing corneal tissue or causing corneal reaction16,17. However, cases of corneal extrusion and infection have been reported for varying periods after this type of implant18,19 and may be related to corneal incompatibility with PMMA.
The types of intracorneal ring segments available globally are all made of PMMA (Intacs®, Intacs-SK®, Ferrara Ring®, Keraring®, and Cornealring®) varying only in diameter, cross-section, arc length, thickness, and the internal and external curvature radii20.
Glycosaminoglycans such as chondroitin sulfate are found in the extracellular corneal matrix and actively participate in the corneal homeostatic process21. Therefore coating the PMMA intracorneal rings with chondroitin sulfate may improve biocompatibility and reduce complications such as corneal extrusion and infection. No studies were found that have compared alterations and complications caused by intracorneal rings coated with chondroitin sulfate (FICRS-CS) versus those commercially available without the coating (FICRS).
The aim of the study was to investigate and to compare the biocompatibility of two types of Ferrara intracorneal ring segment: with and without chondroitin sulfate coating by clinical and histopathological evaluation.
Methods
The study was approved by the Ethics Committee on Animal Research, Botucatu Medical School - UNESP, number 502-05.
A randomized experimental study was performed on 30 healthy female Norfolk albino rabbits weighing from 1550g to 2650g, with normal eyes at clinical examination. The animals were supplied by the Botucatu School of Medicine's Central Animal Colony.
The rabbits were randomly divided into two groups. Group 1 (n=15) was implanted with a PMMA Ferrara intracorneal ring segment without chondroitin sulfate coating (FICRS) in the right eye cornea (RE). Group 2 (n=15) was implanted with a PMMA Ferrara intracorneal ring segment with chondroitin sulfate coating (FICRS-CS) in the right-eye cornea (RE). Left-eye corneas (LE) formed the control group.
The only difference between the Ferrara intracorneal ring segments was the presence of chondroitin sulfate coating in the FICRS-CS and its absence in the FICRS. Intracorneal ring segment dimensions were: 125 micron thickness, 150º arc length, 5mm apex diameter, one orifice at each extremity, and a constant triangular section base of 600µm.
All ring segments were implanted by one of the authors (EA) after intravenous anesthesia with 4% sodium pentobarbital (1ml/Kg) and topical anesthesia with 1% tetracaine hydrochloride. The protocol for intracorneal ring implant surgery was as follows: asepsis, placement of a Barraquer blepharostat, marking the anatomical center of the cornea with a Sinsky hook, marking optical zones at 3, 5, and 7mm from the center mark with an optical zone marker (Storz E 9032), making of a 1.2mm radial incision at 12 o'clock between the 5 and 7mm optical zones with a diamond knife - double cut (Storz E-9055 TAA) to 80% of the corneal thickness, evaluated by ultrasonic pachymetry (Alcon® pachymeter - 8065973401 Serial Nº 3779), introducing a Soares spreader to start the tunnel, followed by a semicircular Ferrara tunnel marker which was rotated 180º counterclockwise, and positioning of the PMMA intracorneal ring with or without the coating according to the group. The radial incision was not sutured.
After surgery, 0.3% ofloxacin eye drops (Oflox 0.3% Allergan®) were applied to the right eye every eight hours for 15 days.
The following clinical parameters were evaluated with the aid of a surgical microscope: presence of edema, presence of vascularization, presence of infection and ring extrusion. These parameters were evaluated at three different moments: M1 - 1 day, M2 - 30 days, and M3 - 60 days after implant.
After M3, animals were euthanized with a lethal dose of pentobarbital and the eyes enucleated. The corneas were removed and bisected through the middle of the ring in the RE (3 o'clock to 9 o'clock section). One of the halves was fixed in 10% buffered formaldehyde whereas the other was sent for scanning electron microscopy which is not covered in this study. Laminas were prepared and stained with hematoxylin eosin and with periodic acid of Schiff. Histological analysis was performed on two different regions of the cornea: over the ring and adjacent (lateral, at 7mm optical zone) to the ring in RE. In control eyes, analyzed regions corresponded to the optical zones from 3 to 5mm. In both zones the analysis included: epithelium, stroma, Descemet membrane and endothelium. Histopathological analysis was performed masked.
The epithelium was evaluated for number of layers, presence of spongiosis, presence of hydropic degeneration, and presence of thinning in the basement membrane. The stroma was evaluated for the presence of neovascularization, inflammatory cells and pseudocapsule.
Statistical analysis was performed using SPSS Version 15 using the parametric paired Student's t and Mann-Whitney tests and the non-parametric Wilcoxon test for dependent samples. Comparisons of proportions were performed by Chi-squared test or Fisher's Exact Test and comparisons for the same individual with MacNemar test. A 5% significance level was adopted (p<0.05).
Results
Clinical parameters
Edema was observed at all three moments in G1 and at two moments in G2. The edema frequencies in M1 were 66.7% and 93.3% respectively for G1 and G2. At M2 edema was observed only in G1 at the frequency of 13.3%. At M3, edema occurred in 20% of G1 and in 6.7% of G2. The difference was not statistically significant (Table 1).
Corneal vascularization occurred only at M3, at lower frequency in G2 but without significant statistical difference (Table 1). Ring extrusion was only observed in G1 (6.7%) (Table 1). Infection was not present in any corneas.
Morphological parameters
Control group corneas (LE) presented an epithelium formed by 5-6 layers of cells, having one line of elongated columnar basal cells, two layers of polygonal cells, two or three layers of wing cells, and one layer of squamous cells. The basement membrane (BM) showed homogenous thickness (Figure 1).
In the region over the ring a diminution was observed in the number of epithelial cell layers, in both experimental groups. The reduction was seen mainly in the middle third, which is formed by wing cells (Figure 2).
The median number of layers was five (5;6) in control eyes and three (3;3) and four (3;5) respectively in G1 and G2, but the reduction was significant only in G1 (Table 2).
The comparison between groups G1 and G2 showed greater reduction in the group implanted with uncoated rings (FICRS) but the difference was not significant (p=0.061).
In the region adjacent to the ring the number of epithelial cell layers was similar to controls in both groups. Analysis also showed no difference in comparison between groups G1 and G2 (Table 2).
In a region localized between the two regions analyzed in this study (over the rings and at 7mm optical zone), epithelial hypertrophy was observed (Figure 2).
Comparison between regions showed a significant reduction in the number of epithelial cell layers in the area over the ring in relation to the region adjacent to the ring in both G1 without coating and G2 with coating (Table 3).
Spongiosis and hydropic degeneration were seen in both corneal regions studied in G1 and G2 (Figure 3).
No statistical difference was seen in the percentage of spongiosis or hydropic degeneration between the groups and between the regions in the same group. Nevertheless, the region over the ring showed higher percentages of these alterations (Table 4).
The basement membrane (MB) displayed thinning in both experimental groups but only in the region over the ring (Figure 4) where its frequency was 22.5% higher in FICRS (Table 4).
Intense stromal compaction was observed in the region over the ring in both groups (Figure 2).
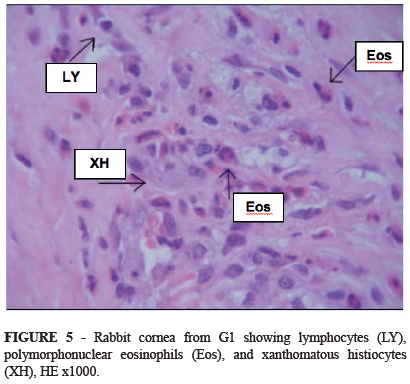
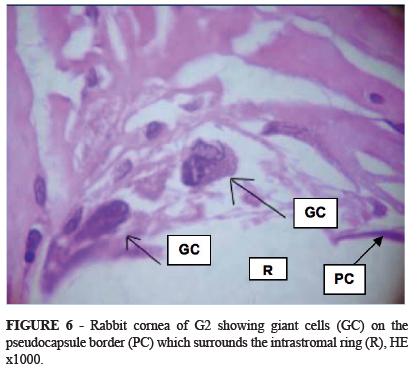
Inflammatory cells (lymphocytes, polymorphonuclear eosinophils and neutrophils and xanthomatous histiocytes) were seen in the stroma adjacent to the ring in G1 (Figure 5). In this region neovascularization was observed 25.2% less in G2 and inflammatory cells 41.7% less in G2 than in G1. Giant cells, histiocytes and pseudocapsule were more frequently observed in G2 by respective margins of 20%, 10% and 26.6% (Table 5 and Figure 6).
Descemet's membrane and the endothelium did not present any alterations in either group.
Discussion
Experimental studies using intracorneal ring implants have been widely performed on the rabbit cornea22-25, due to its similarity to the human cornea even though it lacks the Bowman membrane.
Histopathological studies of humans cornea implanted with an intracorneal ring are extremely rare due to the difficulty of obtaining these corneas and are only possible in cases with indication of corneal transplant or enucleation. Studies in animal species whose corneas are similar to that of humans enable research studies that would be difficult to perform on humans. Therefore, the present study, which analyzes and compares both histopathological and clinical alterations utilizing two Ferrara intracorneal ring types, would be impossible to perform on human beings.
To the best of our knowledge this is the first study of PMMA intracorneal ring coated with chondroitin sulfate which is one of the mucopolysaccharides naturally occurring in the cornea21. Chondroitin sulfate is a glycosaminoglycan present in the matrix of vertebrate connective tissue and is found in the mammalian cornea. In ophthalmology has been used as a wound-healing agent and ocular lubricant. In corneal ulcer and keratoconjunctivitis sicca, chondroitin sulfate exerts anti-inflammatory action. This property of polysulfated glycosaminoglycans is due to its ability to scavenge free radicals, decrease prostaglandin synthesis, block the action of the complement system and reduce interleukin production26,27. These properties may therefore improve ring biocompatibility and reduce complications. Thus, the present study evaluated and compared clinical complications and histopathological alterations in rabbit corneas implanted with two types of Ferrara intracorneal ring segment: the commercially available (without chondroitin sulfate coating) and the Ferrara intracorneal ring segment coated with chondroitin sulfate.
In the present study, clinical evaluation showed that edema decreased in both groups from M1 to M2 and furthermore at M3 (60 days after intracorneal ring implant). The percentage of corneas that showed clinical edema was three times higher in corneas implanted with uncoated rings (FICRS) than those with coated rings (FICRS-CS) (20% versus 6.7%). Corneal edema has been described in many clinical studies in observations that vary from four days to 14 months after surgery28. In a study by Hofling-Lima28,edema occurred in 19.45% of patients who had been implanted with Intacs and Ferrara rings between one week and 22 months post-implant and was associated with infection. The frequency of edema seen in a study by Hofling-Lima28 was to similar our FICRS-implanted group, but in the present study was not associated with infection probably because it was performed on animals with healthy corneas, by a standardized experimental technique and using prophylactic antibiotic treatment with third generation quinolone.
Similarly to edema, clinical corneal vascularization was more frequent with FICRS (26.7%) than with FICRS-CS (6.7%) and occurred only 60 days after the implant in both groups. Case reports studies described corneal vascularization between 12 months and three years after the intracorneal implant17, ,28,29. Nose et al.17 reported vascularization in 50% of cases, a frequency much greater than ours. This discrepancy may be explained by the difference in observation period (60 days in our study), given the possibility that more corneas will develop vascularization with time. But the shorter observation period does not explain the lower percentage of vascularization utilizing rings coated with chondroitin sulfate.
Ring extrusion occurred only in the FICRS-implanted group and was observed 30 days after implant. The extrusion of commercial uncoated intracorneal rings has been described in clinical studies 30,31 where it was reported at 12 months and five years after surgery whereas the extrusion frequency was 13.8% in the Miranda study31. In our FICRS-CS group, the absence of extrusion could be related to the lesser inflammation observed that may be due to the lower antigenicity of rings coated with chondroitin sulfate.
Histopathological analysis showed reduction of corneal epithelium layers in the region over the ring in corneas with FICRS compared to controls. There was also a tendency towards reduction under FICRS-CS. Comparing the number of layers over the ring between the groups, FICRS-CS clearly tended to produce less diminution, suggesting that it may provoke fewer clinical complications such as extrusion. The number of corneal epithelial layers was undiminished in the region adjacent to the ring; the reduction is restricted to the zone over the ring and does not compromise the rest of the cornea. The findings of reduced epithelial layers overlying the intracorneal ring are in agreement with the other four histopathological studies found in the literature25, 32-34, although they did not count the number of layers (quantitative analysis). Samini et al.32, studied eight keratoconic corneas and observed epithelial hypoplasia in five out of eight eyes evaluated after Intacs rings had been removed five to 27 months before keratoplasty. Twa33 and Spirn34 described fewer epithelial layers and hipoplasy over the ring implanted in one Ferrara ring case and one Intacs ring, respectively. The only other study on rabbit corneas was performed on five New Zealand rabbits implanted with Intacs and also found fewer epithelial layers over the ring25. The reduction in epithelial layers seems to be reversible given that several months after the ring explantation, Samini et al.32 observed normal epithelial thickness above the explantation channel scar.
In contrast to the epithelial layer number, similar frequencies of spongiosis and hydropic degeneration, which are signs of edema, were observed in the corneal epithelium regions above and adjacent to the ring in both groups. These alterations, which have not been described in the other histopathological studies, are typical responses of stratified basal epithelium attacked by inflammatory process that may be intra- and especially intercellular. The edema can break the desmosomes, thus forming small vesicles or areas of greater epithelial fragility. The similarity in frequency between groups suggests that such lesions are caused by surgical trauma and not by the rings.
Thinning of the epithelial basement membrane was displayed in both groups but only in the region above the ring, and was less frequent in the FICRS-CS group. No reference to this alteration was found in the four reported studies, but it may be attributable to inflammatory reaction and type II hypersensitivity.
In the corneal stroma, histopathological examination showed three times higher frequency of neovessels in the FICRS group than in FICRS-CS. The fact that a smaller frequency of neovessels was observed at clinical examination demonstrates that the clinical exam underestimated the presence of neovessels. Clinical and histopathological examination showed smaller frequency of neovessels in the chondroitin sulfate group. The latter could be directly related to this lower frequency of neovascularization. In experimental ulcers in horses, the corneas treated with chondroitin sulfate showed lower vascularization than those treated without this compound35.
The frequency of inflammatory cells in corneal stroma was higher in the FICRS group, in which polymorphonuclear eosinophils were predominant, indicating type II hypersensitivity reactions that can be the precursors of stromal neovessels and basement membrane thinning. This lower frequency of inflammatory cells and neovascularization and the absence of extrusion in the chondroitin sulfate group suggest that FICRS-CS is less antigenic. But immunohistochemical study of angiogenesis stimulating factors is required to establish the etiology of these neovessels.
The fact that giant cells were present only in the FICRS-CS group is indicative of macrophage migration, whose interaction with the ring can lead to type IV hypersensitivity late inflammatory reaction that when associated with histiocyte proliferation induced up to a 26.6% higher frequency of pseudocapsule formation. A pseudocapsule may represent an adaptation of the cornea to the ring and we suppose that it provides a possible way for reducing antigenic reaction. The PMMA could be an antigenic agent while the addition of corneal matrix component substances to the rings such as chondroitin sulfate could lead to a closer ideal biocompatibility. PPMA is generally described as inert and well tolerated material but lipidic keratopathy has been a common finding in human implanted intracorneal PPMA rings33 suggesting that it is not totally biocompatible.
Our results showed both clinical and histopathological advantages derived from the chondroitin sulfate coating of the rings in rabbits with healthy corneas. These findings lead us to suppose that such coating may also be beneficial to diseased human corneas. The mechanism by which the chondroitin sulfate leads to these benefits may be through its antiinflammatory action, or via the "encapsulation".
The scarceness of this type of study and the different responses resultant from the two ring types should serve as a stimulus for new experimental studies with longer follow-up and immunohistochemical evaluation in order to obtain better intracorneal implants, especially given their widespread use in very young patients with a long life expectancy.
Conclusion
Ferrara intracorneal rings coated with chondroitin sulfate (FICRS-CS) caused lower frequency of clinical and histopathological alterations than Ferrara intracorneal rings without the coating (FICRS), demonstrating higher biocompatibility of the FICRS-CS.
Received: May 14, 2013
Review: July 15, 2013
Accepted: Aug 16, 2013
Conflict of interest: none
Financial source: none
-
1Krachmer JH, Feder RS, Belin MW. Keratoconus and related non inflamatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293-322.
-
2Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115:37-50.
-
3Buzard K, Fundingsland B. Corneal transplant for keratoconus: results in early and late disease. J Cataract Refract Surg. 1997;23:398-406.
-
4Wang Y, Rabinowitz YS, Rotter JI, Yang H. Genetic epidemiological study of keratoconus: evidence for major gene determination. Am J Med Genet. 2000;93:403-9.
-
5Elias RMS, Lipener C, Uras R, Pavês L. Ceratocone: fatores prognósticos. Arq Bras Oftalmol. 2005;68(4):491-4.
-
6Colin J, Cochener B, Savary G, Malet T. Correcting keratoconus with intracorneal rings. J Cataract Refract Surg. 2000;26(8):1117-22.
-
7Pallikaris GI, Kymionis DG, Astirakakis NI. Corneal ectasia induced by laser in situ keratomileusis. J Refrac Surg. 2001;2711:1796-802.
-
8Rossi L. A clear-eyed view of LASIK Durhammag [Internet]. 2010 Feb/Mar [cited 2011 Oct 17]; 26-29. Available from www.dukehealth.org/eye_center
» link -
9Associação Pan-Americana de Bancos de Olhos. Doação e transplantes de córneas: estatística [internet]. Rio de Janeiro: APABO; 2004 [acesso 29 jan 2012]. Disponível em http://www.apabo.com.br
-
10Troutman RC. Microsurgical control of corneal astigmatism in cataract and keratoplasty. Trans Am Acad Ophthalmol Otolaryngol. 1973;77:OP563-72.
-
11Troutman RC, Swinger C. Relaxing incision for control of postoperative astigmatism following keratoplasty. Ophthalmic Surg. 1980;11:117-20.
-
12Cunha PFA, Alves EAF, Silva FBD, Cunha GHA. Estudo das modificações oculares induzidas pelo implante do anel de FERRARA® em portadores de ceratocone. Arq Bras Oftalmol. 2003;66:417-22.
-
13Alio JL, Shabayek MH, Artola A. Intracorneal ring segments for keratoconus correction: long term follow-up. J Cataract Refract Surg. 2006;32(6):978-85.
-
14Rodrigues LA, Villegas AE, Porras D, Benavides MA, Molina J. Treatment of six cases of advanced ectasia alter LASIK with 6-mm Intacs SK. J Cataract Refract Surg. 2009;25(12):1116-9.
-
15Coskunseven E, Jankov MR 2nd, Hafezi F, Atun S, Arslan E, Kymionis GD. Effect of treatment sequence in combined intrastromal corneal rings and corneal collagen crosslinking for keratoconus. J Cataract Refract Surg. 2009;35(12):2084-91.
-
16Cunha PFA. Técnica cirúrgica para correção de miopia. Anel intraestromal. Rev Bras Oftalmol. 1995;54:19-30.
-
17Nosé W, Neves RA, Burris TE, Schanzlin DJ, Belfort Junior R. Intrastromal corneal ring: 12-month sighted myopic eyes. J Refract Surg. 1996;12(1):20-8.
-
18Hofling-Lima AL, Branco BC, Romano AC, Campos MQS, Moreira H, Miranda D, Kwitko S, de Freitas D, Casanova FH, Sartori M, Schor P, Souza LB. Infections after implantation of intracorneal ring segments. Cornea. 2004;23(6):547-9.
-
19Ferrer C, Alio JL, Montañés AU, Pérez-Santonja JJ, Dias del Rio MA, Toledo JA, Teus MA, Javaloy J. Causes of intraestromal corneal ring segment explantation: clinicopathologic correlation analysis. J Cataract Refract Surg. 2010;36(6):970-7.
-
20Eeartan A, Muftuoglu O. Intracorneal ring segments for keratoconus. Expert Rev Ophthalmol. 2008;3(5):585-91.
-
21Michelacci YM. Collagens and proteoglycans of the corneal extracelular matrix. Braz J Biol Res. 2003;36(8):1037-46.
-
22Barraquer JI. Modification of refraction by means of intracorneal inclusion. Int Ophthalmol Clin. 1966;6:53-78.
-
23Simon G, Barraquer RI. Queratocricoemfitesis: nuevo procedimiento de cirurgia refractiva (estúdio experimental). Arch Soc Esp Oftalmol. 1988;1:87-94.
-
24D'Hermies F, Hartmann C, Von Ey F, Holzkamper C, Renard G, Pouliquen Y. Biocompatibility of a reftractive intracorneal PMMA ring. Fortschr Ophthalmol. 1991;88(6):790-3.
-
25Twa MD, Ruckhofer J, Kash RL, Costello MMS, Schanzlin DJ. Histologic evaluation of corneal stroma rabbits after intrastromal corneal ring implantation. Cornea. 2003;22(2):146-52.
-
26Rashmir-Raven AM, Coyne CP, Fenwich BW, Gaughan EM, Andrews GA, De Bowes RM. Inhibition of equine complement activity by polysulfatated glycosaminiglycans. Am J Vet Res. 1992;53(1):87-90.
-
27Davidson G. Glicosamine and chondroitin sulfate. Compend Contin Educ. 2000;22(5):454-8.
-
28Al-Torbak A, Al-Amri A, Wagner WD. Deep corneal neovascularization after implantation with intracorneal ring segments. Am J Ophthalmol. 2005;140(5):926-7.
-
29Casteluber L, Silva JF, Medeiros HA. Extrusão do anel intra-estromal corneano e vascularização do túnel. Rev Bras Oftalmol. 2007;66(6):403-5.
-
30Bourgues JL, Trong TT, Ellies P, Briat B, Renard G. Intrastromal corneal ring segments and corneal anterior necrosis. J Cataract Refract Surg. 2003;29(6):1228-30.
-
31Miranda D, Sartori M, Francesconi C, Allermann N, Ferrara P, Campos M. Ferrara intrastromal corneal ring segments for severe keratoconus. J Refract Surg. 2003;19(6):645-53.
-
32Samini S, Leger F, Touboul D, Colin J. Histopathological findings after intracorneal ring segment implantation in keratoconic human corneas. J Cataract Refract Surg. 2007;33:247-53.
-
33Twa MD, Kash RL, Costello M, Schanzlin DJ. Morphologic characteristics of lamellar channel deposits in the human eye. A case report. Cornea. 2004;23(4):412-20.
-
34Spirn MJ, Dawson DG, Rubinfeld RS, Burris C, Talamo J, Edelhauser HF, Grossniklaus HE. Histopathological analysis of post - laser - assisted in situ keratomilensis corneal ectasia with intra-stromal corneal ring segment. Arch Ophthalmol. 2005;123:1604-7.
-
35Wouk AFPF, Zulian I, Muller G, Souza ALG. Ação do sulfato de condroitina "A" associado à ciprofloxacina em úlceras de córnea em cavalos. Rev Acad. 2006;4(4):11-20.
Publication Dates
-
Publication in this collection
29 Aug 2013 -
Date of issue
Sept 2013
History
-
Received
14 May 2013 -
Accepted
16 Aug 2013 -
Reviewed
15 June 2013