Abstract
PURPOSE: To investigate the effect of Brassica oleracea herbal balsam on the healing of skin wounds in rats. METHODS: Twenty four rats (Wistar, 60 days, 250 g) were divided into four groups: untreated animals (C) and treated with the ointment (T), subdivided into two experimental times (seven and 16 days). A 3cm² skin wound was made in the back of all animals. 100 ml of the Brassica oleracea was applied twice a day in T group. Biometric analysis was made with images captured at one, four, seven, ten, 13, and 16 days. At seven and 16 days, animals of each group were euthanized. The wound area removed was processed for histological and histomorphometric analysis to quantify birefringent collagen fibers. Statistical analysis was made considering p < 0.05 as significant. RESULTS: Biometric analysis revealed no significant differences between groups in both experimental times studied. However, histomorphometric analysis showed that the number of type I collagen fibers was significantly higher in the specimens of the group T16 compared to the other groups. CONCLUSION: Brassica oleracea accelerated the wound healing process increasing the number of type I collagen fibers and the maturity of the newly formed tissue.
Brassica; Wound Healing; Skin; Rats
acb
7 - ORIGINAL ARTICLE
WOUND HEALING
Effect of Brassica oleracea in rats skin wound healing1 1 Research performed at laboratory of FASBio/University Valley of Paraiba (UNIVAP), Sao Jose dos Campos-SP, Brazil. Part of Master degree thesis, Postgraduate Program in Bioengineering Institute of Research and Development. Tutor: Profa. Dra. Emilia Angela Lo Schiavo Arisawa.
Aline RebollaI; Emilia Angela Lo Schiavo ArisawaII; Paulo Roxo BarjaIII; Maria Belén Salazar PossoIV; Carolina da Silva CarvalhoV
IMaster, Biomedical Engineering, University Valley of Paraiba (UNIVAP), Sao Jose dos Campos-SP, Brazil. Supervised all phases of the study, manuscript writing, critical revision
IIPhD, Full Professor, Oral Biopathology, UNIVAP, Sao Jose dos Campos-SP, Brazil. Conception and design of the study, critical revision
IIIPhD, Associate Professor, Physics, UNIVAP, Sao Jose dos Campos-SP, Brazil. Intellectual and scientific content of the study
IVPhD, Associate Professor, Nursing, UNIVAP, Sao Jose dos Campos-SP, Brazil. Intellectual and scientific content of the study
VMaster, Biomedical Engineering, UNIVAP, Sao Jose dos Campos-SP, Brazil. Technical procedures, statistical analysis
Correspondence Correspondence: Emilia Angela Lo Schiavo Arisawa IP&D, Universidade do Vale do Paraíba (UNIVAP) Avenida Shishima Hifumi, 2411 12244-000 São José dos Campos - SP Brasil Tel/Fax: (55 12)3947-1015 mirela@univap.br
ABSTRACT
PURPOSE: To investigate the effect of Brassica oleracea herbal balsam on the healing of skin wounds in rats.
METHODS: Twenty four rats (Wistar, 60 days, 250 g) were divided into four groups: untreated animals (C) and treated with the ointment (T), subdivided into two experimental times (seven and 16 days). A 3cm2 skin wound was made in the back of all animals. 100 ml of the Brassica oleracea was applied twice a day in T group. Biometric analysis was made with images captured at one, four, seven, ten, 13, and 16 days. At seven and 16 days, animals of each group were euthanized. The wound area removed was processed for histological and histomorphometric analysis to quantify birefringent collagen fibers. Statistical analysis was made considering p < 0.05 as significant.
RESULTS: Biometric analysis revealed no significant differences between groups in both experimental times studied. However, histomorphometric analysis showed that the number of type I collagen fibers was significantly higher in the specimens of the group T16 compared to the other groups.
CONCLUSION:Brassica oleracea accelerated the wound healing process increasing the number of type I collagen fibers and the maturity of the newly formed tissue.
Key words:Brassica. Wound Healing. Skin. Rats.
Introduction
Chronic wounds are a serious public health problem, affecting about 1% of the world population, irrespective of gender, age or race. Despite a multidisciplinary approach, the management of patients with chronic wounds is a major challenge1. In addition to their profound effect on the quality of life of affected individuals, about 2% of the health budget is destined to wound care2-4. Successful wound management requires an understanding of the wound healing process and the identification and prevention of factors that may delay or interrupt wound healing2-5. Early wound treatment permits to reduce public health expenditure and prevents impairment of the quality of life of affected patients2-5.
Impaired skin integrity leads to the development of a wound that could involves different tissues, from the epidermis to deeper layers such as muscles3,4. Immediately after injury, the blood clots forms a scab that protects the injured area2. The consequent release of chemical mediators induces an acute inflammatory response characterized by the presence of neutrophils, followed later by macrophages and lymphocytes2,6-8. The activation, proliferation and differentiation of undifferentiated mesenchymal cells give origin to activated fibroblasts present in the granulation tissue6-8. These fibroblasts become bipolar and secrete extracellular matrix components, including fibronectin, proteoglycans and types I and III collagen fibers6-10. Collagen synthesis is induced hours after injury, but only becomes significant after one week and can continue for 12 to 18 months9-12.
Studies have shown an economically significant effect of plaster made from the leaves of Brassica sp on the healing of skin wounds2-4,13. Brassica oleracea is an edible vegetable whose leaves are used by the population as a skin wound healing agent. Despite its use in folk medicine, scientific studies of this plant are scarce, a fact that has encouraged the present investigation. The herbal balsam Debridan® was fabricated from the active ingredient of Brassica oleracea var. capitata - glycolic extract 10% and is indicated for the treatment of skin wounds14,15.
Biometric analysis permits macroscopic assessment of the efficacy of the repair process based on wound contraction and consequent reduction in the wound area16,17. On the other hand, histomorphometry is an excellent method for microscopic analysis of the wound area, and permits to quantify collagen fibrils during the different stages of wound healing9,10,17-19.
The aim of the present study was to evaluate the effect of Brassica oleracea var. capitata - glycolic extract 10% on the healing of surgical skin wounds in rats using biometric and histomorphometric analyses.
Methods
The present study was approved by the Research Ethics Committee of University Valley of Paraiba (UNIVAP) under the protocol A25/CEUN2010 and in accordance with Federal Law No. 6.638 and the guidelines of the Brazilian College of Animal Experimentation.
All experimental procedures were conducted at the Laboratory of Photodynamic Therapy, Institute of Research and Development (IP&D), UNIVAP.
Twenty-four Wistar rats (Rattus norvegicus, var. albinus), 60 days old, with a mean weight of approximately 280 g, obtained from IP&D, UNIVAP. The animals were housed in individual cages at a controlled temperature under a 12-h light/dark cycle and received a standard diet comprising pelleted food (Labina; Purina Nutrients Ltda, Sao Paulo-SP, Brazil) and mineral water ad libitum. The animals were transferred to the Laboratory of Photoacoustic, UNIVAP, where the experiment was carried out.
For surgical proceedings the animals were weighed and anesthetized by intramuscular administration of 10% ketamine hydrochloride (Dopalen®, 0.1 ml/100 g body weight) and 2% xylazine hydrochloride (Calmium®, 0.1 ml/100 g body weight). An area of 6 x 4 cm (length x width) was shaved in the dorsal region by drawing an imaginary line caudally from the lower margin of the ear. Antisepsis of the area was performed with 4% alcohol-based iodine. In the center of the shaved area, a surgical skin lesion (Ø=3 cm), 1cm below the bone prominence, was created using a metallic circular marker to delimit the area, and with the aid of a surgical scalpel, the dorsal muscle fascia was exposed. For pain control, animals received aspirin (100mg/kg weight) diluted in water, until euthanasis. The animals were randomly divided into two groups of 12 animals each, subdivided in two experimental times: control and treated animals (C and T, respectively), and the experimental times of seven and 16 days. The wound was cleaned daily with 0.9% saline, followed by topical application of 100ml Brassica oleracea var. capitata - glycolic extract 10% (Debridan®) twice a day for the treated group (T).
Biometric and histomorphometric analysis
For biometric analysis, images of the skin wounds at one, four, seven, ten, 13 and 16 days after surgery were captured with an Optical SteadyShot DSC-W350 digital camera (14.1 megapixels), and positioned at a distance of 15 cm. The Image J program was applied to digital images, 25 x magnification, to calculate the diameter of the wound area (cm2).
Six animals of each group (C and T) were sacrificed at days seven and 16, using the anesthetic procedure described above, followed by intracardiac administration of 0.5 ml of potassium chloride. Specimens of the wound area were removed, fixed in 10% formalin, and sent to the Department of Pathological Anatomy, Sao Paulo University Hospital, for slide preparation according to routine procedures. Slices of 5µm were stained with hematoxylin-eosin and picrosirius, and analyzed under a polarized light microscope using the ACT-@U program.
Birefringent collagen fibers were quantified in the dermis (µm2) using digital images of the histological sections (x400) captured with a Nikon Digital Sight DS-5M digital camera coupled to a microscope (Nikon-Optizon x0.8-2.0). The Image Pro Plus Program has been used to measure the wound area and histomorphometric analysis at x25 magnification.
Statistical analysis
The normality of the data was tested by the Kolmogorov-Smirnov test using the OriginPro® 8.0 SRO program. Differences between groups were evaluated by one-way ANOVA for parametric data (Minitab®), followed by the Tukey post-test. p<0.05 was considered to be significant.
Results
Macroscopic biometric analysisz
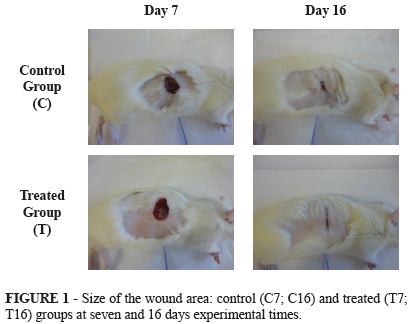
Figure 1 presents the macroscopic aspects of the wounds over the time course of the experiment. No sign of abscess formation in the early phase (seven days) or hypertrophic scars in the final phase (16 days) was observed. The digital images of the wounds permitted to evaluate wound area progression in the groups of the experimental times of seven days and 16 days.
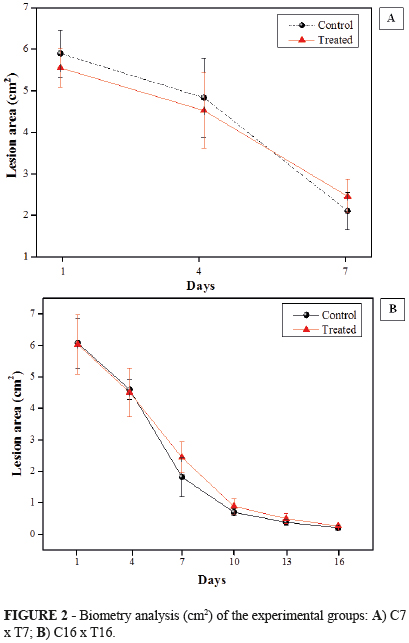
Figure 2 shows the graphic comparative of wound areas between the different groups and experimental times studied. At seven days after surgery, the reduction in wound diameter was greater in group C7 than in T7 group (64.34% versus 55.59%, respectively). The wound was almost completely closed in both groups after 16 days (reduction of 96.7% and 95.85% in C16 and T16, respectively). However, comparison of the biometry results showed no significant differences. It should be noted that the wounds of animals that received herbal treatment were cleaned and treated daily, which may have determined the slight difference observed in the absolute values.
Histomorphometry
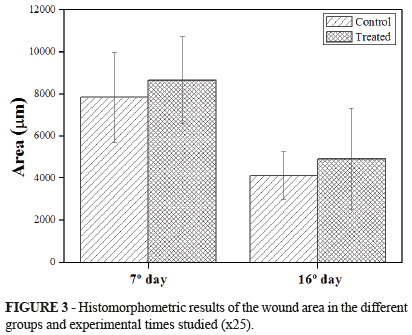
The wound area (µm), number (µm2) and distribution (types I and III) of collagen fibers were evaluated in the histomorphometric analysis. Figure 3 and Table 1 show the absolute values of the wound area. It is possible to observe the lower values in the control groups at seven days (7.83 µm) and 16 days (4.12 µm) compared to the treated groups (T7 = 8.64 µm and T16 = 4.91 µm, respectively). In addition, the best results, i.e., the smallest wound area, were observed for specimens of group C16 (Table 1). However, these results were not statistically significant.
The number and type of birefringent collagen fibers per µm2 were determined by polarized light microscopy at the different experimental times studied (Table 1, Figures 4 and 5). Thin (type III) and thick (type I) collagen fibers could be distinguished. Types I and III collagen fibers appeared by day 4 or 5 of wound healing. Differences in the predominant fiber type and in the number of collagen fibers were observed depending on the stage of the tissue repair process.
The number of thin fibers (Table 1, Figure 4) was higher in group C7 (mean: 0.014) than in group T7 (mean: 0.002), indicating a predominance of type III collagen fibers at the beginning of the inflammatory process.
The predominance of type I collagen fibers (Table 1, Figure 5) was observed on day 16, especially in group T16 (mean: 0.096) (p<0.05), when compared to group C16 (mean: 0.058).
The present results indicate a greater tensile strength of the wounds of animals treated with the herbal ointment when compared to the untreated groups (Figures 5a and 5b).
Discussion
Tissue injuries, when reaching the different layers of the skin, alter their anatomical and physiological structure and trigger the process of tissue repair. Wound healing can occur by first intention or by second intention, when the injury and tissue destruction are extensive2,4,6.
Type I collagen accounts for 80 to 90% of the extracellular matrix of intact skin, whereas type III collagen corresponds to only 10 to 20%11,12. At the beginning of the repair process, the extracellular matrix consists of 30% type III collagen, resulting in a more fragile matrix. Collagen fibers are birefringent and therefore appear bright against a dark background when analyzed under a polarized light microscope9,10,18. This technique permits the use of collagen fibrils to characterize the different stages of wound healing9,18.
In addition, the collagen fibrils present in this matrix are strongly glycosylated, thin and show a parallel and not intermingled orientation1,12,19. After one week, the strength of the extracellular matrix is 3% of that of intact tissue. Collagenases and proteases cleave and degrade collagen fibers. This process is supplied by the deposition of collagen, increasing the thickness and organization of collagen fibrils and contributing to a more resistant matrix. After three weeks, tissue strength has increased by 30% and the typical original strength is reached by three months (80%)1,11,12.
About 80 to 90% of intact skin is formed by type I collagen19. However, in the case of extensive tissue injury, type III collagen predominates in the extracellular matrix during the early stage of wound healing, which is characterized by thin, parallel and not intermingled fibrils9,10. The number of thin fibers was higher in group C7 than in group T7, indicating a predominance of type III collagen fibers at the beginning of the inflammatory process1,11,12,19. These fibers are gradullay absorbed and replaced with type I collagen fibers, increasing the strength, organization and thickness of the extracellular matrix as well as the number of cross-links among fibrils. As a consequence, a predominance of type I collagen fibers was observed on day 16, especially in group T16 when compared to group C16. Since an increase in the number of type I collagen fibers increases the tensile strength of newly formed tissue1,12,16,19.
The monitoring of the progress of wound healing is necessary since wound contraction is greater during the maturation phase and consists of centripetal movement of the edges13,20. Several instruments are used for macroscopic evaluation of this process such as rulers, pachymeters, photographs and mathematical modeling14,20.
We observed a greater tensile strength of the wounds of animals treated with the herbal ointment when compared to the untreated groups. According to Gantwerker and Hom and Montes, the presence of type III collagen fibers indicates the early phase of wound healing, as observed for specimens of the control group.
Since the tissue repair process comprises different stages and the thickness of type I collagen fibers indicates a more advanced stage, the present findings demonstrate that treatment with the herbal ointment yielded the best results. These were confirmed by the predominance of type I collagen fibers in the group treated for 16 days, suggesting that in these specimens the wound healing process was in a more advanced stage and the colagen fibers presented major grade of maturation when compared to the observed in the control group.
Conclusion
Treatment of wounds in skin with Brassica oleracea var. capitata - glycolic extract 10% (Debridan®) showed a significant increase in the number of mature collagen fibers after 16 days of treatment, accelerating the healing process, in rats.
Received: May 27, 2013
Review: July 25, 2013
Accepted: Aug 22, 2013
Conflict of interest: none
Financial source: none
- 1. Schreml S, Szeimies RM, Prantl L, Landthaler M, Babilas P. Wound healing in the 21st century. J Am Acad Dermatol. 2010;63(5):866-81.
- 2. García-Gubern CF, Colon-Rolon L, Bond MC. Essential concepts of wound management. Emerg Med Clin North Am. 2010;28(4):951-67.
- 3. Gorecki C, Lamping DL, Brown JM, Madill A, Firth J, Nixon J. Development of a conceptual framework of health-related quality of life in pressure ulcers: a patient-focused approach. Int J Nurs Stud. 2010;47(12):1525-34.
- 4. de Bruin SR, Heijink R, Lemmens LC, Struijs JN, Baan CA. Impact of disease management programs on healthcare expenditures for patients with diabetes, depression, heart failure or chronic obstructive pulmonary disease: a systematic review of the literature. Health Policy. 2011;101(2):105-21.
- 5. Mandelbaum S, Di Santis E, Mandelbaum M. Cicatrization: current concepts and auxiliary resources - Part I. An Bras Dermatol. 2003;78(4):393-410.
- 6. Kondo T, Ishida Y. Molecular pathology of wound healing. Forensic Sci Int. 2010;203(1-3):93-8.
- 7. Gantwerker EA, Hom DB. Skin: histology and physiology of wound healing. Clin Plast Surg. 2012;39(1):85-97.
- 8. Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35-43.
- 9. Montes GS, Junqueira LC. Biology of collagen. Rev Can Biol Exp. 1982;41(2):143-56.
- 10. Ruszczak Z. Effect of collagen matrices on dermal wound healing. Adv Drug Deliv Rev. 2003;55(12):1595-611.
- 11. Guidugli Neto J. The effect of roentgen radiation on the capillary sprouts and superficial loops of granulation tissue. I. Quantitative study of the vascular volume. Rev Odontol Univ Sao Paulo. 1987;1(4):6-8. De acordo com PubMed
- 12. Guidugli Neto J. The effect of Roentgen radition on the capillary sprouts and superficial loops of the granulation tissue II. Ultrastrucutral aspects. Rev Odontol Univ São Paulo. 1992;6:66-71.
- 13. Mandelbaum S, Di Santis E, Mandelbaum M. Cicatrization: current concepts and auxiliary resources - Part II. An Bras Dermatol. 2003;78(5):525-42.
- 14. Mohammad S, Muhammad M. Evalution op the gastric antiulcerogenic effects of Solanum nigrum, Brassica oleracea and Ocimum basilicum in rats. J Ethnopharmacol. 1989;27(1-2):163-76.
- 15. Monsalves C, Cano A. La familia Brassicaceae em la provincia de Huaylas, Áncash Rev Peru Biol. 2003;10(1):20-32.
- 16. Beldon P. Basic science of wound healing. Surgery. 2010;28(9):409-12.
- 17. Martin P, Teodoro WR, Velosa AP, de Morais J, Carrasco S, Christmann RB, Goldenstein-Schainberg C, Parra ER, Katayama ML, Sotto MN, Capelozzi VL, Yoshinari NH. Abnormal collagen V deposition in dermis correlates with skin thickening and disease activity in systemic sclerosis. Autoimmun Rev. 2012;11(11):827-35.
- 18. Roblyer D, Richards-Kortum R, Sokolov K, El-Naggar AK, Williams MD, Kurachi C, Gillenwater AM. Multispectral optical imaging device for in vivo detection of oral neoplasia. J Biomed Opt. 2008;13(2):024019.
- 19. Teller P, White TK. The physiology of wound healing: injury through maturation. Surg Clin North Am. 2009;89(3):599-610.
- 20. Delode J, Rosow E, Roth C, Adams J, Langevin F. A wound-healing monitoring system. Methode de suivi de la cicatrisation ITBM - RBM. 2001 February, 2001:49-52.
Publication Dates
-
Publication in this collection
29 Aug 2013 -
Date of issue
Sept 2013
History
-
Received
27 May 2013 -
Accepted
22 Aug 2013 -
Reviewed
25 July 2013