Abstracts
PURPOSE: To characterize of the intestinal microbiota of patients with short bowel syndrome (SBS) admitted to the Metabolic Unit of a University Hospital. METHODS: Fecal samples were evaluated, and biochemical tests were conducted only in the case of SBS patients. The nutritional status was assessed via anthropometric measurements and evaluation of food intake by means of a food questionnaire. The pathogenic strains were detected with the aid of cultures and specific biochemical tests in aerobic medium, for determination of species belonging to the Family enterobacteriaceae. Anti-sera were applied to each isolated E. coli strain, for determination of their possible pathogenicity. Molecular methodology was employed for establishment of the intestinal bacterial microbiota profile RESULTS: A lower amount of microorganisms of the family enterobacteriaceae per gram of stool was observed in the case of patients with SBS. However, molecular analysis showed maintenance of the bacterial species ratio, which is equivalent to a healthy intestinal microbiota. CONCLUSION: Despite the massive removal of the small bowel, frequent use of antibiotics, immune system depression, presence of non-digested food in the gastrointestinal tract, and accelerated intestinal transit, the ratio between intestinal bacterial species remain similar to normality.
Short Bowel Syndrome; Parenteral Nutrition; Digestive System Surgical Procedures
OBJETIVO: Caracterizar a microbiota intestinal de pacientes com síndrome do intestino curto (SIC) internados na Unidade Metabólica do Hospital Universitário. MÉTODOS: Foram avaliadas amostras de fezes e exames bioquímicos, estes últimos somente dos pacientes. A avaliação do estado nutricional foi feita a partir de medidas antropométricas e a avaliação do consumo alimentar por meio de inquérito alimentar. Para detecção de cepas patogênicas foram realizados cultivos e testes bioquímicos específicos em meio aeróbico para determinação de espécies da família enterobacteriaceae. Em cada cepa de E. coli isolada foram aplicados anti-soros para determinação de possível patogenicidade. Metodologia molecular também foi utilizada para determinação do perfil da microbiota intestinal bacteriana. RESULTADOS: Observou-se menor quantidade de microorganismos da família enterobacteriaceae por grama de fezes em pacientes com SIC. Porém a análise molecular mostrou a manutenção na proporção de espécies bacterianas, equivalente a microbiota intestinal saudável. CONCLUSÃO: Nossos resultados sugerem que apesar da retirada maciça do intestino delgado, uso freqüente de antibióticos, depressão do sistema imune, presença de alimentos não digeridos no trato gastrintestinal e trânsito intestinal acelerado, a proporção entre as espécies bacterianas intestinais permanecem similares à normalidade.
Síndrome do Intestino Curto; Nutrição Parenteral; Procedimentos Cirúrgicos do Sistema Digestório
5 - ORIGINAL ARTICLE
CLINICAL INVESTIGATION
Cyclic parenteral nutrition does not change the intestinal microbiota in patients with short bowel syndrome1 1 Research performed at Division of Intensive Care of Department of Surgery, Ribeirão Preto Medical School, University of São Paulo (USP), Brazil.
Nutrição parenteral cíclica não altera a microbiota intestinal em pacientes com a síndrome do intestino curto
Eduarda de Castro FurtadoI; Julio Sergio MarchiniII; Carol Kobori da FonsecaIII; Paulo Sérgio Rodrigues CoelhoIV; Mayra Gonçalves MeneguetiV; Maria Auxiliadora-MartinsVI; Anibal Basile-FilhoVII; Vivian Marques Miguel SuenVIII
IMS, Nutritionist. Conducted the research
IIMD, PhD, Professor of the Clinical Nutrition Division, Department of Internal Medicine, Medical School of Ribeirão Preto, University of São Paulo (FMRP-USP). Ribeirão Preto - SP, Brazil. Had primary responsibility for the final content
IIIMD, Biologist. Responsible for data acquisition
IVMD, PhD, Professor of the Department of Cell and Molecular Biology and Pathogenic Bioagents, Medical School of Ribeirão Preto, University of São Paulo (FMRP-USP). Ribeirão Preto - SP, Brazil. Wrote the manuscript
VFellow Master degree. Nursing School of Ribeirão Preto Medical School of the University of São Paulo (FMRP-USP), Ribeirão Preto - SP, Brazil. Responsible for data acquisition and statistical analysis review
VIMD, PhD, Affiliate Professor, Intensive Care Division, Department of Surgery and Anatomy, Medical School of Ribeirão Preto, University of São Paulo, (FMRP-USP). Ribeirão Preto - SP, Brazil. Responsible for data acquisition and critical review of the manuscript
VIIMD, PhD, Associate Professor, Head of Intensive Care Division, Department of Surgery and Anatomy, Medical School of Ribeirão Preto, University of São Paulo, (FMRP-USP). Ribeirão Preto - SP, Brazil. Responsible for data acquisition and critical review of the manuscript
VIIIMD, PhD, Physician of the Nutrition Support Team, Clinical Nutrition Division, Department of Internal Medicine, Medical School of Ribeirão Preto, University of São Paulo, (FMRP-USP). Ribeirão Preto - SP, Brazil. Responsible for study design, intellectual and scientific content, and use of the English language during drafting of the manuscript
Correspondence Correspondence: Vivian Marques Miguel Suen Faculdade de Medicina de Ribeirão Preto-USP. Departmento de Medicina Interna Av. dos Bandeirantes, 3900 14 049 900. - Ribeirão Preto - SP - Brazil Tel.: (55 16) 3636-7804 Fax: (55 16) 3636-7804 vmmsuen@gmail.com
ABSTRACT
PURPOSE: To characterize of the intestinal microbiota of patients with short bowel syndrome (SBS) admitted to the Metabolic Unit of a University Hospital.
METHODS: Fecal samples were evaluated, and biochemical tests were conducted only in the case of SBS patients. The nutritional status was assessed via anthropometric measurements and evaluation of food intake by means of a food questionnaire. The pathogenic strains were detected with the aid of cultures and specific biochemical tests in aerobic medium, for determination of species belonging to the Family enterobacteriaceae. Anti-sera were applied to each isolated E. coli strain, for determination of their possible pathogenicity. Molecular methodology was employed for establishment of the intestinal bacterial microbiota profile
RESULTS: A lower amount of microorganisms of the family enterobacteriaceae per gram of stool was observed in the case of patients with SBS. However, molecular analysis showed maintenance of the bacterial species ratio, which is equivalent to a healthy intestinal microbiota.
CONCLUSION: Despite the massive removal of the small bowel, frequent use of antibiotics, immune system depression, presence of non-digested food in the gastrointestinal tract, and accelerated intestinal transit, the ratio between intestinal bacterial species remain similar to normality.
Key words: Short Bowel Syndrome. Parenteral Nutrition. Digestive System Surgical Procedures.
RESUMO
OBJETIVO: Caracterizar a microbiota intestinal de pacientes com síndrome do intestino curto (SIC) internados na Unidade Metabólica do Hospital Universitário.
MÉTODOS: Foram avaliadas amostras de fezes e exames bioquímicos, estes últimos somente dos pacientes. A avaliação do estado nutricional foi feita a partir de medidas antropométricas e a avaliação do consumo alimentar por meio de inquérito alimentar. Para detecção de cepas patogênicas foram realizados cultivos e testes bioquímicos específicos em meio aeróbico para determinação de espécies da família enterobacteriaceae. Em cada cepa de E. coli isolada foram aplicados anti-soros para determinação de possível patogenicidade. Metodologia molecular também foi utilizada para determinação do perfil da microbiota intestinal bacteriana.
RESULTADOS: Observou-se menor quantidade de microorganismos da família enterobacteriaceae por grama de fezes em pacientes com SIC. Porém a análise molecular mostrou a manutenção na proporção de espécies bacterianas, equivalente a microbiota intestinal saudável.
CONCLUSÃO: Nossos resultados sugerem que apesar da retirada maciça do intestino delgado, uso freqüente de antibióticos, depressão do sistema imune, presença de alimentos não digeridos no trato gastrintestinal e trânsito intestinal acelerado, a proporção entre as espécies bacterianas intestinais permanecem similares à normalidade.
Descritores: Síndrome do Intestino Curto. Nutrição Parenteral. Procedimentos Cirúrgicos do Sistema Digestório.
Introduction
Adult individuals are considered to be colonized by more than 100 trillion microorganisms. Most of these microorganisms colonize the gastrointestinal tract, with more than 400 species of non-pathogenic enterobacteria performing fundamental functions for maintenance of the health status 1. This includes protection against epithelial cell diseases, food digestion, interference with endogenous and exogenous components, immunological potential, and prevention of colonization of the gastrointestinal tract by pathogens2,3.
Intestinal insufficiency, which occurs after extensive resections of the small bowel, with a remaining bowel of less than 200 cm courses with malabsorption and energy-protein malnutrition4 is known as short bowel syndrome (SBS). The survival of patients with SBS depends on parenteral nutrition therapy5. However, infectious complications are not unusual and represent the second cause of death among these individuals6. These infections mainly involve the catheters used for parenteral nutrition infusion. They are possibly related to the translocation of intestinal bacteria, since microorganisms that commonly colonize the gastrointestinal tract such as Enterococcus and Escherichia coli have been detected in 13% and 4% of infected catheters, respectively7. Studies have also pointed out that bacterial translocation may be a probable route for the occurrence of sepsis/septic shock8-10.
The elimination of colonization sites by resection, the frequent use of antibiotics due to recurrent infections, the presence of undigested food in the remaining bowel, and the accelerated intestinal transit may lead to changes in the intestinal microbiota11. On this basis, the objective of the present investigation was to characterize the intestinal microbiota of adult patients with SBS undergoing cyclic parenteral nutrition and to compare it with the microbiota of healthy controls.
Methods
This cross-sectional study was conducted from March 2009 to January 2010 at the Clinics Hospital of the Ribeirão Preto Medical School, University of São Paulo, a Brazilian reference center for the treatment of patients with this syndrome.
Seven SBS patients submitted to cyclic parenteral nutrition at the Metabolic Unit of our institution were enrolled in the study (Patient group), which corresponded to all the patients attended during this period. Exclusion criteria were the presence of infectious conditions with clinical signs and/or the use of antibacterial agents. The Control group consisted of seven healthy and eutrophic individuals from the community, matched with the patients for sex and age. The exclusion criteria for the Control group were smoking, alcohol consumption, previous surgeries of the gastrointestinal tract, chemotherapy, radiotherapy, use of antibiotics, use of probiotics, and the presence of diseases involving the gastrointestinal tract such as Chron's disease and irritable colon syndrome. This study was approved by the Ethics Committee of our Intitution (Protocol 10328/2008), and written informed consent was obtained from all the recruited individuals.
Study design
The experimental design consisted of recruiting controls on a single occasion and recruiting patients at two moments, with an interval of one hospitalization between times. Nutritional status analysis and fecal samples collection for microbiota analysis were performed at each time point.
Nutritional status evaluation
Food consumption was assessed using the methodology described by Pereira et al.12 for the "Habitual Food Intake Day", applied by a trained interviewer. Data were later analyzed with the aid of the Nutwin® software, and the obtained values were compared to those recommended by the United States Department of Agriculture (USDA) in the Dietary Reference Intakes (DRI) document13 referring to the Estimated Average Requirements (EAR) values. In the case of the nutrients for which the recommended value has not yet been defined, the Adequate Intake (AI) values were used as reference. The values obtained from the food surveys were standardized as percentages of the recommended values. Resting energy expenditure (REE) was estimated using the predictive equations proposed by Harris and Benedict14.
The following anthropometric measurements were achieved according to the norms of the World Health Organization (WHO)15: weight, height, tricipital skin folds (TS) and subscapular skin fold (SSF), waist circumference (WC), arm circumference (AC), and arm muscle circumference (AMC). An electronic scale (Filizola PL 200, São Paulo, Brazil) was used for measuring the weight, in kg, with a precision of 0.1 kg.
Only the Patient group was submitted to laboratory assessment of the nutritional status, with the patients being routinely subjected to biochemical examinations as part of the follow-up at the Institution. The following laboratory exams were performed: lipid profile, hemoglobin, hematocrit, mean corpuscular volume, unsaturated iron binding capacity (UIBC), folic acid, serum iron, serum zinc, serum magnesium, total proteins, and albumin. The results were used in this investigation were those that were the closest to the collection of the patient's fecal sample, with a maximum interval of 3 months.
Determination of the intestinal microbiota
- Isolation and identification of the intestinal bacteria by aerobic culture
A fecal sample was employed for analysis of the intestinal microbiota. Two samples were collected from each patient, with one hospitalization interval between them. If any unexpected event occurred during the hospitalization in question, the sample was discarded or not collected, with collection being postponed to the next hospitalization. The individuals were informed about the method of sterile collection16 and the need to evacuate directly into the plastic collector provided for them. They also received a pair of rubber gloves and a sterile collecting spatula. After collection, the material was placed in an isothermal box chilled with ice and immediately transported to the laboratory for microbiota analysis. The process started with the macroscopic observation of the collected feces, which involved analysis of characteristics such as color, texture, and the presence of mucus, blood, and food remains.
The initial procedure for the characterization of the intestinal microbiota was based on the guidelines of the National Committee for Clinical Laboratory Standards16 and consisted of isolating the bacterial colonies that could be cultured in an aerobic environment. Part of the fecal sample was seeded in triplicate on MacConkey agar and Mueller-Hinton blood agar plates and incubated at 37ºC for 24 hours. After this period, macroscopically different colonies were isolated and submitted to biochemical tests for identification.
The isolated strains were first subjected to differential Gram staining. Gram-positive strains were assessed by the catalase, coagulase, and oxidase tests. Gram-negative strains were evaluated in terms of some metabolic activities using indicators contained in Rugai medium and Simmons citrate medium, and were tested for motility on semisolid agar at 37ºC and by the oxidase reaction.
A quantitative method for bacterial count based on plating on solid medium was also employed. To this end, feces (1 g) were diluted in 10 mL filtered phosphate buffer solution (PBS), which was then submitted to serial dilutions ranging from 102 to 109 mg feces/mL PBS. Then, 100 µL of these dilutions were plated onto MacConkey agar, Mueller-Hinton blood agar, Salmonella-Shigella (SS), mannitol, and bile-esculin. After 24 hours of incubation at 37ºC, colony forming units (CFU) were counted, and the presence or absence of positivity was determined in the Agar media used for the specific tests. Thus, considering the dilution, the number of viable bacteria per mL of the original suspension was calculated.
- Determination of the intestinal microbiota by genomic fecal DNA sequencing
Two methods of genomic DNA extraction were used. One of them was based on the method of Ley et al.17, whereas the other followed the instructions of the manufacturer of the QIAamp DNA Stool® product, QIAGEN®.
Using this DNA, the amplification reaction of the intergenic region 16S DNAr was carried out in 50 µL of a reaction mixture containing 5 µL buffer (100 mM HCl ; 500 mM KCl ), 6 µL 25 mM MgCl2, 1 µL 2.5 mM dNTP, and 1 µL (10 pmol) of the universal 1391R (5' - GACGGGCGGTGWGTRCA-3') primer and the specific primer for bacteria 8F (5' - AGAGTTTGATWCTGGCTCAG -3'), 0.40 µL Taq DNA polymerase (5 U µL-1) (Promega®, Madison®, EUA), and 2 µL (100 ng) of DNA template sample. The amplification reactions consisted of an initial denaturation step (95ºC for 3 minutes), followed by 35 intermediate cycles (94ºC for 1 minute, 60 ºC for 1 minute, and 72ºC for 3 minutes), a final extension step (72ºC for 5 minutes), and cooling (15ºC for 15 minutes). The amplified fragments were separated by 1.2% agarose gel electrophoresis in Tris-Acetate-ETSA (TAE) buffer at 100 V for 30 minutes, and the bands resolved on the gel were visualized after ethidium bromide staining under ultraviolet illumination and photographed. The pCR2.1-TOPO vector of the TOPO TA cloning kit®, Invitrogen®, was utilized for the binding reaction, and the reaction product was used for electroporation of electrocompetent cells prepared from the E. coli strain DH10β
Electroporated cells were cultured in LB medium containing 50 µg/mL ampicililin, 40 µg/mL X-Gal, and 0.1 mM IPTGm, selection drugs determined by the protocol of the TOPO vector kit. Plates were incubated at 37ºC, and positive colonies were selected as white colonies. The plasmids with inserted DNA fragments were identified by their ability to form colorless colonies when grown in medium containing X-Gal/IPTG, in contrast to the plasmids with no inserted fragments, which formed blue colonies. White colonies were submitted to plasmid DNA extraction.
A specific volume (1.5 mL) of the culture of the selected drugs in LB medium was employed for plasmid DNA extraction (Miniprep) by the alkaline lysis method18. The extracted DNA was quantitated and sequenced, and chromatograms were recorded and submitted to analysis of similarity using the BLAST19 and Ribosomal Database Project20 software.
Statistical analysis
An exploratory analysis of the data was performed by using the SPSS® software, version 18. The basic objective of this methodology is to describe a series of values of the same nature, providing an overall view of the variation of these values. The rank Spearman correlation coefficient21 was used for determination of the correlation of the results regarding the quantity of bacterial types and other variables possibly affecting this item. The Mann-Whitney test was employed for analysis of unpaired nonparametric samples, and the Wilcoxon signed rank test was utilized for examination of paired nonparametric samples. The level of significance was set at 5%.
Results
The average age of the subjects belonging to the Patient and Control groups were 58 years (SD 12) and 56 (SD 8), respectively. Both groups were composed of three women and four men.
For all the patients, the reason for enterectomy was mesenteric ischemia (100% of the samples), with a large resection of different intestinal segments. The length of the remaining small bowel ranged from 10 to 100 cm after the Treitz angle, with or without preservation of the ileocecal valve. The condition of the large bowel ranged from intact to removal of the transverse colon. The ileocecal valve and colon segments were removed from 3 patients, whereas in patients with a preserved ileocecal valve the colon was also preserved.
The more prevalent secondary diseases in the Patient group were high blood pressure (71% - 5 patients) and chronic renal failure (57% - 4 patients). The classes of medication more frequently prescribed for this group were: nutritional supplements ranging from a single vitamin/mineral to complete polyvitamin supplements (6 patients), a proton pump inhibitor (5 patients), and antihypertensive drugs (4 patients). The mean interval between parenteral nutrition cycles was 23±8 days.
Statistical analysis showed that there was no significant variation in food consumption by the patients during the study period, and that food consumption was similar to that of the Control subjects. Estimated REE did not differ significantly between the Patient and Control groups.
According to the anthropometric evaluation, the Patient group was classified as having protein-energy undernutrition, whilst the Control group was classified as eutrophic. Anthropometric data, energy intake, and total bacterial count for patients and controls are listed in Table 1.
Analysis of the intestinal microbiota revealed that the bacterial diversity of patients and controls was equivalent (p= 0.21). However, when the 2 categories of bacteria (Gram-positive and Gram-negative) were analyzed, the Patient group showed greater diversity of Gram-negative bacteria as compared to the Control group (p= 0.029) and a similar diversity of Gram-positive bacteria (p= 0.072). Bacterial diversity did not differ between the repeated collections from the Patient group (p=0.916), with similar quantities of Gram-negative (p= 0.891) and Gram-positive bacteria (p= 0.713) (Figure 1).
We also observed that the family enterobacteriaceae, represented here by isolates of Gram-negative bacteria, corresponded to about 64% of the total variety of isolated bacterial types. Among these, the genus that was most frequently detected both in both the Control and Patient groups was Escherichia coli, which was present in all the control samples and in all but one patient sample.
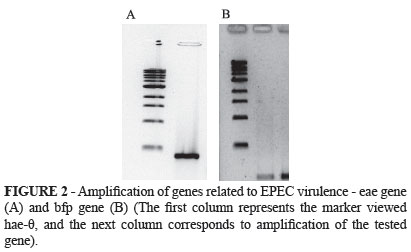
We investigated two genes related to enteropathogenic E. Coli virulence by using the strain E2348/69, namely genes eae and bfp. We also examined the gene ipaH, related to enteroinvasive E.coli, by using the strain MA245/5. Figures 2 and 3 illustrate the agarose gel electrophoresis. The first visualized column represents the θ-hae marker; the following column corresponds to amplification of the tested gene.
We also noted that the absence of the ileocecal valve did not influence the intestinal bacterial variety of the patients. The diversity of both the Gram-negative and Gram-positive intestinal bacteria of patients who took proton pump inhibitors was similar to that of the patients who did not use this medication. The two analysis methods also evidenced the prevalence of the genus Lactobacillus, followed by non-cultivable microorganisms (bacteria and lactobacilli).
Discussion
Microbiological analysis of the feces revealed a higher prevalence of the family enterobacteriaceae in patients with SBS. This is classified as the most important bacterial family because it contains a wide variety of pathogenic species responsible for virtually all hospital and intestinal infections. In about 90% of human infection samples, the causative agent of this condition belongs to this bacterial family, which includes about 30 genera and more than 100 species of facultative anaerobic microorganisms that can be easily cultured in the laboratory1,23. It has been determined that this family may be present in the normal intestinal microbiota without harming the host. An explanation for this fact is that healthy individuals have a competent immune system and an intact intestinal barrier, which prevents the passage of these microorgansims from the intestine to other parts of the body. In addition, the intestine counts on a rigid control of the equilibrium of its microbiota1,24,25.
The greater diversity of bacteria of the family enterobacteriaceae in patients with SBS is a matter of concern when it is associated with the chronic clinical undernutrition status of these patients, which compromises their immunological systems and also facilitates the opening of intercellular channels between the enterocytes26,27. Therefore, such pathogens detected in the fecal samples of the patients may invade the circulation and cause infectious foci or even sepsis28.
Analysis of the influence of the ileocecal valve on the intestinal microbiota of the patients revealed that the present case the diversity of intestinal bacteria was similar, regardless of the existence of the valve. This observation is possibly due to the small sample size. However, the ileocecal valve is known to have a marked influence on microbiological control, preventing excessive bacterial growth in the proximal small bowel8,26,29.
Stomach acidity is also a determining factor in the colonization of the gastrointestinal tract. Thus, the chronic use of antacids may change the pH of the stomach and of the proximal intestine, leading to changes in the local microbiota. Because most of the patients included here were on routine use of proton pump inhibitors, the influence of this medication on the intestinal microbiota was analyzed. However, there were no differences between patients who took the medication or not. This finding is also possibly due to the small sample size and contrasts with literature reports stating that the chronic use of this type of medication may have a negative effect on the change in bacterial diversity in the gastrointestinal tract8,26,30.
Whereas patients had a greater diversity of Gram-negative bacteria, they also had a smaller concentration of bacteria. This fact may be explained by the removal of sites of bacterial colonization upon the intestinal resections. Another fact that may have contributed to the smaller number of bacteria in patients was the frequent use of antibiotics for the treatment of infections, mainly catheter-related bloodstream infections, which are one of the main causes of death in the case of patients with SBS7.
With the determination of the bacterial species by analysis of the sequences of these DNA fragments, we verified that the great majority of the investigated microorganisms had a sequence compatible with that of Lactobacillus ssp, which are common in the human intestinal microbiota. Lactobacillus ssp have vital functions for individuals, such as recovery of non-absorbed ingested energy, transformation of dietary products into energy substrate for enterocytes, fermentation of food fibers, and protection of the intestinal mucosal barrier against aggression by pathogens, among others1-3. Therefore, it is necessary to maintain this genus in the intestinal microbiota, and it is mandatory that the ratio of these microorganisms be kept constant in this medium.
There is a need for continued research in this field, in order to characterize the real intestinal microbiota of patients with SBS, thereby paving the way for new treatments capable of ameliorating symptoms, recovering the nutritional status, and improving survival.
Conclusions
Our findings suggest that despite the massive removal of the small bowel, frequent use of antibiotics, imune system depression, presence of non-digested food in the gastrointestinal tract, and accelerated intestinal transit, the ratio between intestinal bacterial species remain close to normal values. However, patients with short bowel syndrome have a higher diversity of Gram-negative strains belonging to the family Enterobacteriaceae, the most important bacterial family regarding pathogenicity.
Conflict of interest: none
Financial source: none
- 1 Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635-8.
- 2. Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635-8.
- 3. Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol. 2004;70:7220-8.
- 4. Thompson JS, Weseman R, Rochling FA, Mercer DF. Current management of the short bowel syndrome. Sur Clin North Am. 2011;91:493-510.
- 5. Vantini I, Benini L, Bonfante F, Talamini G, Sembenini C, Chiarioni G, Maragnolli O, Benini F, Capra F. Survival rate and prognostic factors in patients with intestinal failure. Dig Liver Dis. 2004;36:46-55.
- 6. Banerjee A, Warwicker P. Acute renal failure and metabolic disturbances in the short bowel syndrome. Q J Med. 2002;95:37-40.
- 7. Machado JC, Suen VMM, Figueiredo JFC, Marchini JS. Biofilms, infection and parenteral nutrition therapy. J Parenter Enteral Nutr. 2009;33:397-403.
- 8. Carlson GL. Surgical management of intestinal failure. Nutrition Society. 2003;62:711-8.
- 9. Dibaise JK, Young RJ, Vanderhoof JA. Enteric microbiota flora, bacterial overgrowth, and short bowel syndrome. Clin Gastroenterol Hepatol. 2006;4:11-20.
- 10. Bongaerts GP, Tolboom JJ, Naber AH, Sperl WJ, Severijnen RS, Bakkeren JA, Willems JL. Role of bacteria in the pathogenesis of short bowel syndrome-associated D-lactic acidemia. Microb Pathog. 1997;22:285-93.
- 11. Kaneko T, Bando Y, Kurihara H, Satomi K, Nonoyama K, Matsuura N. Fecal Microflora in a patient with short-bowel syndrome and identification of dominant lactobacilli. J Clin Microbiol. 1997;353181-5.
- 12. Using food frequency questionnaire in past dietary intake assessment. Pereira RA, Koifman S. Rev. Saúde Pública. 1999;33:610-21.
- 13. Dietary Reference Intakes: Estimated Average Requirements. National Academy of Sciences. Institute of Medicine. Food and Nutrition Board. http://fnic.nal.usda.gov/dietary-guidance/dietary-reference-intakes/dri-tables
- 14. Miles JM. Energy expenditure in hospitalized patients: implications for nutritional support. Mayo Clin Proc. 2006;81:809-16.
- 15. Cogill B. Anthropometric Indicators Measurement Guide. Food and Nutrition Technical Assistance Project, Academy for Educational Development, Washington, D.C., 2003.
-
16National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa, 1997.
- 17. Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070-5.
- 18. Musich PR, Chu W. A hot alkaline plasmid DNA miniprep method for automated DNA sequencing protocols. Biotechniques. 1993;14:958-60.
- 19. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic Local Alignment Search Tool. J Mol Biol. 1990;215:403-10.
- 20. Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2008;37:D141-5.
- 21. Rodgers JL, Nicewander WA. Thirteen Ways to Look at the Correlation Coefficient. The American Statistician. 1988;42:59-66.
- 22. Toigo G, Aparicio M, Attman PO, Cano N, Cianciaruso B, Engel B, Fouque D, Heidland A, Teplan V, Wanner C. Expert working group report on nutrition in adult patients with renal insufficiency. Clin Nutr. 2000;19:197-207.
- 23. Chow JW, Yu VL, Shlaes DM. Epidemiologic perspectives on Enterobacter for the infection control professional. Am J Infect Control. 1994;22:195-201.
- 24. Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, Gordon JI.. Evolution of mammals and their gut microbes. Science. 2008;320:1647-51.
- 25. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;18;449:804-10.
- 26. James WPT. Effects of protein-calorie malnutrition on intestinal absorption. Annals New York Academy of Sciences. 1971;176: 244-61.
- 27. Takeda K, Okumura K. Effects of fermented milk drink containing lactobacillus casei strain Shirota on the human NK-cell activity. J. Nutr. 2007;137:791S-3S.
- 28. Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, Morcock D, McGinty JW, Lifson JD, Lafont BA, Martin MA, Levine AD, Estes JD, Brenchley JM. Compromised gastrointestinal integrity in pigtail macaques in associated with increased microbial translocation, immune activatin, and IL-17 production in the absence of SIV infection. Mucosal Immunol. 2010;3:387-98.
- 29. Dibaise JK, Young RJ, Vanderhoof JA. Enteric Microbial Flora, Bacterial Overgrowth, and Short-Bowel Syndrome. Clin Gastroenterol Hepat. 2006;4(1):11-20.
- 30. Ali T, Roberts DN, Tierney WM. Long-term Safety Concerns with Proton Pump Inhibitors. Am J Med. 2009;122:896-90.
- 31. Matsuda K, Tsuji H, Asahara T, Matsumoto K, Takada T, Nomoto K. Establishment of an analytical system for the human fecal microbiota, based on reverse transcription-quantitative PCR targeting of multicopy rRNA molecules. Appl Environ Microbiol. 2009;75:1961-9.
- 32. Bazzocchi G, Gionchetti P, Almerigi PF, Amadini C, Campieri M. Intestinal microflora and oral bacteriotherapy in irritable bowel syndrome. Dig Liver Dis. 2002;34:S48-53.
- 33. Conte MP, Schippa S, Zamboni I, Penta M, Chiarini F, Seganti L, Osborn J, Falconieri P, Borrelli O, Cucchiara S. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut. 2006;55:1760-7.
Publication Dates
-
Publication in this collection
01 Feb 2013 -
Date of issue
2013