Abstract
PURPOSE:
To compare the biomodulatory effects of LED and ultrasound combined with semipermeable dressing in the repair of cutaneous lesions.
METHODS:
Eighty-four Wistar rats were submitted to surgical injury (2.5 cm) and divided into four groups (n=21): Group I (control), Group II (LED therapy, LED), Group III (LED therapy + dressing, LED+D) and Group IV (ultrasound + dressing, US+D). At seven, 14 and 21 days, the animals were euthanized, and the specimens of interest removed for histological analysis.
RESULTS:
Histological and histomorphometric analysis revealed a greater percent wound regression in animals receiving the dressing (group III: 55.97; group IV: 53.06), as well as a greater reduction in the inflammatory infiltrate (group III: 29.14; group IV: 31.71) since day 7. A later effect, with progression of the tissue repair process only after 14 and 21 days, was observed in the LED group intense fibroblast proliferation and greater collagen fiber production and organization were seen in the LED+D and US+D groups compared to the other groups.
CONCLUSION:
LED combined with a dressing was more effective at accelerating in the repair of cutaneous lesions.
Wound Healing; Phototherapy; Bandages; Rats
Introduction
Several studies have led to the development of increasingly efficient therapeutic approaches to tissue repair, with minimization of the factors that delay or impair this process11. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010 Mar; 89(3):219-29. doi: 10.1177/0022034509359125.
https://doi.org/10.1177/0022034509359125...
2. Mandelbaum SH, Di Santis ÉP, Mandelbaum MHSA. Cicatrização: conceitos atuais e recursos auxiliares - Parte I. An Bras Dermatol. 2003 Jul/Ago;78(4):393-410. doi: 10.1590/S0365-05962003000400002.
https://doi.org/10.1590/S0365-0596200300...
- 33. Balbino CA, Pereira LM, Curi R. Mecanismos envolvidos na cicatrização: uma revisão. Braz J Pharm Sci. 2005 Jan/Mar;41(1):27-51. doi: 10.1590/S1516-93322005000100004.
https://doi.org/10.1590/S1516-9332200500...
. Among the current approaches, light emitting diode (LED) phototherapy and therapeutic ultrasound (TUS) have yielded good results at low cost and have attracted the attention of the scientific community44. Piva JADAC, Abreu EMDC, Silva VDS, Nicolau RA. Effect of low-level laser therapy on the initial stages of tissue repair: basic principles. An Bras Dermatol. 2011 Sept/Oct;86(5):947-54. doi: 10.1590/S0365-05962011000500013.
https://doi.org/10.1590/S0365-0596201100...
5. Júnior MDJM, Arisawa EÂL, Martin AA, de Carvalho JP, da Silva JMN, Silva JF, Silveira JrL. Effects of low-power LED and therapeutic ultrasound in the tissue healing and inflammation in a tendinitis experimental model in rats. Lasers Med Sci. 2014 Jan;29(1):301-11. doi: 10.1007/s10103-013-1327-0.
https://doi.org/10.1007/s10103-013-1327-...
- 66. Maia Filho ALM, Villaverde AB, Munin E, Aimbire F, Albertini R. Comparative study of the topical application of Aloe vera gel, therapeutic ultrasound and phonophoresis on the tissue repair in collagenase-induced rat tendinitis. Ultrasound Med Biol. 2010 Oct;36(10):1682-90. doi: 10.1016/j.ultrasmedbio.2010.06.012.
https://doi.org/10.1016/j.ultrasmedbio.2...
.
The LED is a device that emits spontaneous and non-coherent radiation and has been commercially introduced as an alternative to therapies using low-intensity laser. The action of LED therapy differs from that of non-ablative treatment since it causes no tissue damage due to photothermolysis. LED acts by direct intracellular stimulation based on NADH activation and oxidation and on the alteration of the mitochondrial redox potential, resulting in the so-called photobiostimulatory or photomodulatory effect. This action permits noninvasive wound treatment77. Casalechi HL, Nicolau RA, Casalechi VL, Silveira JrL, De Paula AM, Pacheco MT. The effects of low- level light emitting diode on the repair process of Achilles tendon therapy in rats. Lasers Med Sci. 2009 Jul;24(4):659-65. doi: 10.1007/s10103-008-0607-6.
https://doi.org/10.1007/s10103-008-0607-...
, 88. Neves SMV, Nicolau RA, Maia Filho ALM, Mendes LM.S,Veloso AM. Digital photogrammetry and histomorphometric assessment of the effect of non-coherent light (light-emitting diode) therapy (λ640±20 nm) on the repair of third-degree burns in rats. Lasers Med Sci. 2014 Jan;29(1):203-12. doi: 10.1007/s10103-013-1312-7.
https://doi.org/10.1007/s10103-013-1312-...
.
Ultrasound is also a noninvasive alternative for wound treatment and low-intensity pulsed application is the modality most frequently indicated by researchers. This preference is based on the beneficial effects observed, such as acceleration of the tissue repair process due to increased angiogenesis, the formation of granulation tissue and fibroblasts, and consequent collagen synthesis. In addition, US treatment favors a reduction in the number of leukocytes and macrophages at the site of injury66. Maia Filho ALM, Villaverde AB, Munin E, Aimbire F, Albertini R. Comparative study of the topical application of Aloe vera gel, therapeutic ultrasound and phonophoresis on the tissue repair in collagenase-induced rat tendinitis. Ultrasound Med Biol. 2010 Oct;36(10):1682-90. doi: 10.1016/j.ultrasmedbio.2010.06.012.
https://doi.org/10.1016/j.ultrasmedbio.2...
, 99. O'Brien WD. Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. 2007 Jan/Apr;93(1-3):212-55. doi: 10.1016/j.pbiomolbio.2006.07.010.
https://doi.org/10.1016/j.pbiomolbio.200...
, 1010. Ng Gabriel YF, Fung Dicky TC. The effect of therapeutic ultrasound intensity on the ultrastructural morphology of tendon repair. Ultrasound Med Biol. 2007 Nov;33(11):1750-54. doi: 10.1016/j.ultrasmedbio.2007.05.019.
https://doi.org/10.1016/j.ultrasmedbio.2...
.
Dressings represent an important resource to restore skin continuity by protecting and regenerating the epidermis. Studies have shown that a semipermeable film dressing permits the therapeutic effects which are usually restricted to the wound margins, to extend to the wound bed99. O'Brien WD. Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. 2007 Jan/Apr;93(1-3):212-55. doi: 10.1016/j.pbiomolbio.2006.07.010.
https://doi.org/10.1016/j.pbiomolbio.200...
10. Ng Gabriel YF, Fung Dicky TC. The effect of therapeutic ultrasound intensity on the ultrastructural morphology of tendon repair. Ultrasound Med Biol. 2007 Nov;33(11):1750-54. doi: 10.1016/j.ultrasmedbio.2007.05.019.
https://doi.org/10.1016/j.ultrasmedbio.2...
- 1111. Vermeulen H, Ubbink DT, Goossens A, De Vos R, Legemate DA, Westerbos SJ. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg. 2005 Jun;92(6):665-72. doi: 10.1002/bjs.5055.
https://doi.org/10.1002/bjs.5055...
. In addition, dressings protect the wound against external contamination and permit constant inspection of the insertion site with the least manipulation possible.Vapor-permeable dressings permit gas exchange and maintain the physiology of the epidermis close to normal1111. Vermeulen H, Ubbink DT, Goossens A, De Vos R, Legemate DA, Westerbos SJ. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg. 2005 Jun;92(6):665-72. doi: 10.1002/bjs.5055.
https://doi.org/10.1002/bjs.5055...
12. Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Tech. 2010 Feb;21(2):77-95. doi: 10.1002/pat.1625.
https://doi.org/10.1002/pat.1625...
- 1313. Skórkowska-Telichowska K, Czemplik M, Kulma A, Szopa J. The local treatment and available dressings designed for chronic wounds. J Am Acad. Dermatol. 2013 Apr;68(4):e117-26. doi: 10.1016/j.jaad.2011.06.028.
https://doi.org/10.1016/j.jaad.2011.06.0...
.
Whereas several studies that confirm the isolated action of the LED and TUS in acceleration tissue repair and the importance of the reduction in post surgical time procedures in clinical practice, the objective of the present study was to compare the biomodulatory effect of LED and TUS with combined semipermeable dressing in the repair of cutaneous lesions.
Methods
The study was approved by the Research Ethics Committee of Integral Differential Faculty (FACID) (Protocol No. 246/ 2009) and was conducted according to the guidelines of Arouca Law No. 11.794/2008.
Eighty-four male Wistar rats (Rattus norvegicus) aged 60 days and weighing on average 250g were used.
The animals were housed in polypropylene cages under good hygiene conditions, with free access to standard rat chow (LabinaTM) and water. All experiments were planned in such a way as to minimize animal suffering.
Experimental groups
The animals were divided at random into four groups of 21 rats each: group I (control); group II (LED therapy, applied to the wound bed, total dose of 16 J/cm22. Mandelbaum SH, Di Santis ÉP, Mandelbaum MHSA. Cicatrização: conceitos atuais e recursos auxiliares - Parte I. An Bras Dermatol. 2003 Jul/Ago;78(4):393-410. doi: 10.1590/S0365-05962003000400002.
https://doi.org/10.1590/S0365-0596200300...
; LED); group III (LED therapy applied to the wound bed in combination with a dressing, total dose of 16 J/cm22. Mandelbaum SH, Di Santis ÉP, Mandelbaum MHSA. Cicatrização: conceitos atuais e recursos auxiliares - Parte I. An Bras Dermatol. 2003 Jul/Ago;78(4):393-410. doi: 10.1590/S0365-05962003000400002.
https://doi.org/10.1590/S0365-0596200300...
; LED + D), and group IV (US applied to the wound bed in combination with a dressing; US + D). Each group was then divided into three equal subgroups which were monitored for seven, 14 and 21 days of the experiment, corresponding to the different phases of the healing process.
Forty-eight hours after the surgical procedure and bleeding cessation, the skin was cleaned with 70% alcohol and the animals were submitted to the experimental treatments. The remaining treatments were applied on alternate days in such a way that animals of the 7-day groups received three applications, those of the 14-day groups seven applications, and those of the 21-day groups 10 applications.
At the end of each experimental time, seven animals from each group were euthanized and the skin of the region of interest was submitted to histological analysis.
Surgical procedure
All animals were anesthetized intramuscularly with 10% ketamine hydrochloride (0.1 ml/100 g/kg) and the same dose of 2% xylazine. Next, a 6-cm long by 4-cm wide portion of the dorsolateral region was shaved and disinfected with 4% iodine alcohol. A circular skin wound measuring 2.5 cm in diameter was produced with a circular metal instrument with a cutting metal blade (no. 4) in its lower margin and the tissue was completely removed.
Semipermeable film dressing, LED therapy and therapeutic ultrasound
Forty-eight hours after surgery, the skin was cleaned with 70% alcohol and the dressing was cut according to the size of the wound (exceeding the wound margins) and applied by exerting gentle pressure on the film to guarantee perfect adhesion. For removal of the dressing, one of its edges was raised and the hand positioned under the dressing, carefully pulling it parallel to the skin in the direction of the hairs.
The Tegaderm(r) dressing roll distributed by 3M do Brasil Ltda. is a transparent, elastic, semipermeable, moisture-retaining polyurethane film with tissue repair-stimulating properties that accelerates skin wound healing. The dressing is indicated for uninfected open skin wounds and should be changed at intervals of seven days or according to the saturation of the material.
The LED used in the present study was produced by the Institute of Research and Development (IP&D, Br) of UNIVAP, which emits light in the spectral range of 640 ± 20 nm, with an output power of 30 mW and a total dose of 16 J/cm22. Mandelbaum SH, Di Santis ÉP, Mandelbaum MHSA. Cicatrização: conceitos atuais e recursos auxiliares - Parte I. An Bras Dermatol. 2003 Jul/Ago;78(4):393-410. doi: 10.1590/S0365-05962003000400002.
https://doi.org/10.1590/S0365-0596200300...
. The power of the apparatus was determined in detail before use.
A clinical US apparatus (Sonopulse Special, Ibramed, Br) with a fundamental frequency of 3 MHz, pulsed mode with a power density of 0.5 W/cm22. Mandelbaum SH, Di Santis ÉP, Mandelbaum MHSA. Cicatrização: conceitos atuais e recursos auxiliares - Parte I. An Bras Dermatol. 2003 Jul/Ago;78(4):393-410. doi: 10.1590/S0365-05962003000400002.
https://doi.org/10.1590/S0365-0596200300...
, pulse repetition frequency of 16 Hz to 50%, effective radiating area of 3.5 cm22. Mandelbaum SH, Di Santis ÉP, Mandelbaum MHSA. Cicatrização: conceitos atuais e recursos auxiliares - Parte I. An Bras Dermatol. 2003 Jul/Ago;78(4):393-410. doi: 10.1590/S0365-05962003000400002.
https://doi.org/10.1590/S0365-0596200300...
, and application time of two minutes was used. Ultrasound was applied by the direct coupling method with oscillatory movements. The instrument was calibrated by the manufacturer before and after the experiment in order to determine that there had been no loss of intensity during the treatment of the experimental groups.
Macroscopic evaluation
Images were obtained with a digital camera (Nikon Coolpix P100, with 10.3 megapixel resolution) for measurement of the wound area and for macroscopic evaluation. The camera was fixed to a tripod and kept at a constant 30-cm distance from the surgical wound.
Macroscopic morphometry was performed using the ImageJ software in order to calculate the wound area at the different time points. The initial area was compared to the final one in all specimens of the different groups, with delimitation of the periphery of the wound. Percent wound reduction was calculated by the following formula:
Initial wound area - final wound area/initial wound area × 1001414. Coelho NPMDF, Nogueira VC, Cardoso MAG, Lopes LDS, Nascimento PPD, Rocha EDS, da Silva CLP, Arisawa EÂL Cenostigma macrophyllum Tul. on the healing of skin wounds in rats with diabetes mellitus. Acta Cir Bras. 2013 Aug;28(8):594-600. doi: 10.1590/S0102-86502013000800007.
https://doi.org/10.1590/S0102-8650201300...
.
Histological and histomorphometric analysis
The animals were euthanized with a sodium pentobarbital overdose (60 mg/kg). The anatomical specimen of interest was removed with a sterile scalpel with a 1-cm margin around the wound and the fragments were identified, fixed in 10% formalin and routinely processed for histology. Longitudinal 5-µm histological sections were obtained, stained with hematoxylin-eosin (HE) and Masson's trichrome (TM) and submitted to histological and morphometric analysis based on digital images obtained from three regions of the surgical wound (right margin, central region, and left margin) of each animal using a Leica(r) DMLB2 optical system (x40).
Inflammatory cell counts were performed in an area precisely delimited by the cell counter function of the ImageJ program1414. Coelho NPMDF, Nogueira VC, Cardoso MAG, Lopes LDS, Nascimento PPD, Rocha EDS, da Silva CLP, Arisawa EÂL Cenostigma macrophyllum Tul. on the healing of skin wounds in rats with diabetes mellitus. Acta Cir Bras. 2013 Aug;28(8):594-600. doi: 10.1590/S0102-86502013000800007.
https://doi.org/10.1590/S0102-8650201300...
. The histological parameters analyzed in HE-stained slides were features related to progression of the wound repair process, including morphological modifications in the epidermis and dermis and the degree of epithelialization, cell content, vascularization, fiber position, and collagen fiber grading and remodeling. In view of the variation among groups and among animals within each group, the results are reported as the mean for each group. The qualitative analysis of the slides stained with TM enabled verify grading and remodeling of collagen fibers.
Statistical analysis
Data were analyzed statistically by ANOVA followed by the Tukey test, which presents 95% reliability. The level of significance was set at p<0.05. The analyses were carried out using the GraphPad Prism 3.0 software (Windows platform).
Results
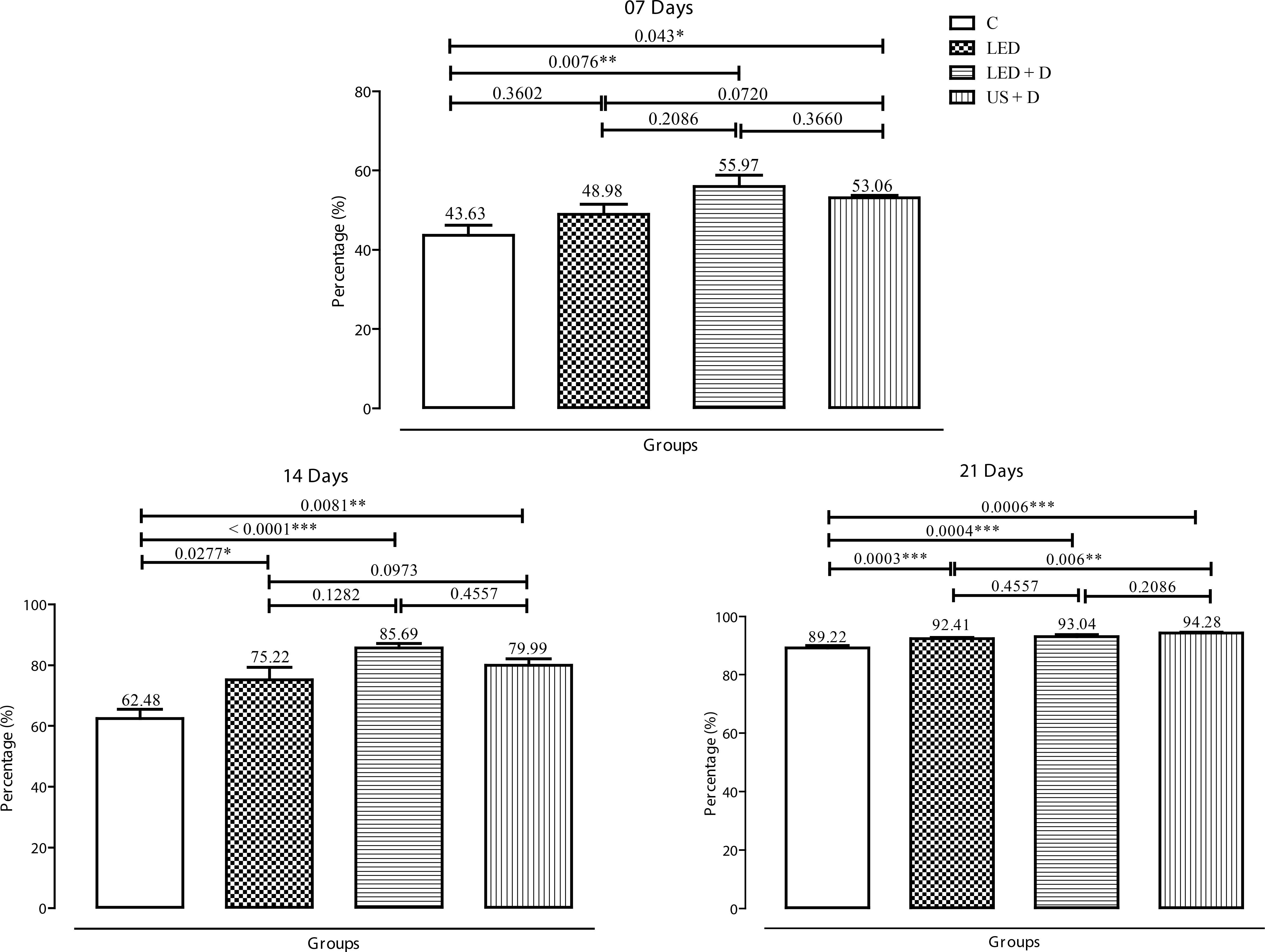
With respect to percent wound regression at seven days, a significant difference in the wound diameter was observed between the groups receiving the dressing and the other groups. The most expressive results were observed for the LED + D (p<0.01) and US + D (p<0.05) compared to control. At 14 days, a significant difference was observed between the treated groups and control (p<0.001 for LED + D, p<0.01 for US + D, and p<0.05 for LED). At 21 days, a significant difference (p<0.001) was observed for the LED, LED + D and US + D groups when compared to control. Intergroup analysis revealed a significant difference (p<0.01) only for the period of 21 days between the US + D and LED groups (Figure 1).
Percentage of wound regression in the experimental groups on days 7, 14 and 21. The p values are indicated by (*p<0.05, **p<0.01, and ***p<0.001).
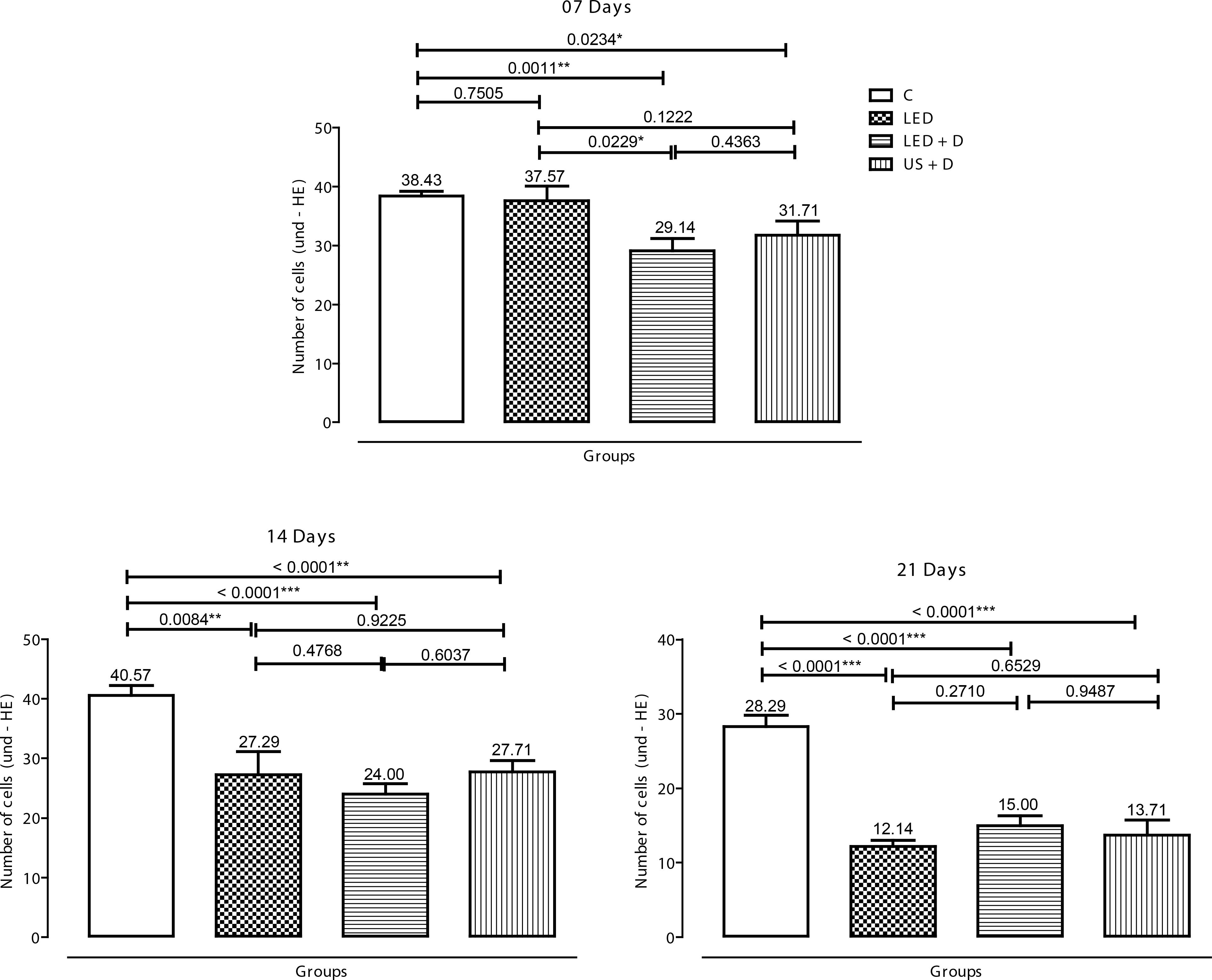
Analysis of the inflammatory cell population at seven days showed a reduction in the inflammatory infiltrate in the LED + D (p<0.01) and US + D groups (p<0.05) compared to control. At 14 days, a significant difference (p<0.001) was observed between the LED + D and control groups. In addition, although at a lower significance level (p<0.01), the US + D and LED groups presented a smaller number of inflammatory cells than the control group. A significant reduction (p<0.001) in the inflammatory cell population was observed after 21 days in all treated groups compared to control animals. Intergroup comparison revealed a significant difference (p<0.05) only at 7 days between the LED + D and LED groups (Figure 2).
Number of inflammatory cells (20.000 µm2) in the experimental groups on days 7, 14 and 21. The p values are indicated by (*p<0.05, **p<0.01, and ***p<0.001).
Qualitative histological analysis based on the degree of reepithelialization and the presence of inflammatory cells revealed results similar to those of histomorphometric analysis. The absence of reepithelialization associated with large numbers of inflammatory cells was observed in the control group at seven days. In contrast, the US + D and LED + D groups exhibited partial reepithelialization with a less evident inflammatory infiltrate. The LED group showed reepithelialization in the early stage and an inflammatory infiltrate similar to that observed in the LED and LED + D groups. Initial remodeling of newly formed collagen fibers was observed in the LED, LED + D and US + D groups, whereas remodeling was still absent in the control group. At 14 days, the control group still showed no reepithelialization and large numbers of inflammatory cells, while partial reepithelialization, more advanced remodeling and a more discrete inflammatory infiltrate were observed in the remaining treated groups (LED, LED + D, and US + D) compared to control. At 21 days, partial reepithelialization and an abundant inflammatory infiltrate were still seen in control specimens. In the LED group, reepithelialization was still incomplete and a small number of inflammatory cells was present. In contrast, the wound was fully reepithelialized in the LED + D and US + D groups, remodeling was in an advanced stage, and only some inflammatory cells were visible in the wound area. In contrast, the process of collagen fiber remodeling was in an intermediate stage in the control group.
The qualitative data regarding the presence of inflammatory cells, fibroblasts, blood vessels, and collagen fiber organization are shown in Figure 3. Intense neovascularization, a reduced inflammatory infiltrate and fibroblast proliferation were observed at 14 and 21 days in the treated groups compared to control. Reepithelialization was minimal in the control specimens, which exhibited large numbers of inflammatory cells, discrete neovascularization, fibroblast proliferation, and disperse newly formed fine collagen fibers.
Qualitative aspects of the inflammatory process of surgical wounds in the experimental groups at 7 (A), 14 (B) and 21 (C) days of treatment (HE, x400). Group I: Control; group II: LED; group III: LED + dressing, and group IV: US + dressing. Yellow arrows: inflammatory cells; red arrows: blood vessels; blue arrows: fibroblasts.
Analysis qualitative of collagen fiber organization in the treated groups revealed partial organization at 14 days and complete organization at 21 days compared to control (Figure 4).
Qualitative aspects of collagen fibers in the experimental groups at 7(A), 14(B) and 21(C) days of treatment (Masson's trichrome, x400). Group I: Control; group II: LED; group III: LED + dressing, and group IV: US + dressing.
Discussion
Macroscopic, qualitative histological and histomorphometric analyses permitted the assessment of the progression of tissue repair and to relate it to the efficacy of the treatments employed. In addition to satisfying the functional objectives of the reconstructed part and the reduction in local morbidity, it is necessary to pay attention to the cosmetic aspect of second-intention healing, since treatment failure can have significant deleterious effects on the esthetic result of skin repair11. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010 Mar; 89(3):219-29. doi: 10.1177/0022034509359125.
https://doi.org/10.1177/0022034509359125...
, 33. Balbino CA, Pereira LM, Curi R. Mecanismos envolvidos na cicatrização: uma revisão. Braz J Pharm Sci. 2005 Jan/Mar;41(1):27-51. doi: 10.1590/S1516-93322005000100004.
https://doi.org/10.1590/S1516-9332200500...
, 1515. Ribeiro JF, dos Anjos EHM, Mello MLS, de Campos Vidal B. Skin collagen fiber molecular order: a pattern of distributional fiber orientation as assessed by optical anisotropy and image analysis. PloS One. 2013 Jan;8(1):e54724. doi: 10.1371/journal.pone.0054724.
https://doi.org/10.1371/journal.pone.005...
.
At seven days after surgery when the tissue repair process has reached the end of the inflammatory phase, a significant difference in percent wound regression and a significant reduction in the inflammatory infiltrate were only observed in animals receiving the dressing (LED + D and US + D). More expressive results were obtained for the LED + D group, demonstrating that phototherapy is more effective than US during this phase, although there is consensus that US can accelerate the inflammatory response, promoting the release of histamine, macrophages and monocytes and increasing collagen synthesis and fibroblast proliferation55. Júnior MDJM, Arisawa EÂL, Martin AA, de Carvalho JP, da Silva JMN, Silva JF, Silveira JrL. Effects of low-power LED and therapeutic ultrasound in the tissue healing and inflammation in a tendinitis experimental model in rats. Lasers Med Sci. 2014 Jan;29(1):301-11. doi: 10.1007/s10103-013-1327-0.
https://doi.org/10.1007/s10103-013-1327-...
, 66. Maia Filho ALM, Villaverde AB, Munin E, Aimbire F, Albertini R. Comparative study of the topical application of Aloe vera gel, therapeutic ultrasound and phonophoresis on the tissue repair in collagenase-induced rat tendinitis. Ultrasound Med Biol. 2010 Oct;36(10):1682-90. doi: 10.1016/j.ultrasmedbio.2010.06.012.
https://doi.org/10.1016/j.ultrasmedbio.2...
, 99. O'Brien WD. Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. 2007 Jan/Apr;93(1-3):212-55. doi: 10.1016/j.pbiomolbio.2006.07.010.
https://doi.org/10.1016/j.pbiomolbio.200...
.
In wound healing, cell proliferation characteristically begins from the margins of the wound and extends to its center, mainly because of the low availability of nutrients and oxygen at this site. The energy provided by LED therapy alters this condition, generating an environment that favors the formation of new blood vessels and proliferation of fibroblasts, which produce collagen fibers and extracellular matrix88. Neves SMV, Nicolau RA, Maia Filho ALM, Mendes LM.S,Veloso AM. Digital photogrammetry and histomorphometric assessment of the effect of non-coherent light (light-emitting diode) therapy (λ640±20 nm) on the repair of third-degree burns in rats. Lasers Med Sci. 2014 Jan;29(1):203-12. doi: 10.1007/s10103-013-1312-7.
https://doi.org/10.1007/s10103-013-1312-...
, 1515. Ribeiro JF, dos Anjos EHM, Mello MLS, de Campos Vidal B. Skin collagen fiber molecular order: a pattern of distributional fiber orientation as assessed by optical anisotropy and image analysis. PloS One. 2013 Jan;8(1):e54724. doi: 10.1371/journal.pone.0054724.
https://doi.org/10.1371/journal.pone.005...
. Several studies have demonstrated the action of LED during the early stages of tissue repair in a variety of clinical situations by the early activation of the inflammatory phase, significantly increasing fibroblast proliferation and reducing the inflammatory infiltrate77. Casalechi HL, Nicolau RA, Casalechi VL, Silveira JrL, De Paula AM, Pacheco MT. The effects of low- level light emitting diode on the repair process of Achilles tendon therapy in rats. Lasers Med Sci. 2009 Jul;24(4):659-65. doi: 10.1007/s10103-008-0607-6.
https://doi.org/10.1007/s10103-008-0607-...
, 1010. Ng Gabriel YF, Fung Dicky TC. The effect of therapeutic ultrasound intensity on the ultrastructural morphology of tendon repair. Ultrasound Med Biol. 2007 Nov;33(11):1750-54. doi: 10.1016/j.ultrasmedbio.2007.05.019.
https://doi.org/10.1016/j.ultrasmedbio.2...
.
Although the combination with a dressing is not obligatory for LED therapy, the use of a film protects the wound against infections. In addition, the present results showed that application of a dressing accelerated the tissue repair process since no significant difference was observed between the LED and control groups, whereas a significant reduction in the inflammatory process was observed in the LED + D compared to the LED group. On the other hand, the combination of a dressing and US is essential for application of the transducer to the wound bed. It is therefore expected to combine the stimulating properties of US and the positive effects of moisture-retaining dressings, which would increase the velocity of tissue repair, with a positive impact on the quality of the scar tissue66. Maia Filho ALM, Villaverde AB, Munin E, Aimbire F, Albertini R. Comparative study of the topical application of Aloe vera gel, therapeutic ultrasound and phonophoresis on the tissue repair in collagenase-induced rat tendinitis. Ultrasound Med Biol. 2010 Oct;36(10):1682-90. doi: 10.1016/j.ultrasmedbio.2010.06.012.
https://doi.org/10.1016/j.ultrasmedbio.2...
, 99. O'Brien WD. Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. 2007 Jan/Apr;93(1-3):212-55. doi: 10.1016/j.pbiomolbio.2006.07.010.
https://doi.org/10.1016/j.pbiomolbio.200...
, 1010. Ng Gabriel YF, Fung Dicky TC. The effect of therapeutic ultrasound intensity on the ultrastructural morphology of tendon repair. Ultrasound Med Biol. 2007 Nov;33(11):1750-54. doi: 10.1016/j.ultrasmedbio.2007.05.019.
https://doi.org/10.1016/j.ultrasmedbio.2...
.
At 14 days, a period corresponding to the phase of cell proliferation, a significant difference in the percentage of wound regression and a significant reduction in the number of inflammatory cells were observed in the treated groups compared to the control group. In agreement with the present study, several investigations have shown that LED therapy and therapeutic US stimulate the healing of skin wounds by second intention, reducing the wound area probably as a result of their action on myofibroblasts present at the site of injury77. Casalechi HL, Nicolau RA, Casalechi VL, Silveira JrL, De Paula AM, Pacheco MT. The effects of low- level light emitting diode on the repair process of Achilles tendon therapy in rats. Lasers Med Sci. 2009 Jul;24(4):659-65. doi: 10.1007/s10103-008-0607-6.
https://doi.org/10.1007/s10103-008-0607-...
8. Neves SMV, Nicolau RA, Maia Filho ALM, Mendes LM.S,Veloso AM. Digital photogrammetry and histomorphometric assessment of the effect of non-coherent light (light-emitting diode) therapy (λ640±20 nm) on the repair of third-degree burns in rats. Lasers Med Sci. 2014 Jan;29(1):203-12. doi: 10.1007/s10103-013-1312-7.
https://doi.org/10.1007/s10103-013-1312-...
- 99. O'Brien WD. Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. 2007 Jan/Apr;93(1-3):212-55. doi: 10.1016/j.pbiomolbio.2006.07.010.
https://doi.org/10.1016/j.pbiomolbio.200...
. More significant results were obtained for LED therapy combined with a dressing, suggesting positive effects of the dressing by maintaining the cellular microenvironment1111. Vermeulen H, Ubbink DT, Goossens A, De Vos R, Legemate DA, Westerbos SJ. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg. 2005 Jun;92(6):665-72. doi: 10.1002/bjs.5055.
https://doi.org/10.1002/bjs.5055...
12. Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Tech. 2010 Feb;21(2):77-95. doi: 10.1002/pat.1625.
https://doi.org/10.1002/pat.1625...
- 1313. Skórkowska-Telichowska K, Czemplik M, Kulma A, Szopa J. The local treatment and available dressings designed for chronic wounds. J Am Acad. Dermatol. 2013 Apr;68(4):e117-26. doi: 10.1016/j.jaad.2011.06.028.
https://doi.org/10.1016/j.jaad.2011.06.0...
associated with the early action of phototherapy which provides the energy to accelerate wound healing77. Casalechi HL, Nicolau RA, Casalechi VL, Silveira JrL, De Paula AM, Pacheco MT. The effects of low- level light emitting diode on the repair process of Achilles tendon therapy in rats. Lasers Med Sci. 2009 Jul;24(4):659-65. doi: 10.1007/s10103-008-0607-6.
https://doi.org/10.1007/s10103-008-0607-...
, 88. Neves SMV, Nicolau RA, Maia Filho ALM, Mendes LM.S,Veloso AM. Digital photogrammetry and histomorphometric assessment of the effect of non-coherent light (light-emitting diode) therapy (λ640±20 nm) on the repair of third-degree burns in rats. Lasers Med Sci. 2014 Jan;29(1):203-12. doi: 10.1007/s10103-013-1312-7.
https://doi.org/10.1007/s10103-013-1312-...
.
The percentage of wound regression and the reduction in the number of inflammatory cells at 21 days of treatment, a period corresponding to the remodeling phase, were statistically significant in all treated groups compared to control. This finding demonstrates that the choice of any of the wound treatment methods studied here is better than awaiting the natural course of wound repair. The present results agree with literature reports showing that US irradiation, especially low-intensity irradiation in the pulsed mode, stimulates tissue repair and reduces the wound area, probably as a result of the acceleration of angiogenesis, matrix deposition, wound contraction, and reepithelization1616. Watson T. Ultrasound in contemporary physiotherapy practice. Ultrsonics. 2008 Aug; 48(4):321-9. doi: 10.1016/j.ultras.2008.02.004.
https://doi.org/10.1016/j.ultras.2008.02...
, 1717. Carvalho PDTCD, Silva IS, Reis FAD, Belchior ACG, Aydos RD, Facco GG, Dourado DM. Histological study of tendon healing in malnourished Wistar rats treated with ultrasound therapy. Acta Cir Bras. 2006;21(4):13-7. doi: 10.1590/S0102-86502006001000004.
https://doi.org/10.1590/S0102-8650200600...
. In this respect, intergroup analysis of wound regression at 21 days revealed more significant results in the US + D group compared to the LED group.
The results of qualitative analysis of the specimens in all experimental groups were similar to the histomorphometric results, with the observation of a reduction in the inflammatory process, fibroblast/collagen neoformation, and acceleration of epidermal maturation in the treated groups. These results were more expressive in the LED + D and US + D groups.
The results of qualitative analysis of collagen fiber grading and remodeling by US irradiation and phototherapy at seven days showed that both treatments favored collagen synthesis, emphasizing the action of the dressing in the LED + D and US + D groups due to its stimulating properties of acceleration of reepithelization1111. Vermeulen H, Ubbink DT, Goossens A, De Vos R, Legemate DA, Westerbos SJ. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg. 2005 Jun;92(6):665-72. doi: 10.1002/bjs.5055.
https://doi.org/10.1002/bjs.5055...
12. Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Tech. 2010 Feb;21(2):77-95. doi: 10.1002/pat.1625.
https://doi.org/10.1002/pat.1625...
- 1313. Skórkowska-Telichowska K, Czemplik M, Kulma A, Szopa J. The local treatment and available dressings designed for chronic wounds. J Am Acad. Dermatol. 2013 Apr;68(4):e117-26. doi: 10.1016/j.jaad.2011.06.028.
https://doi.org/10.1016/j.jaad.2011.06.0...
. These findings agree with experimental studies on the effects of therapeutic US and LED therapy, showing that these treatments stimulate the inflammatory response and consequent collagen synthesis1515. Ribeiro JF, dos Anjos EHM, Mello MLS, de Campos Vidal B. Skin collagen fiber molecular order: a pattern of distributional fiber orientation as assessed by optical anisotropy and image analysis. PloS One. 2013 Jan;8(1):e54724. doi: 10.1371/journal.pone.0054724.
https://doi.org/10.1371/journal.pone.005...
, 1616. Watson T. Ultrasound in contemporary physiotherapy practice. Ultrsonics. 2008 Aug; 48(4):321-9. doi: 10.1016/j.ultras.2008.02.004.
https://doi.org/10.1016/j.ultras.2008.02...
,18.
At 14 days after surgery, a period corresponding to the proliferative phase of wound healing, animals of the treated groups exhibited a more discrete inflammatory infiltrate and remodeling of collagen fibers, which were more numerous and mature and were distributed homogenously and regularly. On the other hand, animals of the control group were in the early stage of the remodeling process. These findings demonstrate that therapeutic US and LED therapy stimulate fibroblast proliferation, in addition to accelerating angiogenesis and increasing collagen secretion1717. Carvalho PDTCD, Silva IS, Reis FAD, Belchior ACG, Aydos RD, Facco GG, Dourado DM. Histological study of tendon healing in malnourished Wistar rats treated with ultrasound therapy. Acta Cir Bras. 2006;21(4):13-7. doi: 10.1590/S0102-86502006001000004.
https://doi.org/10.1590/S0102-8650200600...
,19.
Collagen content and tensile strength increase after 21 days of treatment1515. Ribeiro JF, dos Anjos EHM, Mello MLS, de Campos Vidal B. Skin collagen fiber molecular order: a pattern of distributional fiber orientation as assessed by optical anisotropy and image analysis. PloS One. 2013 Jan;8(1):e54724. doi: 10.1371/journal.pone.0054724.
https://doi.org/10.1371/journal.pone.005...
. At this time point, reepithelization was still not complete in the LED group and the presence of small numbers of inflammatory cells could not be noted. In contrast, in the LED + D and US + D groups, remodeling was complete and the collagen fibers were more differentiated, resembling normal fibers, a finding suggesting a positive effect of the combination with a dressing1111. Vermeulen H, Ubbink DT, Goossens A, De Vos R, Legemate DA, Westerbos SJ. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg. 2005 Jun;92(6):665-72. doi: 10.1002/bjs.5055.
https://doi.org/10.1002/bjs.5055...
12. Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Tech. 2010 Feb;21(2):77-95. doi: 10.1002/pat.1625.
https://doi.org/10.1002/pat.1625...
- 1313. Skórkowska-Telichowska K, Czemplik M, Kulma A, Szopa J. The local treatment and available dressings designed for chronic wounds. J Am Acad. Dermatol. 2013 Apr;68(4):e117-26. doi: 10.1016/j.jaad.2011.06.028.
https://doi.org/10.1016/j.jaad.2011.06.0...
. Taken together, these results suggest that the combination of LED therapy and therapeutic US with a semipermeable dressing accelerates the healing of skin wounds in rats. However, further studies are needed to identify the mechanism of action of this combination in order to permit its clinical application.
Conclusions
The combination of a semi permeable dressing with US or LED stimulates tissue repair by early activation of the inflammatory phase and by acting since the onset of the process. However, LED therapy combined with a dressing was more effective to optimize the healing of skin wounds in rats, permitting greater percent wound regression and a reduction in the number of inflammatory cells and promoting fibroblast proliferation and collagen fiber remodeling.
References
-
1Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010 Mar; 89(3):219-29. doi: 10.1177/0022034509359125.
» https://doi.org/10.1177/0022034509359125 -
2Mandelbaum SH, Di Santis ÉP, Mandelbaum MHSA. Cicatrização: conceitos atuais e recursos auxiliares - Parte I. An Bras Dermatol. 2003 Jul/Ago;78(4):393-410. doi: 10.1590/S0365-05962003000400002.
» https://doi.org/10.1590/S0365-05962003000400002 -
3Balbino CA, Pereira LM, Curi R. Mecanismos envolvidos na cicatrização: uma revisão. Braz J Pharm Sci. 2005 Jan/Mar;41(1):27-51. doi: 10.1590/S1516-93322005000100004.
» https://doi.org/10.1590/S1516-93322005000100004 -
4Piva JADAC, Abreu EMDC, Silva VDS, Nicolau RA. Effect of low-level laser therapy on the initial stages of tissue repair: basic principles. An Bras Dermatol. 2011 Sept/Oct;86(5):947-54. doi: 10.1590/S0365-05962011000500013.
» https://doi.org/10.1590/S0365-05962011000500013 -
5Júnior MDJM, Arisawa EÂL, Martin AA, de Carvalho JP, da Silva JMN, Silva JF, Silveira JrL. Effects of low-power LED and therapeutic ultrasound in the tissue healing and inflammation in a tendinitis experimental model in rats. Lasers Med Sci. 2014 Jan;29(1):301-11. doi: 10.1007/s10103-013-1327-0.
» https://doi.org/10.1007/s10103-013-1327-0 -
6Maia Filho ALM, Villaverde AB, Munin E, Aimbire F, Albertini R. Comparative study of the topical application of Aloe vera gel, therapeutic ultrasound and phonophoresis on the tissue repair in collagenase-induced rat tendinitis. Ultrasound Med Biol. 2010 Oct;36(10):1682-90. doi: 10.1016/j.ultrasmedbio.2010.06.012.
» https://doi.org/10.1016/j.ultrasmedbio.2010.06.012 -
7Casalechi HL, Nicolau RA, Casalechi VL, Silveira JrL, De Paula AM, Pacheco MT. The effects of low- level light emitting diode on the repair process of Achilles tendon therapy in rats. Lasers Med Sci. 2009 Jul;24(4):659-65. doi: 10.1007/s10103-008-0607-6.
» https://doi.org/10.1007/s10103-008-0607-6 -
8Neves SMV, Nicolau RA, Maia Filho ALM, Mendes LM.S,Veloso AM. Digital photogrammetry and histomorphometric assessment of the effect of non-coherent light (light-emitting diode) therapy (λ640±20 nm) on the repair of third-degree burns in rats. Lasers Med Sci. 2014 Jan;29(1):203-12. doi: 10.1007/s10103-013-1312-7.
» https://doi.org/10.1007/s10103-013-1312-7 -
9O'Brien WD. Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. 2007 Jan/Apr;93(1-3):212-55. doi: 10.1016/j.pbiomolbio.2006.07.010.
» https://doi.org/10.1016/j.pbiomolbio.2006.07.010 -
10Ng Gabriel YF, Fung Dicky TC. The effect of therapeutic ultrasound intensity on the ultrastructural morphology of tendon repair. Ultrasound Med Biol. 2007 Nov;33(11):1750-54. doi: 10.1016/j.ultrasmedbio.2007.05.019.
» https://doi.org/10.1016/j.ultrasmedbio.2007.05.019 -
11Vermeulen H, Ubbink DT, Goossens A, De Vos R, Legemate DA, Westerbos SJ. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg. 2005 Jun;92(6):665-72. doi: 10.1002/bjs.5055.
» https://doi.org/10.1002/bjs.5055 -
12Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Tech. 2010 Feb;21(2):77-95. doi: 10.1002/pat.1625.
» https://doi.org/10.1002/pat.1625 -
13Skórkowska-Telichowska K, Czemplik M, Kulma A, Szopa J. The local treatment and available dressings designed for chronic wounds. J Am Acad. Dermatol. 2013 Apr;68(4):e117-26. doi: 10.1016/j.jaad.2011.06.028.
» https://doi.org/10.1016/j.jaad.2011.06.028 -
14Coelho NPMDF, Nogueira VC, Cardoso MAG, Lopes LDS, Nascimento PPD, Rocha EDS, da Silva CLP, Arisawa EÂL Cenostigma macrophyllum Tul. on the healing of skin wounds in rats with diabetes mellitus. Acta Cir Bras. 2013 Aug;28(8):594-600. doi: 10.1590/S0102-86502013000800007.
» https://doi.org/10.1590/S0102-86502013000800007 -
15Ribeiro JF, dos Anjos EHM, Mello MLS, de Campos Vidal B. Skin collagen fiber molecular order: a pattern of distributional fiber orientation as assessed by optical anisotropy and image analysis. PloS One. 2013 Jan;8(1):e54724. doi: 10.1371/journal.pone.0054724.
» https://doi.org/10.1371/journal.pone.0054724 -
16Watson T. Ultrasound in contemporary physiotherapy practice. Ultrsonics. 2008 Aug; 48(4):321-9. doi: 10.1016/j.ultras.2008.02.004.
» https://doi.org/10.1016/j.ultras.2008.02.004 -
17Carvalho PDTCD, Silva IS, Reis FAD, Belchior ACG, Aydos RD, Facco GG, Dourado DM. Histological study of tendon healing in malnourished Wistar rats treated with ultrasound therapy. Acta Cir Bras. 2006;21(4):13-7. doi: 10.1590/S0102-86502006001000004.
» https://doi.org/10.1590/S0102-86502006001000004 -
18Fiório FB, Silveira JrL, Munin E, de Lima CJ, Fernandes KP, Ta-Ferrari RAM, de Carvalho RA. Effect of incoherent LED radiation on third-degree burning wounds in rats. J Cosmet Laser Ther. 2011 Dec;13(6):315-22. doi: 10.3109/14764172.2011.630082.
» https://doi.org/10.3109/14764172.2011.630082 -
19Oliveira RDA, Matos AF, Barros NRB, Fernandes GA, Lima ACG, Nicolau RA. Low-intensity laser therapy and led (light emitting diode) therapy in mechanical resistance of Rattus norvegicus chest incision with implant of steel wire for sternal suture. Rev Bras Eng Biomed. 2013 Jun;29(2):166-74. doi: 10.4322/rbeb.2013.016.
» https://doi.org/10.4322/rbeb.2013.016
-
Financial source: Piaui Research Foundation (FAPEPI)
-
1
Research performed at Institute of Research and Development (IPD), Vale do Paraiba University (UNIVAP), Sao Jose dos Campos-SP and Integral Differential Faculty (FACID), Teresina-PI, Brazil. Part of PhD degree thesis, Postgraduate Program in Biomedical Engineering, UNIVAP. Tutor: Emilia Angela Loschiavo Arisawa.
Publication Dates
-
Publication in this collection
Sept 2014
History
-
Received
22 Apr 2014 -
Reviewed
23 June 2014 -
Accepted
18 July 2014