Abstract
PURPOSE:
To analyze microscopically the effects of different concentrations of oxygen in the lungs of rats.
METHODS:
There were 20 rats distributed in three experimental groups (concentration of oxygen to 40%, 70% and 100%) and a control group. The animals were exposed to the oxygen in a chamber of acrylic during three days and after exposition, the animals were submitted to median thoracotomia to remove the lungs. The lung tissue of all of the animals was analyzed as regards presence of acute and chronic inflammation, capillary congestion, alveolar walls thick, interstitial and alveolar edema, alveolar hemorrhage, denudation capillary and alveolar endothelium areas and atelectasis.
RESULTS:
The analysis histopathologic revealed significant statistics difference for acute and chronic inflammation, capillary congestion, alveolar walls thick, interstitial and alveolar edema, alveolar hemorrhage, denudation capillary and alveolar epithelium areas.
CONCLUSIONS:
Exposition to the oxygen during 72 hours in the concentration of 40% does not produce significant histopathologic alterations in the lung tissue; in the concentration of 70%, can promotes the alveolar walls thick and capillary congestion and in the concentration of 100% can cause death and originate diffuse pulmonary lesion.
Oxygen; Lung; Rats
Introduction
Oxygen is one of the most abundant chemical elements on earth, occupying about 21% of
the atmosphere. Discovered around 200 years ago, is the essential substance that has
fascinated man for all time, without which life on earth would not be possible11. Lucchiari P, Sugizaki M, Malucelli M, Bacila M. Desenvolvimento de
métodos físicos para o estudo do comportamento bioquímico e fisiológico de organismos
antárticos. Arch Vet Scienc. 1998;3:115-8.. However, its use for therapeutic purposes is more
recent, being described by Joseph Priestley in the early nineteenth century. In 90
years, began to be used for acute situations. At this time, there were also reports of
reduced mortality of patients with chronic obstructive pulmonary disease when subjected
to oxygen therapy22. Muhe L, Webert M. Oxygen delivery to children with hypoxaemia in
small hospitals in developing countries. Int J Tuberc Lung Dis. 2001 Jun;5:527-32.
PMID: 11409579.
,
33. Hay WW, Bell EF. Oxygen therapy, oxygen toxicity, and the STOP-ROP
trial. Pediatrics. 2000 Feb;105:424-5. doi: 10.1542/peds.105.2.424.
https://doi.org/10.1542/peds.105.2.424...
.
Oxygen therapy should be based on technical knowledge of equipment, as well as pathophysiological knowledge of the disease in question and also must consider the complications that may treatment trigger11. Lucchiari P, Sugizaki M, Malucelli M, Bacila M. Desenvolvimento de métodos físicos para o estudo do comportamento bioquímico e fisiológico de organismos antárticos. Arch Vet Scienc. 1998;3:115-8. , 22. Muhe L, Webert M. Oxygen delivery to children with hypoxaemia in small hospitals in developing countries. Int J Tuberc Lung Dis. 2001 Jun;5:527-32. PMID: 11409579..
Based on the above statement and in order that the Intensive Care Unit, used high concentrations of oxygen into sick patients and the procedure can cause damage to the lung tissue, it is important to demonstrate and to analyze experimentally the real effect of oxygen, and in what concentrations it can be used without causing damage to lung tissue.
Methods
The project was evaluated and approved by the Ethics in Research Committee of Federal University of Maranhao (UFMA), protocol 11/08.
This is an experimental study where we used a sample of 20 female rats (Rattus norvegicus albinus, Rodentia Mammalia) Wistar, supplied by the UFMA vivarium, mean age 60 days and mean body weight of 191 grams.
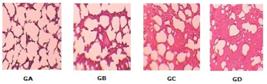
The sample was distributed randomly into four groups of five animals, control (GA) remained under atmospheric oxygen (FiO2 21%), group (GB) was subjected to an O2 concentration of about 40%, group C (GC) to an O2 concentration of about 70% and group D (GD) to an O2 concentration of approximately 100% for three days for a total of seventy - two hours.
During the experiment, the animals were kept in a chamber of transparent acrylic glass, which allowed the observation of the sample inside. The camera is designed for the purpose of this research in the laboratory of mechanics of the Federal Institute of Education, Science and Technology of Maranhao -cIFMA, measuring 34cm high, 57cm long and 34cm wide, with a volume of 0.065 m³. On his side were made with two holes 1 cm in diameter. The first hole was used for the entry of two polyethylene tubes, connected to the outputs of compressed air and oxygen, the latter controlled by a manometer. The second hole connected to a pressure reducer, responsible for controlling the pressure inside the chamber. The camera had enough space to accommodate the five animals in each group, which received standard diet for rats (Purina(r)) and filtered water ad libitum during the three-day experiment (Figure 1).
The animals received dosages of gases through the continuous flow of oxygen and compressed air (gas mixture with average molecular weight of 28.966 obtained by using filters and compressors) that were installed in the chamber and controlled by flow meters.
The experiment took place in this sequence: the control group was not subjected to high concentrations of oxygen remained in the cages under atmospheric oxygen (FiO2 21%). Group B (FiO2 40%) was pre-fitted with 6 liters (L) compressed air and 2L of the oxygen, group C (FiO2 70%) with 3L compressed air and 5L of the oxygen and group D (FiO2 100%) with 0L compressed air and 8L of the oxygen. The values were estimated from the formula of FiO2 and can be calculated from the flow of gas mixture.
The pressure inside the chamber was measured using a mixture of H2O with artificial coloring liquid (MIX(r)) contained in a silicone tube with a length of 52 cm and 0.5 cm in diameter. One end was introduced into the chamber while the other being free, under pressure from the atmospheric air at the sea level (1 Atm). Next to the hose drew attention two points of balance between atmospheric pressure and the pressure inside the chamber. Thus, as the water with artificial coloring was moving, it was concluded that there was an increase in pressure inside the acrylic box. This pressure was controlled by the reductive pressure valve, allowing balance or reducing the pressure gradient between the chamber and atmospheric pressure.
After exposure to oxygen, the animals were anesthetized and did the surgical procedure
of thoracotomy combined with abdominal approach for removal of pulmonary tissue. The
operation was performed by the same experimenter and followed methodization for the
animals of the four experimental groups55. Pereira MLL, Teles AP, Pereira Neto J. Hyperbaric chamber of acrylic
to small animals. Acta Cir Bras. 2001 Oct/Nov/Dec;16:267-70. doi:
10.1590/S0102-86502001000400014.
https://doi.org/10.1590/S0102-8650200100...
.
The animals were kept in the laboratory fed a basal diet for rats and water ad libitum and evaluated daily.
Each animal underwent anesthesia with a mixture of ketamine 5% (Vetanarcol(r)) and xylazine 2% (kensol(r)) in a 1:1 ratio, at a dose of 0.2 ml/100g body weight, administered intramuscular.
After exposure to oxygen, underwent the surgical procedure, performed median thoracotomy with bilateral subcostal incision 5 cm starting from the tip of the xiphoid process and following the bottom edge of the tenth rib to remove the lung tissue.
The animals were induced to death at 24h, 48h and 72h following as the group, with a lethal dose of xylazine (12 mg\kg) and ketamine (42 mg\kg).
The lungs were identified and carefully removed. Each surgical specimen was washed in saline 0.9% (ADV Tayuyna(r)) and placed in anatomical position. Subsequently, the lungs were placed in polystyrene and dipped in collectors for exam (COLLECTOR Universal(r)) with identification, dissection order and right or left lung, which contained a solution fixative (formaldehyde 10%) and sent for histological analysis at the Laboratory of Histopathology, Presidente Dutra University Hospital, Federal University of Maranhao - HUPD/UFMA.
Was performed histopathological evaluation of qualitative criteria including
inflammation (classified in degrees), chronic inflammation, capillary congestion,
thickening of the alveolar walls, interstitial edema, alveolar edema, alveolar
hemorrhage denuded areas of the capillary endothelium, denuded areas of the alveolar
epithelium, atelectasis, classified as absent, mild, moderate and severe, according to
the protocols of histological analysis histológica55. Pereira MLL, Teles AP, Pereira Neto J. Hyperbaric chamber of acrylic
to small animals. Acta Cir Bras. 2001 Oct/Nov/Dec;16:267-70. doi:
10.1590/S0102-86502001000400014.
https://doi.org/10.1590/S0102-8650200100...
.
For data processing software was used Graphpad Instat version 3.05., 32 bit for Win 95 / NT, Create Sep. 27, 2000.
To evaluate the histopathological data, we used the Kruskal Wallis test with Dunnett test for multiple comparisons (nonparametric data). With respect to categorical variables, we applied the Chi-square. Despite having been used nonparametric tests, put the mean and standard deviation in Table 1 in order to inform about the variability of values. In all tests was set at 0.05 or 5% (p<0.05) the level of rejection of the null hypothesis.
Histopathological evaluation of acute inflammation (classified in degrees), chronic inflammation, capillary congestion, thickening of the alveolar walls, interstitial edema, alveolar edema, alveolar hemorrhage, denuded areas of the capillary endothelium, denuded areas of the alveolar epithelium and atelectasis.
Results
During the experiment, two rats of group D with FiO2 100%, died before of the 72 hours, the first with 61 hours and 54 minutes and the second death in 66 hours and 40 minutes of exposure.
The intensity of acute inflammation in the lungs of rats tested in the GC showed mild in most animals, while in GD the intensity of acute inflammation was moderate in all animals. The intensity of acute inflammation was absent in three groups (Table 1).
The analysis of indicators of chronic inflammation, capillary congestion and thickening of the alveolar walls showed a statistically significant difference between groups (Table 1 and Figure 2).
In the groups A, B and C there were absent of alveolar and interstitial edema and alveolar hemorrhage, with a statistically significant difference (Table 1).
The analysis of the presence of denuded areas of the capillary endothelium and alveolar epithelial areas showed a statistically significant difference (Table 1). Regarding the presence of atelectasis, there was no significant differences between groups (Table 1).
Discussion
Continuous exposure to oxygen for 48 to 72 hours produces progressive pulmonary disorders44. Patel D, Goel A, Agarwal S, Garg P, Lakhani K. Oxygen toxicity. J Indian Acad Clin Med. 2003;4:234-7.. Then, in the present study, we used a period of 72 hours of exposure to oxygen for all groups.
To expose the animals to oxygen, we used a normobaric chamber based on a type of acrylic
sealed chamber designed for small animals, keeping them without anesthesia or analgesia,
in a fit condition to receive oxygen in different fractions, uneventful 55. Pereira MLL, Teles AP, Pereira Neto J. Hyperbaric chamber of acrylic
to small animals. Acta Cir Bras. 2001 Oct/Nov/Dec;16:267-70. doi:
10.1590/S0102-86502001000400014.
https://doi.org/10.1590/S0102-8650200100...
. Previous studies demonstrated the use of acrylic
chamber11. Lucchiari P, Sugizaki M, Malucelli M, Bacila M. Desenvolvimento de
métodos físicos para o estudo do comportamento bioquímico e fisiológico de organismos
antárticos. Arch Vet Scienc. 1998;3:115-8.
,
66. Pereira MLL, Scheidt TC, Simões MJ, Mosquette R, Gomes PO. Hiperbaric
oxygen in actinic lesions of rat colon: morphological and morphometric aspects. Acta
Cir Bras. 2004 Nov/Dec;19:658-63. doi:
10.1590/S0102-8650200400060001.
https://doi.org/10.1590/S0102-8650200400...
7. Rojas LM, Suárez SM, Lemus M, Benítez K, Ramírez Y, Mitchell M,
Boada-Sucre A. Oxidative stress as possible cause of retinopathy in developing rats
submitted to hyperoxia. INCI. 2004;29:556-61.
-
88. Mataloun MM, Rebello CM, Mascaretti RS, Dohlnikoff M, Leone CR.
Pulmonary responses to nutritional restriction and hyperoxia in premature rabbits. J
Pediatr (Rio J). 2006 May/Jun;82:179-85. doi:10.2223/JPED.1471.
https://doi.org/10.2223/JPED.1471...
. However these studies used a model of
hyperbaric chamber.
The results showed that two rats in group D, with FiO2 100%, progressed to death 72
hours before the experiment, as observed in previous experimental studies99. Barazzone C, Horowitz S, Donati YR, Rodriguez I, Piguet PF. Oxygen
toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol. 1998
Oct;19:573-81. doi: 10.1165/ajrcmb.19.4.3173
https://doi.org/10.1165/ajrcmb.19.4.3173...
,
1010. Waxman AB, Einarsson O, Seres T, Knickelbein RG, Warshaw JB,
Johnston R, Homer RJ, Elias JA. Targeted lung expression of interleukin-11 enhances
murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J
Clin Invest. 1998 May;101:1970-82. doi: 10.1172/JCI1337.
https://doi.org/10.1172/JCI1337...
.
We did not observe the presence of acute inflammation in rats in groups A and B, shown
in only one animal in group C and in most animals in group D. While chronic
inflammation, reported by several studies, and performed in all animals of group D was
not found in other groups1111. Suguihara C, Lessa AC. Strategies to minimize lung injury in
extremely low birth weight infants. J Pediatr (Rio J). 2005 Mar;81:S69-S78. doi:
10.1590/S0021-75572005000200009.
https://doi.org/10.1590/S0021-7557200500...
.
Group A did not show capillary congestion, but four rats in group B and all animals in
groups C and D, presented this event. It was found thickening of the alveolar walls in
group A, it was revealed in three animals in group B and all animals in groups C and D,
these changes were found in a previous experimental study with 17 preterm rabbits88. Mataloun MM, Rebello CM, Mascaretti RS, Dohlnikoff M, Leone CR.
Pulmonary responses to nutritional restriction and hyperoxia in premature rabbits. J
Pediatr (Rio J). 2006 May/Jun;82:179-85. doi:10.2223/JPED.1471.
https://doi.org/10.2223/JPED.1471...
.
A previous study in rats exposed to 100% concentration, showed interstitial edema and
alveolar edema and hemorrhage99. Barazzone C, Horowitz S, Donati YR, Rodriguez I, Piguet PF. Oxygen
toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol. 1998
Oct;19:573-81. doi: 10.1165/ajrcmb.19.4.3173
https://doi.org/10.1165/ajrcmb.19.4.3173...
. Similar to this
research, the GD was evidenced this characteristics in all rats.
Hyperoxic exposure continued for 48 to 72 hours produces progressive cellular changes and may had denudation of the areas of the capillary endothelium44. Patel D, Goel A, Agarwal S, Garg P, Lakhani K. Oxygen toxicity. J Indian Acad Clin Med. 2003;4:234-7.. Unlike this affirmation, in this experiment, all groups showed this change discrete or absent.
Some studies indicate that after 48 hours of exposure to high concentrations of oxygen, there is increased permeability of the alveolar epithelium22. Muhe L, Webert M. Oxygen delivery to children with hypoxaemia in small hospitals in developing countries. Int J Tuberc Lung Dis. 2001 Jun;5:527-32. PMID: 11409579.. Corroborating this statement, it was the denuded of the areas of the alveolar epithelium in three animals in group D, group exposed to highest concentration. Another study indicates that higher fraction of inspired oxygen in short courses of mechanical ventilation ameliorates the parameters studied in elderly lungs. In elderly group observed alveolar septa dilatation and significative increase in neutrofiles1313. Cavassani SS, Junqueira VB, Moraes JB, Luzo KK, Silva CM, Barros M, Marinho M, Simões RS, Oliveira-Júnior IS. Short courses of mechanical ventilation with high-O2 levels in elderly rat lungs. Acta Cir Bras. 2011 Apr;26(2):107-13. PMID: 21445472..
There are studies that report the occurrence of atelectasis after exposure to high
concentrations of oxygen1212. Bateman NT, Leach RM. ABC of oxygen. Acute oxygen therapy. BMJ. 1998
Sep;317(7161):798-801. PMID: 9740573.. In a contradictory
statement to this author, in this study, there was a statistically significant
difference in atelectasis between groups C and D, found in only one animal of group C. A
likely hypothesis for this result is that inside the chamber were the presence of
nitrogen, since during the experiment was not possible to have a pulse of air to the
measurement of gases88. Mataloun MM, Rebello CM, Mascaretti RS, Dohlnikoff M, Leone CR.
Pulmonary responses to nutritional restriction and hyperoxia in premature rabbits. J
Pediatr (Rio J). 2006 May/Jun;82:179-85. doi:10.2223/JPED.1471.
https://doi.org/10.2223/JPED.1471...
. The nitrogen did not
participate in gas exchange between alveoli and alveolar capillaries; then it helps
maintain lung volume. When replaced by oxygen in the alveoli, which participates in gas
exchange, triggering a decrease in volume leading alveolar atelectasis1212. Bateman NT, Leach RM. ABC of oxygen. Acute oxygen therapy. BMJ. 1998
Sep;317(7161):798-801. PMID: 9740573..
Conclusions
The exposure to oxygen for 72 hours with a concentration of 40% produces histopathological changes discrete to moderate in the alveolar walls and capillaries. The concentration of 70% promotes a moderate to severe thickening of the alveolar walls and capillary congestion. The concentration of 100% over 60 hours of exposure can lead to death and lead to diffuse lung lesions characterized by acute and chronic inflammation, interstitial and alveolar edema, thickening of the alveolar walls, alveolar hemorrhage, capillary congestion and denuded alveolar epithelium areas.
References
-
1Lucchiari P, Sugizaki M, Malucelli M, Bacila M. Desenvolvimento de métodos físicos para o estudo do comportamento bioquímico e fisiológico de organismos antárticos. Arch Vet Scienc. 1998;3:115-8.
-
2Muhe L, Webert M. Oxygen delivery to children with hypoxaemia in small hospitals in developing countries. Int J Tuberc Lung Dis. 2001 Jun;5:527-32. PMID: 11409579.
-
3Hay WW, Bell EF. Oxygen therapy, oxygen toxicity, and the STOP-ROP trial. Pediatrics. 2000 Feb;105:424-5. doi: 10.1542/peds.105.2.424.
» https://doi.org/10.1542/peds.105.2.424 -
4Patel D, Goel A, Agarwal S, Garg P, Lakhani K. Oxygen toxicity. J Indian Acad Clin Med. 2003;4:234-7.
-
5Pereira MLL, Teles AP, Pereira Neto J. Hyperbaric chamber of acrylic to small animals. Acta Cir Bras. 2001 Oct/Nov/Dec;16:267-70. doi: 10.1590/S0102-86502001000400014.
» https://doi.org/10.1590/S0102-86502001000400014 -
6Pereira MLL, Scheidt TC, Simões MJ, Mosquette R, Gomes PO. Hiperbaric oxygen in actinic lesions of rat colon: morphological and morphometric aspects. Acta Cir Bras. 2004 Nov/Dec;19:658-63. doi: 10.1590/S0102-8650200400060001.
» https://doi.org/10.1590/S0102-8650200400060001 -
7Rojas LM, Suárez SM, Lemus M, Benítez K, Ramírez Y, Mitchell M, Boada-Sucre A. Oxidative stress as possible cause of retinopathy in developing rats submitted to hyperoxia. INCI. 2004;29:556-61.
-
8Mataloun MM, Rebello CM, Mascaretti RS, Dohlnikoff M, Leone CR. Pulmonary responses to nutritional restriction and hyperoxia in premature rabbits. J Pediatr (Rio J). 2006 May/Jun;82:179-85. doi:10.2223/JPED.1471.
» https://doi.org/10.2223/JPED.1471 -
9Barazzone C, Horowitz S, Donati YR, Rodriguez I, Piguet PF. Oxygen toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol. 1998 Oct;19:573-81. doi: 10.1165/ajrcmb.19.4.3173
» https://doi.org/10.1165/ajrcmb.19.4.3173 -
10Waxman AB, Einarsson O, Seres T, Knickelbein RG, Warshaw JB, Johnston R, Homer RJ, Elias JA. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J Clin Invest. 1998 May;101:1970-82. doi: 10.1172/JCI1337.
» https://doi.org/10.1172/JCI1337 -
11Suguihara C, Lessa AC. Strategies to minimize lung injury in extremely low birth weight infants. J Pediatr (Rio J). 2005 Mar;81:S69-S78. doi: 10.1590/S0021-75572005000200009.
» https://doi.org/10.1590/S0021-75572005000200009 -
12Bateman NT, Leach RM. ABC of oxygen. Acute oxygen therapy. BMJ. 1998 Sep;317(7161):798-801. PMID: 9740573.
-
13Cavassani SS, Junqueira VB, Moraes JB, Luzo KK, Silva CM, Barros M, Marinho M, Simões RS, Oliveira-Júnior IS. Short courses of mechanical ventilation with high-O2 levels in elderly rat lungs. Acta Cir Bras. 2011 Apr;26(2):107-13. PMID: 21445472.
-
Financial source: Maranhao Research Foundation (FAPEMA)
-
1
Research performed at Department of Medicine II, Laboratory of Academic League of Experimental Surgery of Maranhao (LACEMA), School of Medicine, Federal University of Maranhao (UFMA), Sao Luis-MA, Brazil.
Publication Dates
-
Publication in this collection
Dec 2014
History
-
Received
19 Aug 2014 -
Reviewed
17 Oct 2014 -
Accepted
21 Nov 2014