Abstract
PURPOSE:
To compare sciatic nerve regeneration in rats using three different techniques of repair.
METHODS:
Fifteen isogonics rats were divided into three groups according to the method used to repair a 5-mm long defect created in the sciatic nerve: autogenous graft (Group A), polyglycolic acid tube (PGAt) (Group B), and of the association of PGAt with the graft (Group C). Histological analysis, regenerated myelinated axon number count and functional analysis were used to compare after six weeks.
RESULTS:
There was no difference in fiber diameter and degree of myelinization presented by Groups A, B and C. Group B presented the lowest number of regenerated axons. The groups did not display any significant functional difference after walking track analysis (p<0.05).
CONCLUSION:
No differences between the three groups in terms of functional recovery, although there were histological differences among them.
Peripheral Nerves; Surgery, Plastic; Nerve Regeneration; Polyglycolic Acid; Therapeutic Uses; Rats
Introduction
Extensive losses of neural tissue due to tumor resection or traumatic injuries do not allow for repair by primary anastomosis. In such cases, nerve autografting is the treatment of choice. It guides axonal growth and joins the distal and proximal ends, therefore reducing tension along the suture line11. Terzis J, Faibisoff B, Williams HB. The nerve gap: suture under tension vs. graft. Plast Reconstr Surg. 1975 Aug;56(2):166-70. PMID: 1096197..
However, autografting may pose some considerable problems: 1) donor-site morbidity; 2) the large amount of autologous tissue required to repair extensive losses of neural tissue (which is sometimes insufficient); 3) functional deficits that remain despite adequate surgical treatment, whose primary goal is the improvement in functional recovery and the decrease of sequelae .
Several techniques with this purpose have been described. Bridging the gap between the ends of a sectioned nerve with tubular conduits is an alternative technique with theoretical advantages such as better confinement of the regenerating axonal fibers, reduced inflammatory reaction and formation of cicatricial tissue at the repair site, proper guiding of fiber growth towards the distal end, prevention of donor-site associated sequelae, and shortened duration of surgery22. Brunelli GA, Vigasi A, Brunelli GR. Different conduits in peripheral nerve surgery. Microsurgery. 1994;15(3):176-8. PMID: 8015422. , 33. Keeley RD, Nguyen KD, Stephanides MJ, Padilla J, Rosen JM. The artificial nerve graft: a comparison of blended elastomer-hydrogel with polyglicolic acid conduits. J Reconstr Microsurg. 1991 Apr;7(2):93-100. PMID: 1646884..
Studies on extensive losses of neural tissue and their repair by bridging the gap between the proximal and distal stumps have been performed since the second half the 19th century44. Glück T. Uber neuroplastik auf dem weg der transplantation. Arch Klinik Chir. 1880;25:606-16.. Several replacements for nerve grafts have been employed such as vessels55. Cunha AS, Costa MP, Silva CF. Peroneal nerve reconstruction by using glycerol-preserved veins: histological and functional assessment in rats. Acta Cir Bras. 2013 Feb;28(2):94-101. PMID: 23370921., fascia66. Kirk EG, Lewis D. Fascial tubulization in the repair of nerve defects. JAMA. 1915;65(6):486-92., plastic tubes77. Garrity RW. The use of plastics and rubber tubing in the management of irreparable nerve injuries. Surg Forum. 1955;6:517-20. PMID: 13391538., absorbable tubes88. Mackinnon SE, Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglicolic acid tube. Plast Reconstr Surg. 1990 Mar;85(3):419-24. PMID: 2154831., silicone tubes99. Lundborg G, Gelberman RH, Longo RM, Powell, Varon S. In vivo regeneration of cut nerves encased in silicone tubes: growth across a six millimeter gap. J Neuropathol Exp Neurol. 1982 Jul;41(4):412-22. PMID: 7086464., muscle1010. Sanes JR, Marshall LM, McMahan UJ. Reinnervation of muscle fiber basal lamina after removal of myofibers. Differentiation of regenerating axons at original synaptic sites. J Cell Biol. 1978 Jul;78(1):176-98. PMID: 307554., synthetic tubes77. Garrity RW. The use of plastics and rubber tubing in the management of irreparable nerve injuries. Surg Forum. 1955;6:517-20. PMID: 13391538. and allogeneous nerve1111. Lemos SPS, Hayashi I, Cunha AS, Silva CF, Barros Filho TEP, Costa MP, Ferreira MC. Glycerol-preserved allogenous nerve: an experimental study with rats. Acta Ortop Bras. 2008;16(3): 133-7. doi.org/10.1590/S1413-78522008000300001.
https://doi.org/10.1590/S1413-7852200800...
.
Peripheral nerve repair with absorbable polyglycolic acid tube has been extensively investigated88. Mackinnon SE, Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglicolic acid tube. Plast Reconstr Surg. 1990 Mar;85(3):419-24. PMID: 2154831. with no significant difference in comparison to nerve grafting 1212. Hentz VR, Rosen JM, Xiao SJ, Mcgill KC, Abraham G. A comparison of suture and tubulization nerve repair techinique in a primate. J Hand Surg Am. 1991 Mar;16(2):251-61. PMID: 1850770..
To date, there are no available studies regarding the use of combined neurotubes and autografting .The objective of this study was to assess the degree of neural regeneration in rats obtained by the interposition of combined autograft and polyglycolic acid tube (PGAt) and compare it with autografting or polyglicolic acid tube alone through histological and functional analysis.
Methods
Research comply with laboratory animals advisors by the Council for International Organization of Medical Sciences (CIOMS) ethical code for animal experimentation (WHO Chronicle 1985; 39(2):51-6).
Fifteen 8-week-old Lewis rats, weighing between 200 and 300g were used. Five millimeter-long defects were created by microsurgical technique in the sciatic nerve of the right hind paw. The animals were then divided into three study groups according to the mode of treatment. Repeated measures analysis of variance followed by Tukey's multiple comparisons was performed as a statistic method.
Group A animals (control group) were treated by having the removed nerve segment sutured back in place (autografting), maintaining its original orientation. Group B animals were treated with a 2.3 mm-diameter PGAt conduit 10mm in length (Neurotube, manufactured by Neurogen L.L.C., Baltimore, USA), which was interposed between the sectioned segments. Finally, group C animals also had the removed nerve fragment repositioned, maintaining its original orientation (as in group A), but the graft was enveloped by the PGAt (Table 1).
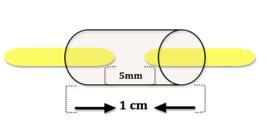
All study animals were anesthetized with intraperitoneal injection of sodium pentobarbital (5 mg/kg) before surgical procedure. The sciatic nerve was dissected at the dorsal surface of the right hind paw and a 5mm segment of the nerve was removed (Figure 1).
In group A animals, the removed segment was sutured back in its normal position with 4 separate epineural 10-0 nylon stitches (Figure 2).
In group B animals, the PGA tube was anchored in its position by a "U" stitch in each extremity starting from the outside to the inside of the tube, then through the epineural sheath of the stump and finally from the inside to the outside of the tube again, so as to enclose a 2.5 mm segment at each end. Once sutured at one end, the tube was filled with heparinized (10 IU/ml) saline solution. The other end was sutured likewise and again the tube was filled with heparinized (10 IU/ml) saline solution (Figures 3 and 4).
In group C animals, the resected segment was sutured back in its position (autogenous graft) in the same manner as in the control group. Following the suture of the autograft, the polyglycolic acid tube was sectioned lengthwise with microsurgical scissors and then placed in order to enclose the autograft plus a 2.5 mm segment of both the proximal and distal stumps of the injured nerve, just as in group B animals (Figures 5 and 6). The PGA tube incision was sutured with 3 simple 8-0 nylon stitches (proximal, median and distal) to ensure proper coaptation of the tube edges. The polyglycolic acid tube was then fixated to the muscle by two separate 8-0 nylon stitches (Figures 5 and 6).
The animals were given water and food ad libitum and sacrificed six weeks after the surgery for histological analysis and regenerated myelinated axon count. Functional recovery was evaluated on the third week after surgery and just before the sacrifice of the animals (on the sixth week) by walking track analysis, which assesses hind limb performance by examining footprints, and the results were compared to the analysis performed before surgery.
Results
All animals remained healthy throughout the 6-week period of study, and no surgical wound infection or neuropathic plantar ulcerations were observed.
Histological analysis
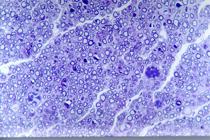
Microscopic analysis of Group A (autograft) animals showed that the graft was bordered by an epineurium of fusiform cells. Inside the epineurium there was a large amount of myelinated axons of different diameters and homogenous distribution. Tissue reaction around the graft was more intense in group A animals when compared to the other groups. Escaping regenerated fibers outside the limits of the epineurium were seen in all five animals in group A. In addition, a limited amount of axonal bundles was observed in group A (smaller than in group B). Signs of Wallerian degeneration were similar in groups A and C (Figures 7 and 8).
In Group B (PGAt) animals, the cells had a fusiform appearance, and contained inside of the tube neural stroma-like tissue with a significant amount of myelinated axons of different diameters, grouped in small fasciculi of various sizes and heterogeneous distribution, with interposing connective tissue. Remarked neoangiogenesis was observed inside the tube all around the neural tissue with blood vessels that pierced the polyglycolic acid tube mesh. Intensive neoangiogenesis within the regenerated nerve was observed in group B, but not in Group A.
Reactive fibrosis around the tube was less evident than in Group A (autograft). Escaping regenerated fibers were not observed outside the tube (Figures 9 and 10).
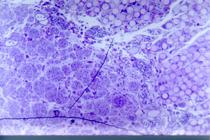
In Group C (PGAt + graft) animals, the cells also had a fusiform appearance and contained inside of the tube a considerable amount of neural tissue which corresponded to the nerve graft. A large number of myelinated axons, of different sizes and heterogeneous distribution were seen. Fusiform cells with long cytoplasmic processes were observed in addition to pronounced neoangiogenesis inside the tube all around the neural tissue with blood vessels that pierced the polyglycolic acid tube mesh. Neoangiogenesis was less intense and the number of axonal bundles in group C was smaller than in Group B (PGAt) but similar to Group A (autograft). Several giant multinucleated cells could be seen with PGA-like matter in their cytoplasm. Reactive fibrosis around the tube was less pronounced in Group C (PGAt + graft) than in Group A (autograft) but similar to group B (PGAt). Escaping regenerated axons were not observed outside the tube (Figures 11 and 12).
There was no difference in fiber diameter and the degree of myelination between Groups A (autograft), B (PGAt) and C (PGAt + graft).
Regenerated myelinated axon count
The mean regenerated myelinated axon count, standard deviation and standard error of each study group are shown in Table 2.
In Group A, the average count of regenerated myelinated axons was 7225.6 ± 617.5 (mean ± SD). Group B had an average 4225.2 ± 376.8 of regenerated myelinated axons and group C had 7488.4 ± 384.54. One-way analysis of variance (ANOVA) followed by Tukey's multiple comparison of means were performed (p<0.05). There were no significant differences between groups A and C regarding the regenerated axon count 6 weeks after nerve section. However, the average count of regenerated myelinated axons in Group B was significantly lower than in groups A and C. These data are summarized in Table 3.
Functional evaluation
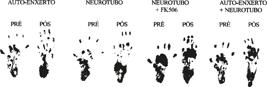
Footprints of the study rats before and after surgery in the three groups are shown in Figure 13.
Mean sciatic function indexes (SFI) before surgery were -8.002 ± 5.26 (Group A); -5.928 ± 13.144 (Group B) and -4.936 ± 3.712 (Group C).
Kruskal-Wallis analysis (p<0.05) did not show any significant difference in the sciatic function index (SFI) between the three groups before surgery.
Mean SFI values and their standard errors in the postoperative period (immediate, three and six weeks) of each group are shown in Table 4.
SFI in the postoperative period (immediate, 3 and 6 weeks) according to the group of treatment.
Repeated measures analysis of variance followed by Tukey's multiple comparisons was performed to assess SFI regarding postoperative times (p<0.05). In the immediate postoperative period there was no significant difference in the SFI between the three study groups. Three weeks after surgery, there was a significant difference between group B (PGAt) and groups A (autograft) and C (PGAt + graft). However, no significant difference between groups A and C was detected. These data are summarized in Table 5.
There was no significant difference in SFI between the study groups six weeks after surgery.
Discussion
Whenever the extension of tissue loss in peripheral nerve lesions do not allow for primary suture, the best repairing method seems to be autografting22. Brunelli GA, Vigasi A, Brunelli GR. Different conduits in peripheral nerve surgery. Microsurgery. 1994;15(3):176-8. PMID: 8015422. , 1313. Costa HJ, Ferreira Bento R, Salomone R, Azzi-Nogueira D, Zanatta DB, Paulino Costa M, da Silva CF, Strauss BE, Haddad LA. Mesenchymal bone marrow stem cells within polyglycolic acid tube observed in vivo after six weeks enhance facial nerve regeneration. Brain Res. 2013 May;1510:10-21. PMID: 23542586.. However, some important aspects to be considered include: 1) the need for resecting autologous tissue; 2) the large amount of autogenous tissue required which may not be available; 3) duration of surgery may be shortened with use of synthetic conduits22. Brunelli GA, Vigasi A, Brunelli GR. Different conduits in peripheral nerve surgery. Microsurgery. 1994;15(3):176-8. PMID: 8015422.; 4) autografting results are not completely satisfactory1414. Kitahara AK, Nishimura Y, Shimizu Y, Endo K. Facial nerve repair accomplished by interposition of a collagen nerve guide. J Neurosurg. 2000 Jul;93(1):113-20. PMID: 10883913..
Employing synthetic nerve graft conduits to bridge the gap between the two ends of the injured nerve has shown encouraging results. In small defects where the distance of the nerve won't allow for chemotactic and chemotrophic attraction exerted by the distal stump in the region of axonal growth, the results are comparable to autografting22. Brunelli GA, Vigasi A, Brunelli GR. Different conduits in peripheral nerve surgery. Microsurgery. 1994;15(3):176-8. PMID: 8015422. , 88. Mackinnon SE, Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglicolic acid tube. Plast Reconstr Surg. 1990 Mar;85(3):419-24. PMID: 2154831. , 1212. Hentz VR, Rosen JM, Xiao SJ, Mcgill KC, Abraham G. A comparison of suture and tubulization nerve repair techinique in a primate. J Hand Surg Am. 1991 Mar;16(2):251-61. PMID: 1850770..
In selected cases, the polyglycolic acid tube may be an alternative to nerve grafting1212. Hentz VR, Rosen JM, Xiao SJ, Mcgill KC, Abraham G. A comparison of suture and tubulization nerve repair techinique in a primate. J Hand Surg Am. 1991 Mar;16(2):251-61. PMID: 1850770. , 1515. Dellon Al, Mackinnon SE. An alternative to the classical nerve graft for the management of the short nerve gap. Plast Reconstr Surg. 1988 Nov;82(5):849-56. PMID: 2845455.. Synthetic absorbable nerve graft conduits have shown better results than non-absorbable conduits1616. Madison R, Da Silva CF, Dikkes P, Chiu TH, Sidman RL. Increased rate of peripheral nerve regeneration using bioresorbable nerve guides and a laminin-containing gel. Exp Neurol. 1985 Jun;88(3):767-72. PMID: 3996520.. Absorbable conduits promote little reactive fibrosis and do not hinder the increase in nerve diameter1616. Madison R, Da Silva CF, Dikkes P, Chiu TH, Sidman RL. Increased rate of peripheral nerve regeneration using bioresorbable nerve guides and a laminin-containing gel. Exp Neurol. 1985 Jun;88(3):767-72. PMID: 3996520., as opposed to non-absorbable conduits.
Autograft as employed in group A of our study is considered the treatment of choice for repairing neural defects with tissue loss22. Brunelli GA, Vigasi A, Brunelli GR. Different conduits in peripheral nerve surgery. Microsurgery. 1994;15(3):176-8. PMID: 8015422.. Polyglycolic acid tube used in group B has already been studied in primates as an alternative to nerve grafting when tissue loss was shorter than 30 mm1111. Lemos SPS, Hayashi I, Cunha AS, Silva CF, Barros Filho TEP, Costa MP, Ferreira MC. Glycerol-preserved allogenous nerve: an experimental study with rats. Acta Ortop Bras. 2008;16(3): 133-7. doi.org/10.1590/S1413-78522008000300001.
https://doi.org/10.1590/S1413-7852200800...
, 1515. Dellon Al, Mackinnon SE. An alternative to the classical nerve graft for the management of the short nerve gap. Plast Reconstr Surg. 1988 Nov;82(5):849-56. PMID: 2845455.. Finally, the combination of PGA tube and autografting seen in group C hasn't been reported so far. Our objective was to add the good results obtained with both techniques. At the same time, the PGA tube is expected to decrease inflammatory reaction and formation of cicatricial tissue around the graft, thus preventing neuroma formation and axonal escape. It should also help a larger amount of axons to regenerate and reach their target organs which would consequently result in better functional recovery.
Neuroma formation has only been described in rats submitted to autografting18. In our study, tube collapse was not observed in either group B or C despite the description of this possible complication22. Brunelli GA, Vigasi A, Brunelli GR. Different conduits in peripheral nerve surgery. Microsurgery. 1994;15(3):176-8. PMID: 8015422. , 1111. Lemos SPS, Hayashi I, Cunha AS, Silva CF, Barros Filho TEP, Costa MP, Ferreira MC. Glycerol-preserved allogenous nerve: an experimental study with rats. Acta Ortop Bras. 2008;16(3): 133-7. doi.org/10.1590/S1413-78522008000300001.
https://doi.org/10.1590/S1413-7852200800...
.
Our results have shown axonal fibers beyond the limits of the epineurium and neuroma formation along the suture lines in group A animals, which had already been reported by some authors88. Mackinnon SE, Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglicolic acid tube. Plast Reconstr Surg. 1990 Mar;85(3):419-24. PMID: 2154831. , 1717. Gold BG, Katoh K, Storm-Dickerson T. The immunosuppressant FK 506 increases the rate of axonal regeneration in rat sciatic nerve. J Neurosci. 1995 Nov;15(11):7509-16.. On the other hand, this was not observed in the study groups in which PGA tubes were used.
Groups A and C were similar in terms of the amount of myelinated axons, the various diameters of axonal fibers and their homogeneous distribution, the presence of neoangiogenesis, the degree of Wallerian degeneration and the amount of axonal groups. Our results have shown that there were differences between groups A and C regarding the absence of axonal escape and a slight decrease in tissue reaction. It wasn't possible to compare our results with those of previous studies, because the association of PGA tube and autografting has never been reported.
Study data has shown that group B had the lowest average count of regenerated myelinated axons. The combination of PGA tube and autografting in group C led to a significantly greater increase in the average count of regenerated myelinated axons. Group C axon count was also higher than in the control group (A - autograft only), but this increase was not significant.
Concerning functional evaluation, there was no significant difference between the study groups in the immediate and late (six weeks) postoperative periods. However, three weeks after surgery, mean SFI in group B was significantly better than in the other groups, whereas there was no difference between groups A and C. Discrepancy between functional findings and axon counts may be explained by the preciseness of restored connections between the regenerated axons and their target organs, which is more important than the overall number of axonal fibers18. In fact, perspectives for future research on neurotubes1919. di Summa PG, Kingham PJ, Campisi CC, Raffoul W, Kalbermatten DF. Collagen (NeuraGen(r)) nerve conduits and stem cells for peripheral nerve gap repair. Neurosci Lett. 2014 Jun;572:26-31. PMID: 24792394. , 2020. Demir A, Simsek T, Acar M, Aktaş A, Vlamings R, Ayyıldız M, Yıldırım M, Temel Y, Kaplan S. Comparison between flexible collagen and vein conduits used for size-discrepant nerve repair: an experimental study in rats. J Reconstr Microsurg. 2014 Jun;30(5):329-34. PMID: 24683135. are very promising, with more suitable and less expensive materials that will become easily available and produce better functional results, in addition to new medications that will be developed to promote neural regeneration and prevent iatrogenic effects.
Conclusions
There were no significant alterations in the histologic patterns between the group of autograft with polyglycolic acid tube and the group with autograft alone. In the polyglycolic acid tube group a lower number of regenerated myelinated axons were observed. There was no significant functional evaluation difference between the studied groups.
References
-
1Terzis J, Faibisoff B, Williams HB. The nerve gap: suture under tension vs. graft. Plast Reconstr Surg. 1975 Aug;56(2):166-70. PMID: 1096197.
-
2Brunelli GA, Vigasi A, Brunelli GR. Different conduits in peripheral nerve surgery. Microsurgery. 1994;15(3):176-8. PMID: 8015422.
-
3Keeley RD, Nguyen KD, Stephanides MJ, Padilla J, Rosen JM. The artificial nerve graft: a comparison of blended elastomer-hydrogel with polyglicolic acid conduits. J Reconstr Microsurg. 1991 Apr;7(2):93-100. PMID: 1646884.
-
4Glück T. Uber neuroplastik auf dem weg der transplantation. Arch Klinik Chir. 1880;25:606-16.
-
5Cunha AS, Costa MP, Silva CF. Peroneal nerve reconstruction by using glycerol-preserved veins: histological and functional assessment in rats. Acta Cir Bras. 2013 Feb;28(2):94-101. PMID: 23370921.
-
6Kirk EG, Lewis D. Fascial tubulization in the repair of nerve defects. JAMA. 1915;65(6):486-92.
-
7Garrity RW. The use of plastics and rubber tubing in the management of irreparable nerve injuries. Surg Forum. 1955;6:517-20. PMID: 13391538.
-
8Mackinnon SE, Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglicolic acid tube. Plast Reconstr Surg. 1990 Mar;85(3):419-24. PMID: 2154831.
-
9Lundborg G, Gelberman RH, Longo RM, Powell, Varon S. In vivo regeneration of cut nerves encased in silicone tubes: growth across a six millimeter gap. J Neuropathol Exp Neurol. 1982 Jul;41(4):412-22. PMID: 7086464.
-
10Sanes JR, Marshall LM, McMahan UJ. Reinnervation of muscle fiber basal lamina after removal of myofibers. Differentiation of regenerating axons at original synaptic sites. J Cell Biol. 1978 Jul;78(1):176-98. PMID: 307554.
-
11Lemos SPS, Hayashi I, Cunha AS, Silva CF, Barros Filho TEP, Costa MP, Ferreira MC. Glycerol-preserved allogenous nerve: an experimental study with rats. Acta Ortop Bras. 2008;16(3): 133-7. doi.org/10.1590/S1413-78522008000300001.
» https://doi.org/10.1590/S1413-78522008000300001 -
12Hentz VR, Rosen JM, Xiao SJ, Mcgill KC, Abraham G. A comparison of suture and tubulization nerve repair techinique in a primate. J Hand Surg Am. 1991 Mar;16(2):251-61. PMID: 1850770.
-
13Costa HJ, Ferreira Bento R, Salomone R, Azzi-Nogueira D, Zanatta DB, Paulino Costa M, da Silva CF, Strauss BE, Haddad LA. Mesenchymal bone marrow stem cells within polyglycolic acid tube observed in vivo after six weeks enhance facial nerve regeneration. Brain Res. 2013 May;1510:10-21. PMID: 23542586.
-
14Kitahara AK, Nishimura Y, Shimizu Y, Endo K. Facial nerve repair accomplished by interposition of a collagen nerve guide. J Neurosurg. 2000 Jul;93(1):113-20. PMID: 10883913.
-
15Dellon Al, Mackinnon SE. An alternative to the classical nerve graft for the management of the short nerve gap. Plast Reconstr Surg. 1988 Nov;82(5):849-56. PMID: 2845455.
-
16Madison R, Da Silva CF, Dikkes P, Chiu TH, Sidman RL. Increased rate of peripheral nerve regeneration using bioresorbable nerve guides and a laminin-containing gel. Exp Neurol. 1985 Jun;88(3):767-72. PMID: 3996520.
-
17Gold BG, Katoh K, Storm-Dickerson T. The immunosuppressant FK 506 increases the rate of axonal regeneration in rat sciatic nerve. J Neurosci. 1995 Nov;15(11):7509-16.
-
19di Summa PG, Kingham PJ, Campisi CC, Raffoul W, Kalbermatten DF. Collagen (NeuraGen(r)) nerve conduits and stem cells for peripheral nerve gap repair. Neurosci Lett. 2014 Jun;572:26-31. PMID: 24792394.
-
20Demir A, Simsek T, Acar M, Aktaş A, Vlamings R, Ayyıldız M, Yıldırım M, Temel Y, Kaplan S. Comparison between flexible collagen and vein conduits used for size-discrepant nerve repair: an experimental study in rats. J Reconstr Microsurg. 2014 Jun;30(5):329-34. PMID: 24683135.
-
Financial source: none
-
1
Research performed at Microsurgery Laboratory, Department of Plastic Surgery, Faculty of Medicine, University of Sao Paulo (USP), Brazil.
Publication Dates
-
Publication in this collection
Jan 2015
History
-
Received
19 Sept 2014 -
Reviewed
17 Nov 2014 -
Accepted
18 Dec 2014