Abstract
PURPOSE:
To investigate hemostatic effects of supplementary factor XIII and desmopressin (DDAVP) in resuscitation of uncontrolled bleeding.
METHODS:
Fifty-four rabbits were randomized in nine groups: G1: Sham; G2: FXIII and normotensive resuscitation (NBP); G3: FXIII and permissive hypotension (PH) (MAP 60% baseline); G4: FXIII/DDAVP/NBP; G5: FXIII/DDAVP/PH; G6: NBP only; G7: FXIII no hemorrhage; G8: FXIII/DDAVP no hemorrhage; G9: PH only. Thromboelastometry and intra-abdominal blood loss were assessed. Scanning electron microscopy (EM) of the clots was performed.
RESULTS:
Compared to Sham, only G8 (FXIII/DDAVP w/o hemorrhage) showed clotting time (CT) significantly lower (p<0.05). NBP alone (G6) resulted in significantly prolonged CT compared to G2, G3 and G5 (p<0.05). Similarly, median alpha angle was significantly larger in G3,4,5, and 9 compared to G6 (p<0.05). Area under the curve was significantly greater in G5 than G2. Intra-abdominal blood loss was lower in G5 and G9 compared to G2 and G6. FXIII/DDAVP and PH resulted in more robust fibrin mesh by EM.
CONCLUSIONS:
Normotensive resuscitation provokes more bleeding and worsens coagulation compared to pH, that is partially reversed by factor XIII and desmopressin. FXIII and DDAVP can synergistically improve coagulation. Permissive hypotension reduces bleeding regardless of those agents.
Hemorrhage; Shock; Resuscitation; Factor XIII; Deamino Arginine Vasopressin; Rabbits
Introduction
Hemorrhagic shock is responsible for 30 to 40% of all injury related deaths, and is the most common cause of potentially preventable death from trauma. Approximately 50% of trauma deaths within the first 48 hours after injury are caused by uncontrolled bleeding and hemostatic dysfunction11. Geeraedts LM Jr, Kaasjager HA, van Vugt AB, Frölke JP. Exsanguination in trauma: A review of diagnostics and treatment options. Injury. 2009 Jan;40(1):11-20. doi: 10.1016/j.injury.2008.10.007.
https://doi.org/10.1016/j.injury.2008.10...
. Thus, expeditious hemorrhage control is important to decrease mortality and prevent severe coagulopathy. Trauma associated coagulopathy is an early and primary event in hemorrhaging trauma victims. Approximately 25% of severely injured patients are coagulopathic at admission22. Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003 Jun;54(6):1127-30. PMID: 12813333.. Studies have shown that the presence of coagulopathy is associated with a 4 fold increased mortality in trauma22. Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003 Jun;54(6):1127-30. PMID: 12813333. , 33. MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003 Jul;55(1):39-44. PMID: 12855879.. Unraveling the mechanisms of trauma associated coagulopathy is a current topic of research.
Trauma associated coagulopathy has several key precursors, consequently it is difficult to determine the precise mechanism2. Hence, pre-emptive treatment of this condition has gained considerable support44. Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, Flaherty SF, Grathwohl KW, Spinella PC, Perkins JG, Beekley AC, McMullin NR, Park MS, Gonzalez EA, Wade CE, Dubick MA, Schwab CW, Moore FA, Champion HR, Hoyt DB, Hess JR. Damage control resuscitation: directly adressing the early coagulopathy of trauma. J Trauma. 2007 Feb;62(2):307-10. PMID: 17297317. , 55. Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. Surg Clin N Am. 2012 Aug;92(4):877-91 doi: 10.1016/j.suc.2012.06.001.
https://doi.org/10.1016/j.suc.2012.06.00...
. The addition of procoagulant pharmacological agents to that treatment strategy has shown promising results66. Porta CR, Nelson D, McVay D, Salgar S, Eckert M, Izenberg S, Martin MJ. The effects of tranexamic acid and prothrombin complex concentrate on the coagulopathy of trauma: an in vitro analysis of the impact on severe acidosis. J Trauma. 2013 Dec;75(6):954-60. doi: 10.1097/TA.0b013e31829e20bf.
https://doi.org/10.1097/TA.0b013e31829e2...
, 77. CRASH-2 Trial Collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichatshuili T, Guptas S, Herrera J, Hunt B, Iribhogbe P, Izurieta M, Khamis H, Komolafe E, Marrero MA, Metia-Mantilla J, Miranda J, Morales C, Olaomi O, Olldashi F, Perel P, Peto R, Ramana PV, Ravi RR, Yuttakasemsunt S. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomized, placebo-controlled trial. Lancet. 2010 Jul 3;376(9734):23-32. doi: 10.1016/S0140-6736(10)60835-5 .
https://doi.org/10.1016/S0140-6736(10)60...
. Because of the multifactorial nature of TAC, it is conceivable that pharmacological agents with distinct pro-coagulant effects may be employed synergistically to treat this condition .
Coagulation factor XIII is a protransglutaminase that was initially called fibrin stabilizing factor, and labeled factor XIII 20 years after its discovery88. Hsieh L, Nugent D. Factor XIII deficiency. Haemophilia.2008 Nov;14(6):1190-200. doi: 10.1111/j.1365-2516.2008.01857.x.
https://doi.org/10.1111/j.1365-2516.2008...
, 99. Lorand K, Konishi K. Activation of fibrin-stabilizing factor of plasma by thrombin. Arch Biochem Biophys. 1964 Apr;105:58-67. PMID: 14165504.. Even though, FXIII was originally described for its role on fibrin cross-linkage and clot stabilization, additional important hemostatic functions have been attributed to this protein1010. Bagoly Z, Koncz Z, Harsfalvi J, Muszbek L. Factor XIII, clot structure, thrombosis. Thromb Res. 2012 Mar;129(3):382-7. doi: 10.1016/j.thromres.2011.11.040.
https://doi.org/10.1016/j.thromres.2011....
11. Levy JH, Greenberg C. Biology of Factor XIII and clinical manifestations of Factor XIII deficiency. Transfusion. 2013 May;53(5):1120-31. doi:10.1111/j.15372995.2012.03865.x.
https://doi.org/10.1111/j.15372995.2012....
12. Nielsen VG, Gurley WQ Jr, Burch TM. The impact of factor XIII on coagulation kinetics and clot strength determined by thromboelastography. Anesth Analg. 2004 Jul;99(1):1203. PMID: 15281516. - 1313. Theusinger OM, Baulig W, Asmis LM, Seifert B, Spahn DR. In vitro factor XIII supplementation increases clot firmmness in Rotation Thromboelastometry (Rotem). Thromb Haemost. 2010 Aug;104(2):385-91. doi: 10.1160/TH09-12-0858.
https://doi.org/10.1160/TH09-12-0858...
. A purified concentrate of FXIII has been marketed in Europe for more than 20 years. Replacement therapy with that concentrate is a widely accepted treatment for acquired and congenital deficiency of FXIII, as well as, supportive therapy in cases of poor wound healing88. Hsieh L, Nugent D. Factor XIII deficiency. Haemophilia.2008 Nov;14(6):1190-200. doi: 10.1111/j.1365-2516.2008.01857.x.
https://doi.org/10.1111/j.1365-2516.2008...
, 1111. Levy JH, Greenberg C. Biology of Factor XIII and clinical manifestations of Factor XIII deficiency. Transfusion. 2013 May;53(5):1120-31. doi:10.1111/j.15372995.2012.03865.x.
https://doi.org/10.1111/j.15372995.2012....
, 1414. Lusher J, Pile SW, Alexander S, Nugent D. Prophylactic therapy with Fibrogammin P is associated with a decreased incidence of bleeding episodes: a retrospective study. Haemophilia. 2010 Mar;16(2):316-21. doi: 10.1111/j.1365-2516.2009.02123.x.
https://doi.org/10.1111/j.1365-2516.2009...
. Similarly, desmopressin acetate (1-deamino-8-d-arginine vasopressin; DDAVP) has been used in the management of patients with hemophilia A and von Willebrand disease for more than 30 years1515. Mannucci PM. Desmopressin (DDAVP) in the treatment of bleeding disorders: the first 20 years. Blood. 1997 Oct;90(7):2515-21. PMID: 9326215.. DDAVP increases plasma levels of both factor VIII (FVIII) and von Willebrand factor and enhances platelet adhesion to injured endothelium1515. Mannucci PM. Desmopressin (DDAVP) in the treatment of bleeding disorders: the first 20 years. Blood. 1997 Oct;90(7):2515-21. PMID: 9326215. , 1616. Sakariassen KS, Cattaneo M, vd Berg A, Ruggeri ZM, Mannucci PM, Sixma JJ. DDAVP enhances platelet adherence and platelet aggregate growth on human artery subendothelium. Blood. 1984 Jul;64(1):229-36. PMID: 6428488..
In the present study, we sought to investigate the acute hemostatic effect of FXIII and DDAVP in a model of uncontrolled hemorrhagic shock that incorporates fluid resuscitation strategies currently used.
Methods
The study was approved by the Animal Research Committee, Federal University of Minas Gerais, Brazil (protocol number 261/2010).
Fifty-four male New Zealand rabbits (2.000-2.900g) were acclimated for two weeks, individually housed, and maintained at 25oC on 12-hour light/day cycles. The animals were fed rabbit chow (Nutricoelho-Purina; Cotia-SP, Brazil), and water ad libitum.
Pre-hemorrhage procedures
Animals were surgically prepared for hemorrhage. Briefly, ketamine 60mg/kg and xylazine 8mg/kg (Fort Dodge Animal Health, Fort Dodge, IA) was administered intramuscularly; additional doses were given intravenously as needed. A tracheostomy was performed, and subsequently the right internal jugular vein and the right carotid artery were cannulated with polyethylene tubing (PE10; Clay Adams, Sparks, MD), previously filled with lactated Ringers (LR) solution. A midline laparotomy (5cm) was performed to expose the left side of the infrarenal aorta. Full thickness 2-0 nylon running sutures were placed through the edges of the laparotomy for later abdominal closure. At this time, baseline blood samples (3ml total) were obtained with 1ml syringes. The first milliliter was discarded and the other samples were immediately transferred to 1ml tubes containing sodium citrate as anticoagulant (MiniCollect(r), Vacuette, Monroe, NC). The samples were obtained from the right carotid artery for blood gases and thromboelastometry (ROTEM(r)Coagulation Analyzer, Pentapharm, Munich, Germany). This was performed on temperature-corrected blood samples using NATEM(r) (Non-Activated TEM) and star-tem(r) for recalcifying citrated blood. The thromboelastometry parameters were calculated using the coagulation dynamics evaluation software (DyCoDerivAn; Avordusol, Rissov, Denmark).
Experimental groups
Fifty-four (n=54) animals were randomly divided into nine groups (n=6 per group) according to the fluid resuscitation regimen used and intravenous bolus of FXIII (35 U/Kg) (Fibrogammin(r) - CSL Bhering GmbH, Marburg, Germany) and or 0.3 µg/kg of DDAVP (Ferring Pharmaceuticals, Limhamn, Sweden). Normotensive resuscitation groups (NBP) groups received intravenous LR to maintain mean arterial pressure (MAP) at baseline (pre-hemorrhage) levels. Permissive hypotension (PH) groups received LR to maintain MAP at 60% of baseline. Resuscitation in each group were the following:
-
- Group 1 (Sham): Animals underwent surgical procedures but no hemorrhage.
-
- Group 2: Hemorrhage and FXIII infusion during NBP resuscitation.
-
- Group 3: Hemorrhage and FXIII infusion during PH resuscitation.
-
- Group 4: Hemorrhage and FXIII infusion in conjunction with DDAVP during NBP resuscitation.
-
- Group 5: Hemorrhage and FXIII infusion in conjunction with DDAVP during PH resuscitation.
-
- Group 6: Hemorrhage and NBP resuscitation only.
-
- Group 7: Animals underwent surgical procedures, received FXIII bolus infusion, but no hemorrhage.
-
- Group 8: Animals underwent surgical procedures, received FXIII in conjunction with DDAVP bolus infusion, but no hemorrhage.
-
- Group 9: Hemorrhage and PH resuscitation only.
Hemorrhage procedures
All animals were placed on a heating pad to maintain rectal temperature above 35oC during the experiment as previously described1717. Rezende-Neto JB, Rizoli S, Andrade M V, Ribeiro D, Lisboa T, Camargos ER, Martins P, Cunha-Melo JR. Permissive hypotension and desmopressin enhance clot formation. J Trauma. 2010 Jan;68(1):42-50. doi: 10.1097/TA.0b013e3181c66393.
https://doi.org/10.1097/TA.0b013e3181c66...
. In brief, MAP was continuously monitored with a Pro-Paq (Protocol Systems, Beaverton, OR) connected to the right carotid artery. Bleeding was induced by a single puncture injury to the left side of the infra-renal aorta using a 16-gauge needle (Jelco, Medex, Carlsbad, CA). The site of injury was 2mm below the lower pole of the left kidney and it did not go through the other side of the aorta. The abdomen was completely closed immediately after the injury by pulling on the previously placed sutures. To simulate Emergency Medical Services time to arrival, no fluids or drugs were infused for the next 15 minutes. Afterwards, fluid resuscitation was maintained for 90 minutes (maximum rate 5ml/min) through the right jugular vein with an infusion pump (Stoelting, Wood Dale, IL). The resuscitation regimen was predetermined for each group and the total amount of fluid recorded. At the end of the resuscitation phase, new set of blood samples were obtained for final tests. Subsequently, the abdomen was opened and the total blood loss calculated as the difference between blood-soaked sponges minus the weight of pre-weighed dry sponges. The clot closest to the aortic injury was removed. A 1x1cm fragment of the clot was placed in 2.5% glutaraldehyde 0.1mol/L phosphate buffer solution for 12h at 4oC, post-fixed in buffered 1% osmium tetroxide for 1h. Samples were washed, cut into small pieces in 70% ethanol, dehydrated, critical point dried, and sputtered-coated in gold for scanning electron microscopy (FEI Quanta 200, Hillsboro, OR). All animals were killed at the end of the experiment by ketamine and xylazine overdose and sectioning of the aorta.
Statistical analysis
Thromboelastometry results were compared using the percentages of the differences between the baseline values of the medians. Whereby, differences between the baseline and the final value of each parameter were calculated. The percentage used for comparison to the initial value was calculated by the formula: (final value - baseline value)/baseline value. Hence, negative results indicated reduction of a parameter (baseline value > final value) and positive results indicated increase of a parameter (baseline value < final value). The Mann-Whitney test was used for comparison between groups. Software R version 2.7.1 and Epi Info version 6.04 (Atlanta, GA) were used for analysis. Other data are reported as mean ± SEM. One-way analysis of variance (ANOVA) was performed with post hoc analysis using Tukey's test for multiple comparisons between experimental means. A p<0.05 was considered statistically significant.
Results
Hemodynamic and metabolic response to hemorrhage
Mean baseline MAP was 75.1 ± 1.2mmHg. Hemorrhage from the aortic injury resulted in a significant decrease of the MAP compared to nonhemorrhaged animals that persisted throughout the first 15 minutes after injury 23.4 ± 1.5mmHg (p<0.05) (Figure 1). Normotensive fluid resuscitation (NBP) successfully restored MAP at approximately 30 minutes after injury compared to nonhemorrhaged animals; respectively 71 ± 1.5mmHg vs. 78 ± 1.8mmHg (p>0.05). However, MAP in the PH resuscitation groups persisted lower than nonhemorrhaged animals and NBP resuscitation groups (48.7 ± 2.5mmHg, p<0.05); this was maintained throughout the experiment (Figure 1).
Mean arterial pressure of the animals during the experiment. Fluid resuscitation and pharmacologic agents where started at 15 minutes. Data represent mean ± SEM (six animals per group). *p
The mean baseline pH was similar among the groups (7.42 ± 0.08). Nonhemorrhaged animals had normal pH at the end of the experiment. Whereas, hemorrhage resulted in reduced pH and decreased HCO- 3 compared to baseline and nonhemorrhaged animals; respectively 7.33 ± 0.12 and 18.25 ± 0.7mEq/L (p<0.05). Ten animals died among the 48 that underwent bleeding. Seven of those died within the first 15 minutes after the aortic injury, and the other three died immediately after the beginning of fluid resuscitation; all those animals were replaced.
Thromboelastometry
Clotting time
The median clotting time (CT) among nonhemorrhaged animals was 43.4% longer in Group 1 (Sham) compared to Group 8 (95%CI: 0.57 - 96; p<0.05). Comparison among other nonhemorrhage groups showed no statistically significant difference. Hemorrhage followed by NBP resuscitation alone (Group 6) resulted in a more prolonged median CT (52.2%), compared to NBP resuscitation with infusion of FXIII (Group 2) (95%CI: 22-204; p<0.05). Significantly more prolonged median CT was also shown in group 6 compared to group 3 (38.9%; 95%CI: -195.7 - -2, p<0.05), and compared to group 5 (53.6%; 95CI%: 16-197.4; p<0.05). There were no other significant differences in the median CT among the groups.
Alpha angle, area under the curve, and maximum clot firmness
There were no statistically significant differences of those parameters among nonhemorrhaged animals. Whereas, hemorrhage followed by PH resuscitation alone (Group 9) resulted in a median alpha angle 27.6% larger compared to NBP resuscitation (Group 6) (95%CI: 2.1 - 92.6, p<0.05). NBP resuscitation with infusion of FXIII in conjunction with DDAVP (Group 4) resulted in a median alpha angle 43.3% larger compared to group 6 (95%CI: -71.1 - -9.4%, p <0.05). Similarly, group 5 had a median alpha angle 39.7% larger than group 6 (95%IC:-67.6 - -4.2%, p<0.05). The median alpha angle of group 3 was 24.7% larger than that of group 6 (95%CI: 0.1 - 50%, p<0.05). The only statistically significant difference with respect to the area under the curve (AUC) was between group 5 and group 2. Where the median AUC was 14.3% larger in group 5 (95%IC: -31.3 - -0.7, p<0.05). There were no statistically significant differences in maximum clot firmness (MCF) among the groups, regardless of the resuscitation strategy used.
Intra-abdominal blood loss, scanning electron microscopy, and fluid infusion
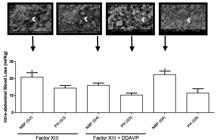
Fluid resuscitation strategy aimed at restoring MAP to baseline levels (Group 6) resulted in significantly higher intra-abdominal blood loss (22.3 ml/Kg ± 2.1; p<0.05) compared to permissive hypotension alone (Group 9; 11.5ml/Kg ± 2.5). Similar results were obtained with permissive hypotension and supplementary infusion of FXIII in conjunction with DDAVP (Group 5) (10.3ml/Kg ± 1.1) (Figure 2). The addition of FXIII to normotensive resuscitation (Group 2) did not reduced intra-abdominal blood loss significantly (20.8ml/Kg ± 2.6; p<0.05). However, no statistically significant differences were noticed between groups 5 and 9 compared to group 4, where normotensive resuscitation was associated with supplementary infusion of FXIII in conjunction with DDAVP (15.9ml/Kg ± 1.3). Although only illustrative, scanning electron microscopy demonstrated much more robust fibrin mesh in permissive hypotension resuscitated animals with supplementary FXIII and DDAVP (Group 5) compared to normotensive resuscitation alone (Group 6). Interestingly, the addition of those pharmacological agents seemed to have improved the fibrin mesh in normotensive resuscitated animals as well; groups 2 and 4 (Figure 2).
Scanning electron microscopy of clots and intra-abdominal blood loss at 105 minutes after aortic injury. Arrowheads indicate fibrin mesh within the clots; magnification 2500x. Data represent mean ± SEM; *p
As expected, animals included in normotensive resuscitation groups required significantly more intravenous fluids compared with those that underwent PH resuscitation (p<0.05). Overall, the mean volume of LR infused in NBP resuscitated groups was 213ml/Kg ± 13.6 and only 81.5ml/Kg ± 6.6 in pH (p<0.05).
Discussion
Damage control resuscitation in trauma encompasses strategies aimed at hemorrhage control, adequate systemic perfusion, and expeditious addressment of coagulopathy44. Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, Flaherty SF, Grathwohl KW, Spinella PC, Perkins JG, Beekley AC, McMullin NR, Park MS, Gonzalez EA, Wade CE, Dubick MA, Schwab CW, Moore FA, Champion HR, Hoyt DB, Hess JR. Damage control resuscitation: directly adressing the early coagulopathy of trauma. J Trauma. 2007 Feb;62(2):307-10. PMID: 17297317.. The latter, ultimately consists of judicious use of crystalloids and high ratio of balanced blood products to PRBC55. Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. Surg Clin N Am. 2012 Aug;92(4):877-91 doi: 10.1016/j.suc.2012.06.001.
https://doi.org/10.1016/j.suc.2012.06.00...
. More recently, early fibrinolysis, platelet dysfunction, clotting factor inactivation and depletion, were described in acute traumatic coagulopathy1818. Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, Pittet, JF. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann. Surg. 2012 Feb;255(2):379-85. doi:10.1097/SLA.0b013e318235d9e6.
https://doi.org/10.1097/SLA.0b013e318235...
19. Cohen MJ, Kutcher M, Redick B, Nelson M, Call M, Knudson MM, Schreiber MA, Bulger EM, Muskat P, Alarcon LH, Myers JG, Rahbar MH, Braser KJ, Phelan HA, del Junco DJ, Fox EE, Wade CE, Holcomb JB, Cotton BA, Matijevic N. Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma. 2013 Jul;75(1 Suppl 1):S40-7. doi: 10.1097/TA.0b013e31828fa43d.
https://doi.org/10.1097/TA.0b013e31828fa...
- 2020. Rizoli SB, Scarpelini S, Callum J, Nascimento B, Mann KG, Pinto R, Jansen J, Tien HC. Clotting factor deficiency in early trauma-associated coagulopathy. J Trauma. 2011 Nov;71(5 Suppl 1):S427-34. doi: 10.1097/TA.0b013e318232e5ab.
https://doi.org/10.1097/TA.0b013e318232e...
. To approach those multiple conditions, pharmacological agents have been incorporated in the management of coagulopathy66. Porta CR, Nelson D, McVay D, Salgar S, Eckert M, Izenberg S, Martin MJ. The effects of tranexamic acid and prothrombin complex concentrate on the coagulopathy of trauma: an in vitro analysis of the impact on severe acidosis. J Trauma. 2013 Dec;75(6):954-60. doi: 10.1097/TA.0b013e31829e20bf.
https://doi.org/10.1097/TA.0b013e31829e2...
, 77. CRASH-2 Trial Collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichatshuili T, Guptas S, Herrera J, Hunt B, Iribhogbe P, Izurieta M, Khamis H, Komolafe E, Marrero MA, Metia-Mantilla J, Miranda J, Morales C, Olaomi O, Olldashi F, Perel P, Peto R, Ramana PV, Ravi RR, Yuttakasemsunt S. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomized, placebo-controlled trial. Lancet. 2010 Jul 3;376(9734):23-32. doi: 10.1016/S0140-6736(10)60835-5 .
https://doi.org/10.1016/S0140-6736(10)60...
.
The main findings of this study were that FXIII and DDAVP used concomitantly with permissive hypotension resuscitation (group 5) significantly improved thromboelastometry parameters compared to normotensive fluid resuscitation strategy used alone (group 6). Moreover, the former strategy also resulted in less intra-abdominal bleeding. Interestingly, CT significantly improved with supplementary FXIII (group 2), and alpha angle improved with infusion of FXIII in conjunction with DDAVP (group 4), despite normotensive resuscitation in both groups. These findings suggest that detrimental effects of high volume infusion on initial coagulation parameters were partially abrogated by FXIII and DDAVP. These results are consistent with other reports, which showed that, even though FXIII is primarily responsible fibrin cross-liking and final clot stabilization, it also participates in earlier stages of coagulation1212. Nielsen VG, Gurley WQ Jr, Burch TM. The impact of factor XIII on coagulation kinetics and clot strength determined by thromboelastography. Anesth Analg. 2004 Jul;99(1):1203. PMID: 15281516. , 1313. Theusinger OM, Baulig W, Asmis LM, Seifert B, Spahn DR. In vitro factor XIII supplementation increases clot firmmness in Rotation Thromboelastometry (Rotem). Thromb Haemost. 2010 Aug;104(2):385-91. doi: 10.1160/TH09-12-0858.
https://doi.org/10.1160/TH09-12-0858...
, 2121. Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002 Jul 1;100(1):148-52. PMID: 12070020. , 2222. Jambor C, Reul V, Schnider TW, Degiacomi P, Metzner H, Korte WC. In vitro inhibition of factor XIII retards clot formation, reduces clot firmness, and increases fibrinolytic effects in whole blood. Anesth Analg. 2009 Oct;109(4):1023-8. doi: 10.1213/ANE.0b013e3181b5a263.
https://doi.org/10.1213/ANE.0b013e3181b5...
.
We hypothesize that the improvement in early thromboelastometry parameters in our model could be attributed to the rapid activation of supplementary FXIII by thrombin; whereby pro-transglutaminase plasma FXIII is converted into activated FXIII2121. Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002 Jul 1;100(1):148-52. PMID: 12070020.. Additionally, DDAVP reduces the lag phase of thrombin generation by increasing FVIII, von Willebrand factor, and platelet organelle calcium (Ca2+) 2323. Ibbotson SH, Davies JA, Grant PJ. The influence of infusions of 1-deamino-8-D-arginine vasopressin (DDAVP) in vivo on thrombin generation in vitro. Thromb Haemost. 1992 Jul 6;68(1):37-9. PMID: 1514171.. Therefore, more thrombin is available to activate both FXIII and platelets, and to initiate fibrin polymerization from fibrinopeptide cleavage2424. Wisel JW, Litvinov RI. Mechanisms of fibrin polymerization and clinical implications. Blood. 2013 Mar 7;121(10):1712-9. doi: 10.1182/blood-2012-09-306639.
https://doi.org/10.1182/blood-2012-09-30...
. In accordance with those findings, our results showed that even in the absence of primed hemostasis, as in non-hemorrhaged animals, CT was significantly improved, possibly by high thrombin generation as a result of FXIII and DDAVP used concomitantly (group 8).
In the present study, concomitant infusion of FXIII and DDAVP (group 4) led to significant improvement in platelet function, as indicated by increase in the alpha angle, despite normotensive fluid resuscitation. In contrast, infusion of FXIII without DDAVP was not enough to improve alpha angle (group 2) compared to normotensive resuscitation used alone (group 6). This finding is consistent with previous research which showed that fibrinogen, and ultimately fibrin, lowers the concentration of thrombin required to activate FXIII1313. Theusinger OM, Baulig W, Asmis LM, Seifert B, Spahn DR. In vitro factor XIII supplementation increases clot firmmness in Rotation Thromboelastometry (Rotem). Thromb Haemost. 2010 Aug;104(2):385-91. doi: 10.1160/TH09-12-0858.
https://doi.org/10.1160/TH09-12-0858...
. In another study, improvement in thromboelastometric parameters of healthy volunteers, was only detected in blood samples that were incubated with FXIII and fibrinogen concomitantly2525. Schlimp CJ, Cadamuro J, Solomon C, Redl H, Schochl H. The effect of fibrinogen concentrate and factor XIII on thromboelastometry in 33% diluted blood with albumin, hydroxyethyl starch or saline in vitro. Blood Transf. 2013 Oct;11(4):510-7. doi: 10.2450/2012.0171-12.
https://doi.org/10.2450/2012.0171-12...
.
It is important to bear in mind that there are limitations to the effectiveness of FXIII on coagulation. Factor XIII activity should be increased to 20 to 30% to prevent surgical bleeding and poor wound healing, and an activity level of less than 50% adequately responds to treatment with FXIII concentrate88. Hsieh L, Nugent D. Factor XIII deficiency. Haemophilia.2008 Nov;14(6):1190-200. doi: 10.1111/j.1365-2516.2008.01857.x.
https://doi.org/10.1111/j.1365-2516.2008...
, 1111. Levy JH, Greenberg C. Biology of Factor XIII and clinical manifestations of Factor XIII deficiency. Transfusion. 2013 May;53(5):1120-31. doi:10.1111/j.15372995.2012.03865.x.
https://doi.org/10.1111/j.15372995.2012....
. However, supplementary infusion of FXIII once activity levels are above 50% does not necessarily improve thromboelastographic parameters1212. Nielsen VG, Gurley WQ Jr, Burch TM. The impact of factor XIII on coagulation kinetics and clot strength determined by thromboelastography. Anesth Analg. 2004 Jul;99(1):1203. PMID: 15281516. , 1313. Theusinger OM, Baulig W, Asmis LM, Seifert B, Spahn DR. In vitro factor XIII supplementation increases clot firmmness in Rotation Thromboelastometry (Rotem). Thromb Haemost. 2010 Aug;104(2):385-91. doi: 10.1160/TH09-12-0858.
https://doi.org/10.1160/TH09-12-0858...
. This could explain the unanticipated lack of improvement in MCF after supplementary FXIII infusion in our study1212. Nielsen VG, Gurley WQ Jr, Burch TM. The impact of factor XIII on coagulation kinetics and clot strength determined by thromboelastography. Anesth Analg. 2004 Jul;99(1):1203. PMID: 15281516. , 1313. Theusinger OM, Baulig W, Asmis LM, Seifert B, Spahn DR. In vitro factor XIII supplementation increases clot firmmness in Rotation Thromboelastometry (Rotem). Thromb Haemost. 2010 Aug;104(2):385-91. doi: 10.1160/TH09-12-0858.
https://doi.org/10.1160/TH09-12-0858...
, 2222. Jambor C, Reul V, Schnider TW, Degiacomi P, Metzner H, Korte WC. In vitro inhibition of factor XIII retards clot formation, reduces clot firmness, and increases fibrinolytic effects in whole blood. Anesth Analg. 2009 Oct;109(4):1023-8. doi: 10.1213/ANE.0b013e3181b5a263.
https://doi.org/10.1213/ANE.0b013e3181b5...
, 2626. Nielsen VG, Kirklin JK, Hoogenddorn H, Ellis TC, Holman WL. Thromboelastographic method to quantify the contribution of factor XIII to coagulation kinetics. Blood Coagul Fibrinolysis. 2007 Mar;18(2):145-50 PMID: 17287631.. That was also noticed despite supplementary DDAVP infusion, and regardless of the fluid resuscitation strategy.
Even though, DDAVP activation of plasminogen activator (PA) could have played a role in abrogating an increase in MCF we do not believe that this was the case in the study herein2727. Mannucci PM, Rota L. Plasminogen activator response after DDAVP: a clinico-pharmacological study. Thromb Res. 1980 Oct 1;20(1):69-76. PMID: 7193918.. In support to that stance, previous research showed that crosslinking of fibrin by FXIII facilitates binding of α-plasmin inhibitor, decreases binding of plasminogen, and protects against fibrinolysis; hence, suppressing any fibrinolytic effect of DDAVP that could have occurred1010. Bagoly Z, Koncz Z, Harsfalvi J, Muszbek L. Factor XIII, clot structure, thrombosis. Thromb Res. 2012 Mar;129(3):382-7. doi: 10.1016/j.thromres.2011.11.040.
https://doi.org/10.1016/j.thromres.2011....
, 2323. Ibbotson SH, Davies JA, Grant PJ. The influence of infusions of 1-deamino-8-D-arginine vasopressin (DDAVP) in vivo on thrombin generation in vitro. Thromb Haemost. 1992 Jul 6;68(1):37-9. PMID: 1514171.. Furthermore, research by our group has previously demonstrated improvement in MCF with DDAVP infusion in a similar model1717. Rezende-Neto JB, Rizoli S, Andrade M V, Ribeiro D, Lisboa T, Camargos ER, Martins P, Cunha-Melo JR. Permissive hypotension and desmopressin enhance clot formation. J Trauma. 2010 Jan;68(1):42-50. doi: 10.1097/TA.0b013e3181c66393.
https://doi.org/10.1097/TA.0b013e3181c66...
. Moreover, electron microscopy of the clots, in the present study, qualitatively demonstrated more robust fibrin mesh with supplementary infusion of FXIII, and or DDAVP, compared to normotensive fluid resuscitation used alone. Essentially, we believe that thromboelastometric assessment of MCF by fibrinogen specific assay (FIBTEM(r)), as demonstrated by others, could have provided different results1313. Theusinger OM, Baulig W, Asmis LM, Seifert B, Spahn DR. In vitro factor XIII supplementation increases clot firmmness in Rotation Thromboelastometry (Rotem). Thromb Haemost. 2010 Aug;104(2):385-91. doi: 10.1160/TH09-12-0858.
https://doi.org/10.1160/TH09-12-0858...
, 2525. Schlimp CJ, Cadamuro J, Solomon C, Redl H, Schochl H. The effect of fibrinogen concentrate and factor XIII on thromboelastometry in 33% diluted blood with albumin, hydroxyethyl starch or saline in vitro. Blood Transf. 2013 Oct;11(4):510-7. doi: 10.2450/2012.0171-12.
https://doi.org/10.2450/2012.0171-12...
.
In this study, fluid replacement was a major determinant of blood loss. Permissive hypotension clearly led to decreased bleeding compared to normotensive fluid resuscitation. Mechanistically, the reduction in blood loss with permissive hypotension can be attributed to a combination of events. According to Laplace's law, permissive hypotension decreases vascular wall tension and delays peak pulse pressure. Consequently, more time is allowed before undesirable pressure is applied to an initially vulnerable clot2828. Li T, Zhu Y, Hu Y, Li L, Diago Y, Tang I, Liu L. Ideal permissive hypotension to resuscitate uncontrolled hemorrhagic shock and the tolerance time in rats. Anesthesiology. 2011 Jan;114(1):111-9. doi: 10.1097/ALN.0b013e3181fe3fe7.
https://doi.org/10.1097/ALN.0b013e3181fe...
. Moreover, permissive hypotension contributes to vasoconstriction and reduces dilutional effect on coagulopathy44. Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, Flaherty SF, Grathwohl KW, Spinella PC, Perkins JG, Beekley AC, McMullin NR, Park MS, Gonzalez EA, Wade CE, Dubick MA, Schwab CW, Moore FA, Champion HR, Hoyt DB, Hess JR. Damage control resuscitation: directly adressing the early coagulopathy of trauma. J Trauma. 2007 Feb;62(2):307-10. PMID: 17297317. , 1919. Cohen MJ, Kutcher M, Redick B, Nelson M, Call M, Knudson MM, Schreiber MA, Bulger EM, Muskat P, Alarcon LH, Myers JG, Rahbar MH, Braser KJ, Phelan HA, del Junco DJ, Fox EE, Wade CE, Holcomb JB, Cotton BA, Matijevic N. Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma. 2013 Jul;75(1 Suppl 1):S40-7. doi: 10.1097/TA.0b013e31828fa43d.
https://doi.org/10.1097/TA.0b013e31828fa...
. Interestingly, our results failed to demonstrate additional decrease in intra-abdominal blood loss with supplementary infusion of FXIII and or DDAVP to pH compared to pH used alone. This finding is consistent with other research which showed that the major determinant of early hemorrhage and rebleeding from clot dislodgment, in uncontrolled hemorrhagic shock models, is the mechanical effect of increased blood pressure, not coagulopathy2929. Sondeen JL, Coppes VG, Holcomb JB. Blood Pressure at which rebleeding occurs after resuscitation in swine with aortic injury. J Trauma. 2003 May;54(5 Suppl):S110-7 PMID: 12768112..
This study has several limitations. Even though, our model simulates some conditions encountered in traumatic hemorrhage, rabbits have different hemodynamic and hemostatic responses compared to humans3030. Frith D, Cohen MJ, Brohi K. Animal models of trauma-induced coagulopathy. Thromb Res. 2012 May;129(5):551-6. doi: 10.1016/j.thromres.2011.11.053
https://doi.org/10.1016/j.thromres.2011....
.
Severe hypothermia and blood product transfusion were not included in this model, decreasing clinical relevance. Furthermore, only the acute phase of resuscitation from hemorrhage was assessed. Understanding the late effects of the resuscitation strategies used herein require further research. Lastly, lack of assessment of clotting factors, fibrinolysis, and platelet activity precluded a more comprehensive mechanistic analysis of our results.
Conclusions
Normotensive resuscitation provokes more bleeding and worsens coagulation compared to pH, that is partially reversed by factor XIII and desmopressin. FXIII and DDAVP can synergistically improve coagulation. Permissive hypotension reduces bleeding regardless of those agents.
Acknowledgements
Joao Baptista de Rezende Neto acknowledges the Minas Gerais Research Foundation (FAPEMIG) (Project APQ-000393-10) the research grant.
Jose Renan da Cunha Melo acknowledges the Brazilian National Council for Research and Scientific Development (CNPq) for a research scholarship.
References
-
1Geeraedts LM Jr, Kaasjager HA, van Vugt AB, Frölke JP. Exsanguination in trauma: A review of diagnostics and treatment options. Injury. 2009 Jan;40(1):11-20. doi: 10.1016/j.injury.2008.10.007.
» https://doi.org/10.1016/j.injury.2008.10.007 -
2Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003 Jun;54(6):1127-30. PMID: 12813333.
-
3MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003 Jul;55(1):39-44. PMID: 12855879.
-
4Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, Flaherty SF, Grathwohl KW, Spinella PC, Perkins JG, Beekley AC, McMullin NR, Park MS, Gonzalez EA, Wade CE, Dubick MA, Schwab CW, Moore FA, Champion HR, Hoyt DB, Hess JR. Damage control resuscitation: directly adressing the early coagulopathy of trauma. J Trauma. 2007 Feb;62(2):307-10. PMID: 17297317.
-
5Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. Surg Clin N Am. 2012 Aug;92(4):877-91 doi: 10.1016/j.suc.2012.06.001.
» https://doi.org/10.1016/j.suc.2012.06.001 -
6Porta CR, Nelson D, McVay D, Salgar S, Eckert M, Izenberg S, Martin MJ. The effects of tranexamic acid and prothrombin complex concentrate on the coagulopathy of trauma: an in vitro analysis of the impact on severe acidosis. J Trauma. 2013 Dec;75(6):954-60. doi: 10.1097/TA.0b013e31829e20bf.
» https://doi.org/10.1097/TA.0b013e31829e20bf -
7CRASH-2 Trial Collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichatshuili T, Guptas S, Herrera J, Hunt B, Iribhogbe P, Izurieta M, Khamis H, Komolafe E, Marrero MA, Metia-Mantilla J, Miranda J, Morales C, Olaomi O, Olldashi F, Perel P, Peto R, Ramana PV, Ravi RR, Yuttakasemsunt S. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomized, placebo-controlled trial. Lancet. 2010 Jul 3;376(9734):23-32. doi: 10.1016/S0140-6736(10)60835-5 .
» https://doi.org/10.1016/S0140-6736(10)60835-5 -
8Hsieh L, Nugent D. Factor XIII deficiency. Haemophilia.2008 Nov;14(6):1190-200. doi: 10.1111/j.1365-2516.2008.01857.x.
» https://doi.org/10.1111/j.1365-2516.2008.01857.x -
9Lorand K, Konishi K. Activation of fibrin-stabilizing factor of plasma by thrombin. Arch Biochem Biophys. 1964 Apr;105:58-67. PMID: 14165504.
-
10Bagoly Z, Koncz Z, Harsfalvi J, Muszbek L. Factor XIII, clot structure, thrombosis. Thromb Res. 2012 Mar;129(3):382-7. doi: 10.1016/j.thromres.2011.11.040.
» https://doi.org/10.1016/j.thromres.2011.11.040 -
11Levy JH, Greenberg C. Biology of Factor XIII and clinical manifestations of Factor XIII deficiency. Transfusion. 2013 May;53(5):1120-31. doi:10.1111/j.15372995.2012.03865.x.
» https://doi.org/10.1111/j.15372995.2012.03865.x -
12Nielsen VG, Gurley WQ Jr, Burch TM. The impact of factor XIII on coagulation kinetics and clot strength determined by thromboelastography. Anesth Analg. 2004 Jul;99(1):1203. PMID: 15281516.
-
13Theusinger OM, Baulig W, Asmis LM, Seifert B, Spahn DR. In vitro factor XIII supplementation increases clot firmmness in Rotation Thromboelastometry (Rotem). Thromb Haemost. 2010 Aug;104(2):385-91. doi: 10.1160/TH09-12-0858.
» https://doi.org/10.1160/TH09-12-0858 -
14Lusher J, Pile SW, Alexander S, Nugent D. Prophylactic therapy with Fibrogammin P is associated with a decreased incidence of bleeding episodes: a retrospective study. Haemophilia. 2010 Mar;16(2):316-21. doi: 10.1111/j.1365-2516.2009.02123.x.
» https://doi.org/10.1111/j.1365-2516.2009.02123.x -
15Mannucci PM. Desmopressin (DDAVP) in the treatment of bleeding disorders: the first 20 years. Blood. 1997 Oct;90(7):2515-21. PMID: 9326215.
-
16Sakariassen KS, Cattaneo M, vd Berg A, Ruggeri ZM, Mannucci PM, Sixma JJ. DDAVP enhances platelet adherence and platelet aggregate growth on human artery subendothelium. Blood. 1984 Jul;64(1):229-36. PMID: 6428488.
-
17Rezende-Neto JB, Rizoli S, Andrade M V, Ribeiro D, Lisboa T, Camargos ER, Martins P, Cunha-Melo JR. Permissive hypotension and desmopressin enhance clot formation. J Trauma. 2010 Jan;68(1):42-50. doi: 10.1097/TA.0b013e3181c66393.
» https://doi.org/10.1097/TA.0b013e3181c66393 -
18Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, Pittet, JF. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann. Surg. 2012 Feb;255(2):379-85. doi:10.1097/SLA.0b013e318235d9e6.
» https://doi.org/10.1097/SLA.0b013e318235d9e6 -
19Cohen MJ, Kutcher M, Redick B, Nelson M, Call M, Knudson MM, Schreiber MA, Bulger EM, Muskat P, Alarcon LH, Myers JG, Rahbar MH, Braser KJ, Phelan HA, del Junco DJ, Fox EE, Wade CE, Holcomb JB, Cotton BA, Matijevic N. Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma. 2013 Jul;75(1 Suppl 1):S40-7. doi: 10.1097/TA.0b013e31828fa43d.
» https://doi.org/10.1097/TA.0b013e31828fa43d -
20Rizoli SB, Scarpelini S, Callum J, Nascimento B, Mann KG, Pinto R, Jansen J, Tien HC. Clotting factor deficiency in early trauma-associated coagulopathy. J Trauma. 2011 Nov;71(5 Suppl 1):S427-34. doi: 10.1097/TA.0b013e318232e5ab.
» https://doi.org/10.1097/TA.0b013e318232e5ab -
21Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002 Jul 1;100(1):148-52. PMID: 12070020.
-
22Jambor C, Reul V, Schnider TW, Degiacomi P, Metzner H, Korte WC. In vitro inhibition of factor XIII retards clot formation, reduces clot firmness, and increases fibrinolytic effects in whole blood. Anesth Analg. 2009 Oct;109(4):1023-8. doi: 10.1213/ANE.0b013e3181b5a263.
» https://doi.org/10.1213/ANE.0b013e3181b5a263 -
23Ibbotson SH, Davies JA, Grant PJ. The influence of infusions of 1-deamino-8-D-arginine vasopressin (DDAVP) in vivo on thrombin generation in vitro. Thromb Haemost. 1992 Jul 6;68(1):37-9. PMID: 1514171.
-
24Wisel JW, Litvinov RI. Mechanisms of fibrin polymerization and clinical implications. Blood. 2013 Mar 7;121(10):1712-9. doi: 10.1182/blood-2012-09-306639.
» https://doi.org/10.1182/blood-2012-09-306639 -
25Schlimp CJ, Cadamuro J, Solomon C, Redl H, Schochl H. The effect of fibrinogen concentrate and factor XIII on thromboelastometry in 33% diluted blood with albumin, hydroxyethyl starch or saline in vitro. Blood Transf. 2013 Oct;11(4):510-7. doi: 10.2450/2012.0171-12.
» https://doi.org/10.2450/2012.0171-12 -
26Nielsen VG, Kirklin JK, Hoogenddorn H, Ellis TC, Holman WL. Thromboelastographic method to quantify the contribution of factor XIII to coagulation kinetics. Blood Coagul Fibrinolysis. 2007 Mar;18(2):145-50 PMID: 17287631.
-
27Mannucci PM, Rota L. Plasminogen activator response after DDAVP: a clinico-pharmacological study. Thromb Res. 1980 Oct 1;20(1):69-76. PMID: 7193918.
-
28Li T, Zhu Y, Hu Y, Li L, Diago Y, Tang I, Liu L. Ideal permissive hypotension to resuscitate uncontrolled hemorrhagic shock and the tolerance time in rats. Anesthesiology. 2011 Jan;114(1):111-9. doi: 10.1097/ALN.0b013e3181fe3fe7.
» https://doi.org/10.1097/ALN.0b013e3181fe3fe7 -
29Sondeen JL, Coppes VG, Holcomb JB. Blood Pressure at which rebleeding occurs after resuscitation in swine with aortic injury. J Trauma. 2003 May;54(5 Suppl):S110-7 PMID: 12768112.
-
30Frith D, Cohen MJ, Brohi K. Animal models of trauma-induced coagulopathy. Thromb Res. 2012 May;129(5):551-6. doi: 10.1016/j.thromres.2011.11.053
» https://doi.org/10.1016/j.thromres.2011.11.053
-
Financial source: Minas Gerais Research Foundation (FAPEMIG) (Project APQ-000393-10)
-
1
Research performed at Laboratory Prof. Lineu de Freire Maia, Department of Surgery. Scanning Electron Microscopy, Center for Image Acquisition and Processing (CAPI) ICB, Federal University of Minas Gerais (UFMG), Belo Horizonte-MG, Brazil. Part of PhD degree thesis, Postgraduate Program in Sciences Applied to Surgery and Ophthalmology, UFMG. Tutor: Prof. João Baptista de Rezende-Neto.
Publication Dates
-
Publication in this collection
Mar 2015
History
-
Received
18 Nov 2014 -
Reviewed
20 Jan 2015 -
Accepted
16 Feb 2015