Abstract
PURPOSE:
To evaluate the effect of Black cumin (Nigella sativa Linn.) pre-treatment on renal ischemia/reperfusion (I/R) induced injury in the rats.
METHODS:
A total of 40 male Wistar rats were randomly allocated into five equal groups including Sham, I/R model and three I/R+ Black cumin (0.5, 1 and 2%)-treated groups. I/R groups' kidneys were subjected to 60 min of global ischemia at 37°C followed by 24 h of reperfusion. At the end of reperfusion period, the rats were euthanized. Superoxide dismutase, catalase and glutathione peroxidase activities as well as reduced glutathione and renal malondialdehyde contents were determined in renal tissues. Kidney function tests and histopathological examination were also performed.
RESULTS:
High serum creatinine, blood urea nitrogen and uric acid as well as malondialhehyde (MDA) levels, and low antioxidant enzyme activities were observed in I/R rats compared to the sham rats. Pre-treatment with Black cumin for three weeks prior to IR operation improved renal function and reduced I/R induced renal inflammation and oxidative injury. These biochemical observations were supported by histopathological test of kidney sections.
CONCLUSION:
Black cumin significantly prevented renal ischemia/reperfusion induced functional and histological injuries.
Nigella sativa; Ischemia; Reperfusion; Kidney; Rats
Introduction
Renal ischemia and reperfusion (I/R) injury is the major cause of acute renal failure (ARF) and may also be involved in the development and progression of some forms of chronic kidney disease11. Radhakrishnan J, Kiryluk K. Acute renal failure outcomes in children and adults. Kidney Int. 2006 Jan;69(1):17-9. doi: 10.1038/sj.ki.5000094.
https://doi.org/10.1038/sj.ki.5000094...
, which is encountered in many clinical conditions such as kidney transplantation, partial nephrectomy, hemorrhagic shock, certain hypotensive states, and elective urological operations major vascular surgery such as renal artery angioplasty and aortic aneurysm surgery22. Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007 Oct;376:1-43. doi: 10.1007/s00210-007-0183-5.
https://doi.org/10.1007/s00210-007-0183-...
3. Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007 Oct;123(1):7-13. doi: 10.1016/j.clim.2006.09.008.
https://doi.org/10.1016/j.clim.2006.09.0...
- 44. Legrand M, Mik EG, Johannes T, Payen D, Ince C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med. 2008 Jul-Aug;14(7-8):502-16. doi: 10.2119/2008-00006.
https://doi.org/10.2119/2008-00006...
.
The post-ischemic kidney usually undergoes a series of complicated pathophysiological changes, including inflammation, regeneration, apoptosis and interstitial fibrosis. Renal ischemia induces oxidative stress, which results in exacerbated and prolonged systemic inflammatory response and the presumptive death of renal cells after reperfusion22. Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007 Oct;376:1-43. doi: 10.1007/s00210-007-0183-5.
https://doi.org/10.1007/s00210-007-0183-...
, 33. Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007 Oct;123(1):7-13. doi: 10.1016/j.clim.2006.09.008.
https://doi.org/10.1016/j.clim.2006.09.0...
. Reperfusion injury is one of the main causes of acute renal injury, which can manifest histologically as acute tubular necrosis55. Nafar M, Parvin M, Sadeghi P, Ghoraishian M, Soleimani M, Tabibi A, Nouralizadeh A, Amirkhanlou S, Barzi F, Alipour B. Effects of stem cells and granulocyte colony stimulating factor in reperfusion injury. Iran J Kidney Dis. 2010 Jul;4(3):207-13. PMID: 20622308.. Reactive oxygen species (ROS) which are generated in high concentration in ischemic organs after reperfusion have various cytotoxic effects, including DNA damage, protein oxidation and nitrosylation, lipid peroxidation, and induction of apoptosis66. Rodrigo R, Bosco C. Oxidative stress and protective effects of polyphenols: Comparative studies in human and rodent kidney. A review. Comp Biochem Physiol C Toxicol Pharmacol. 2006 Mar-Apr;142(3-4):317-27. doi:10.1016/j.cbpc.2005.11.002.
https://doi.org/10.1016/j.cbpc.2005.11.0...
. Increased reactive oxygen species during renal reperfusion, directly endanger glomerular and tubular epithelium integrity, one of the factors in the development of acute tubular necrosis77. Senturk H, Kabay S, Bayramoglu G, Ozden H, Yaylak F, Yucel M, Olgun EG, Kutlu A. Silymarin attenuates the renal ischemia/reperfusion injury-induced morphological changes in the rat kidney. World J Urol. 2008 Aug;26(4):401-7. doi: 10.1007/s00345-008-0256-1.
https://doi.org/10.1007/s00345-008-0256-...
.
Free radical depletion for the treatment of reperfusion injury has found its clinical benefits in the prevention of post-ischemic tissue injury following organ transplantation88. Amersi F, Nelson SK, Shen XD, Kato H, Melinek J, Kupiec-Weglinski JW, Horwitz LD, Busuttil RW, Horwitz MA. Bucillamine, a thiol antioxidant, prevents transplantation-associated reperfusion injury. Proc Natl Acad Sci USA. 2002 Jun;99(13):8915-20. doi: 10.1073/pnas.132026099.
https://doi.org/10.1073/pnas.132026099...
, 99. Seo MY, Lee SM. Protective effect of low dose of ascorbic acid on hepatobiliary function in hepatic ischemia/reperfusion in rats. J Hepatol. 2002 Jan;36(1):72-7. doi: 10.1016/S0168-8278(01)00236-7.
https://doi.org/10.1016/S0168-8278(01)00...
. ROS collectively are instrumental in impairing overall renal function and in inducing apoptosis in renal cells1010. Burns AT, Davies DR, McLaren AJ, Cerundolo L, Morris PJ, Fuggle SV. Apoptosis in ischemia/reperfusion injury of human renal allografts. Transplantation. 1998 Oct;66(7):872-6. PMID: 9798696.. Antioxidant therapy has been well documented to help in the improvement of organ functions and to prevent apoptosis1111. Witenberg B, Kalir HH, Raviv Z, Kletter Y, Kravtsov V, Fabian I. Inhibition by ascorbic acid of apoptosis induced by oxidative stress in HL-60 myeloid leukemia cells. Biochem Pharmacol. 1999 Apr;57(7):823-32. doi: 10.1016/S0006-2952(98)00351-7.
https://doi.org/10.1016/S0006-2952(98)00...
.
Herbal medicines derived from plant extracts are being increasingly utilized to treat a wide variety of clinical disease. More attention has been paid to the protective effects of natural antioxidants. Natural antioxidants strengthen the endogenous antioxidant defenses from reactive oxygen species (ROS) and restore and optimal balance by neutralizing the reactive species. They are gaining immense importance by virtue of their critical role in disease prevention. In recent years achievement of new types of plant-based antioxidants to overcome the damages caused by various factors have been seriously considered by researchers1212. Hajhashemi V, Ghannadi A, Jafarabadi H. Black cumin seed essential oil, as a potent analgesic and anti-inflammatory drug. Phytother Res. 2004 Mar;18(3):195-9. doi: 10.1002/ptr.1390.
https://doi.org/10.1002/ptr.1390...
.
There are a wide range of plants with medicinal properties and according to the World Health Organization, 70 to 80 percent of the world's population believes in traditional medicine to maintain health.
Nigella sativa Linn. from the Ranunculaceae family, is commonly known as Black cumin in traditional medicine. It has been shown that the biological activity of the black cumin is related to the composition of its essential oil1414. Padhye S, Banerjee S, Ahmad A, Mohammad R, Sarkar FH. From here to eternity-the secret of Pharaohs: Therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008 Sep;6(b):495-510. PMID: 19018291., which is containing of 30 to 48% Thymoquinone, 7 to 15% P-cymene, 6 to 12% Carvacrol, 2 to 7% 4-terpineol, 1 to 4% Tanethole and 1 to 8% Sesquiterpene1313. Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000 Aug;14(5):323-8. PMID: 10925395.. Pharmacologically, Thymoquinone and its derivatives are the most important components of Black cumin constituents1414. Padhye S, Banerjee S, Ahmad A, Mohammad R, Sarkar FH. From here to eternity-the secret of Pharaohs: Therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008 Sep;6(b):495-510. PMID: 19018291..
In India, Arabic countries, Europe and Iran Black cumin seeds and oil are traditionally used in treating diseases such as asthma, hypertension, diabetes, inflammation, tumor, cough, bronchitis, headache, eczema, fever, dizziness, gastrointestinal disturbances, impotence, painful menstruation and flu. Black cumin is also used as carminative, diuretic and anti-parasitic agent1515. Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003 Apr;17(4):299-305. doi: 10.1002/ptr.1309.
https://doi.org/10.1002/ptr.1309...
. The beneficial effects of black cumin and its constituents have been shown by clinical and especially experimental studies, from which hypotension1616. Khattab MM, Nagi MN. Thymoquinone supplementation attenuates hypertension and renal damage in nitric oxide deficient hypertensive rats. Phytother Res. 2007 May;21(5):410-4. doi: 10.1002/ptr.2083.
https://doi.org/10.1002/ptr.2083...
, 1717. Dehkordi FR, Kamkhah AF. Antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam Clin Pharmacol. 2008 Aug;22(4):447-52. doi: 10.1111/j.1472-8206.2008.00607.
https://doi.org/10.1111/j.1472-8206.2008...
, hypoglycemic1818. Kaleem M, Kirmani D, Asif M, Ahmed Q, Bano B. Biochemical effects of Nigella sativa L seeds in diabetic rats. Indian J Exp Biol. 2006 Sep;44(9):745-8. PMID: 16999030. , 1919. Meddah B, Ducroc R, El Abbes Faouzi M, Eto B, Mahraoui L, Benhaddou-Andaloussi A, Martineau LC, Cherrah Y, Haddad PS. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol. 2009 Jan;121(3):419-24. doi: 10.1016/j.jep.2008.10.040.
https://doi.org/10.1016/j.jep.2008.10.04...
, hypolipidemic2020. Bamosa AO, Ali BA, al-Hawsawi ZA. The effect of thymoquinone on blood lipids in rats. Indian J Physiol Pharmacol. 2002 Apr;46(2):195-201. PMID: 12500494., antioxidant2121. Uz E, Bayrak O, Uz E, Kaya A, Bayrak R, Uz B, Turgut FH, Bavbek N, Kanbay M, Akcay A. Nigella sativa oil for prevention of chronic cyclosporine nephrotoxicity: an experimental model. Am J Nephrol. 2008 Apr;28(3):517-22. doi: 10.1159/000114004.
https://doi.org/10.1159/000114004...
, 2222. Kanter M, Coskum O, Uysal H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch Toxicol. 2006 Apr;80(4):217-24. doi: 10.1007/s00204-005-0037-1.
https://doi.org/10.1007/s00204-005-0037-...
, anti-inflammatory2323. Al-Ghamdi MS. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol. 2001 Jun;76(1):45-8. doi: 10.1016/S0378-8741(01)00216-1.
https://doi.org/10.1016/S0378-8741(01)00...
and anti-tumor2424. Ait Mbarek L, Ait Mouse H, Elabbadi N, Bensalah M, Gamouh A, Aboufatima R, Benharref A, Chait A, Kamal M, Dalal A, Zyad A. Anti tumor properties of blackseed (Nigella sativa L.) extracts. Braz J Med Biol Res. 2007 Jun;40(6):839-47. doi: 10.1590/S0100-879X2006005000108.
https://doi.org/10.1590/S0100-879X200600...
, 2525. Al-Johar D, Shinwari N, Arif J, Al-Sanea N, Jabbar AA, El-Sayed R, Mashhour A, Billedo G, El-Doush I, Al-Saleh I. Role of Nigella sativa and a number of its antioxidant constituents towards azoxymethane-induced genotoxic effects and colon cancer in rats. Phytother Res. 2008 Oct;22(10):1311-23. doi: 10.1002/ptr.2487.
https://doi.org/10.1002/ptr.2487...
effects can be noted.
In many diseases, such as cirrhosis, hepatitis and chemical poisoning in which free radicals are produced; the antioxidant properties of Black cumin could be very useful. It has been shown that antioxidant effects of Black cumin oil are 4-5 times greater than its essence2626. Machmudah S, Shiramizu Y, Goto M, Sasaki M, Hirose T. Extraction of Nigella sativa L. using supercritical Co2: A study of antioxidant activity of the extract. Sep Sci Technol. 2005 Jan;40(6):1267-75. doi: 10.1081/SS-200053005.
https://doi.org/10.1081/SS-200053005...
. Also, due to the presence of fatty acids and compounds such as tocopherols, carotenoids, metal ions and phosphorus components in smaller amounts, fresh and intact plant most of its oil is resistant against to oxidation2727. Famadan MF. Oxidative stability of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.) and niger (Guizotia abyssinica cass.) Crude seed oils upon stripping. Eur J Lipid Sci Technol. 2004 Jan;106(1):35-43. doi: 10.1002/ejlt.200300895.
https://doi.org/10.1002/ejlt.200300895...
.
Considering the numerous beneficial effects and particularly antioxidant activity of Black cumin, it is believed that the seeds of this plant are capable to protect the kidneys against oxidative stress during I/R injury. Therefore, the present study evaluated the beneficial effects of Black cumin on kidney histopathology, function markers and antioxidant activities in renal ischemia/reperfusion (I/R) induced injury in the rats.
Methods
Investigations were conducted in accordance with the internationally accepted principles for laboratory animal use and care as found in the United States guidelines (United States National Institutes for Health publication no. 85-23, revised in 1985) and our Ethical committee on animal care approved the protocol.
Plant materials
Black cumin seeds were purchased from a local herb store in Tabriz, Iran. The seed was identified and authenticated by Professor Dr. Abbas Delazar, head of Pharmacognosy department. After cleaning the seeds under running tap water for 10 min, they were rinsed twice with distilled water and air dried in an oven at 40°C overnight until a constant weight was attained. The seeds were grounded to a powder shape using an electric grinder (Zhejiang, China) for 6 minutes and were mixed with rat chow pellet powder into different doses as 0.5% (low dose), 1% (medium dose) and 2% (High dose).
Animals
A total of forty male Wistar rats (about 180-200 g body weight) obtained from Pasteur Institute of Iran were selected for the study and were acclimatized to their environment for one week prior to experimentation. Animals randomly allocated into five equal groups including Sham operation group, IR group and three I/R+ Black cumin (0.5, 1 and 2%)-treatment groups. The animals were housed under standard environmental conditions (23 ± 1 °C, with 55 ± 5% humidity and a 12 h light/12 h dark cycle) and maintained with free access to water and a standard laboratory diet ad libitum. Three weeks before the experiment, the diet of I/R+Black cumin-treatment animals were supplemented with Black cumin and these three groups of rats received different percentages (0.5, 1 and 2%) of Black cumin in their standard diet. These doses were selected on the basis of Tauseef Sultan et al.2828. Tauseef Sultan M, Masood Sadiq B, Rabia Shabeer A, Rizwana B, Ambreen N, Hafiz Ansar RS. Supplementation of powdered black cumin (Nigella sativa) seeds reduces the risk of hypercholesterolemia. Funct Foods Health Dis. 2011 Dec;12(1):516-24., study in 2011, who investigated the effects of powdered Black cumin seeds on hypercholesterolemia in the rats.
The experiments and surgical procedures performed in the different groups of animals are described below. A previous study showed that supplementation of Black cumin up to the dose of 1 g/kg supplemented for a period of 28 days resulted no changes in liver enzymes level and did not cause any toxicity effect on the liver function2929. Dollah MA, Parhizkar S, Latiff LA, Hafanizam Bin Hassan M. Toxicity effect of Nigella Sativa on the liver function of rats. Adv Pharm Bull. 2013 Feb;3(1):97-102. doi: 10.5681/apb.2013.016.
https://doi.org/10.5681/apb.2013.016...
.
Chemicals
All chemicals used in this study were of analytical grade and obtained from Nanjing Jiancheng Bioengineering Institute, Nanjing, China.
Surgery and experimental design
Animals were anesthetized by with intraperitoneal injection of ketamine-xylazine (50 mg/kg and 10 mg/kg, respectively). The abdominal area was prepared with povidone iodine. Autoclave-sterilized surgical instruments were used for the procedure. A laparotomy was performed with a vertical midline incision. The renal pedicles, containing the artery, vein and nerve supply of each kidney were isolated. A hemostatic micro clamps were applied on the renal pedicle for 60 minutes to create complete renal ischemia. The clamp was removed later to allow restoration of blood flow to the kidney.
Additionally, sham-operated rats underwent a simple laparotomy under identical conditions and served as the operation controls. After removing the clamp, the abdomen was closed in two layers. In all groups, the animals were kept in metabolic cages for 24 hours to collect urine and also to measure water consumption. At the end of the 24 hours, the rats were killed by decapitation. Blood samples was obtained from abdominal aorta into dried tubes and immediately centrifuged at 4°C, 1.000g for 15 min to collect sera. Serum and urine samples were stored at -20°C until analysis.
After sacrificing the animals, the kidney was quickly harvested and weighted for measurement of the ratio of kidney weight to body weight. Renal tissue was perfused immediately with ice cold hypertonic saline solution, and homogenate 10% prepared in 1.15% w/v of potassium chloride for measurement of antioxidant activity, using a glass Teflon homogenizer3030. Bhalodia Y, Kanzariya N, Patel R, Patel N, Vaghasiya J, Jivani N, Raval H. renoprotective activity of benincasa cerifera fruit extract on ischemia/reperfusion-induced renal damage in rat. Iran J Kidney Dis. 2009 Apr;3(2):80-5. PMID: 19395782.. A portion of the kidney was fixed in 10% neutral-buffered formalin solution for histopathologic evaluations.
Kidney function study
Serum creatinine (Scr), blood urea nitrogen (BUN), and uric acid levels were measured by commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) using semiautomatic analyzer.
Estimation of antioxidant activity
The kidney homogenate was centrifuged in 7000 ×g for 10 minutes at 4˚C and supernatant were used for measurement of oxidative stress by estimation of reduced glutathione (GSH) and determination of malondialdehyde (MDA) as well as antioxidant enzymes (AOE) such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px). GSH, MDA, SOD, CAT and GSH-Px were measured by using commercially available kits according to the manufacturer's protocol (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Protein contents were measured using the dye-binding assay of Bradford method3131. Bradford MM. A rapid and sensitive method of the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976 May;72:248-54. PMID: 942051..
GSH reacts with 5,5′-dithiobis-2-nitrobenzoic acid, and the absorbance spectra of the product have a maximum absorbance at 410 nm. The results were expressed as μmol/gwt protein. Kidney homogenate MDA levels were expressed as nmol MDA per mg protein and antioxidant activity was expressed as arbitrary units per mg protein. Degree of lipid peroxidation in kidney tissue homogenates was determined in terms of thiobarbituric acid reactive substances (TBARSs) formation by following the protocol of Esterbauer and Cheesman3232. Esterbauer H, Cheesman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407-21. doi: 10.1016/0076-6879(90)86134-H.
https://doi.org/10.1016/0076-6879(90)861...
. SOD activity was measured by Nishikimi method3333. Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun. 1972 Jan;46(2):849-54. PMID: 4400444. and was modified by Kakkar method3434. Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984 Apr;21(2):130-2. PMID: 6490072.. Each unit of SOD activity was determined as required enzyme concentration for prohibition of creation color at 1 minute, under study conditions. CAT activity was measured by and was based on hydrogen peroxide breakdown. GSH-Px activity was measured by Rotruck method3535. Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973 Feb;179(73):588-90. doi:10.1126/science.179.4073.588.
https://doi.org/10.1126/science.179.4073...
and was expressed as micromole of GSSG /minute/milligram of protein, based on blew reaction: 2H2O+GSSG→H2 O2+2GSH
Microscopic studies
The renal sample was fixed by immersing it in 10% neutral-buffered formalin. The sample was then embedded in paraffin, sliced into 5 μm sections, and stained with hematoxylin-eosin. The renal sections were examined blindly by an expert pathologist for tubular cell swelling, interstitial edema, tubular dilatation, and moderate to severe necrosis in all treatments. A minimum of 10 fields for each kidney slide were examined and assigned for severity of changes using scores on a scale of mild (1+), moderate (2+), and severe (3+) damage3636. Thiemermann C, Patel NS, Kvale EO, Cockerill GW, Brown PA, Stewart KN, Cuzzocrea S, Britti D, Mota-Filipe H, Chatterjee PK. High density lipoprotein (HDL) reduces renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003 Jul;14(7):1833-43. PMID: 12819243.
37. Singh D, Chander V, Chopra K. Protective effect of catechin on ischemia-reperfusion-induced renal injury in rats. Pharmacol Rep. 2005 Jan-Feb;57(1):70-6. PMID: 15849379. - 3838. Chen H, Xing B, Liu X, Zhan B, Zhou J, Zhu H, Chen Z. Ozone oxidative preconditioning inhibits inflammation and apoptosis in a rat model of renal ischemia/reperfusion injury. Eur J Pharmacol. 2008 Mar;581(3):306-14. PMID: 18093583..
Statistical analysis
The Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA), version 17.0, was used for statistical analysis. All data are presented as mean ± SEM. Before statistical analysis, all variables were checked for normality and homogeneity of variance by using the Kolmogorov-Smirnoff and Levene tests, respectively. The data obtained were tested by ANOVA followed by Tukey's post-hoc multiple comparison test.
Results
Kidney function study
Table 1 shows the effects of Black cumin on the serum levels of markers of kidney function (Serum creatinine, blood urea nitrogen, and uric acid). The serum concentrations of creatinine (p<0.001), urea (p<0.001), and uric acid (p<0.001) in the I/R group were found to be significantly higher than those in the sham rats, suggesting a significant degree of glomerular dysfunction mediated by renal I/R. Pretreatment with Black cumin (1 and 2%) dose-dependently significantly decreased serum creatinine (p<0.05 and p<0.01, respectively), urea (p<0.05 and p<0.01, respectively), and uric acid (p<0.05 and p<0.01, respectively) levels compared with the reperfusion group, although these levels were still significantly higher than the sham control. Three wk treatment of the other eight rats with Black cumin 5% led to a non-significantly reductions in the mentioned serum biochemical parameters.
Water intake and urinary excretion rates and kidney-body weight ratio in experimental rats are depicted in Table 2. Urinary excretion rate significantly decreased in I/R rats as compared with the sham animals (p<0.001). It dose-dependently significantly (p<0.05 and p<0.01, respectively) increased by pretreatment with Black cumin in the I/R+ Black cumin 1 and 2% groups. Pretreatment with Black cumin 0.5% led to non-significant elevation in the urinary excretion rate (Table 2). Water intake decreased in animals with reperfusion injury as compared with the rats in sham operation group (p<0.001) and it dose-dependently significantly (p<0.05 and p<0.01, respectively) increased by pretreatment with Black cumin in I/R+ Black cumin 1 and 2%) groups. Pretreatment with Black cumin 0.5% led to a non-significant elevation in the water intake rate (Table 2). Finally, the ratio of kidney weight to body weight also increased in the reperfusion group as compared with sham group (p<0.001). Pretreatment with Black cumin 1 and 2% dose-dependently significantly (p<0.05 and p<0.01, respectively) decreased this ratio compared with no Black cumin pretreatment in the reperfusion group. Pretreatment with Black cumin 0.5% led to a non-significant reduction in the kidney-body weight ratio.
Antioxidant activity
Effect of Black cumin pretreatment on renal MDA and GSH levels and SOD, CAT and GSH-Px activities in experimental rats are presented in Table 3. In I/R group, ischemia and reperfusion caused significant decreases in tissue GSH levels and antioxidant enzymes (SOD, CAT and GSH-Px) activities (p<0.001) when compared with the sham control group. In the I/R+Black cumin 1 and 2% groups, GSH levels and these antioxidant enzymes activities were found to be dose-dependently significantly increased (p<0.05 and p<0.01, respectively) compared to IR model group. Pretreatment with Black cumin 0.5% led to a non-significantly increases in the levels of mentioned renal antioxidant agents (Table 3). As expected, the level of renal MDA was significantly increased in IR model rats compared with sham rats as shown in Table 3. In the I/R+ Black cumin 1 and 2% groups, levels of renal MDA were dose-dependently significantly decreased (p<0.05 and p<0.01, respectively) in comparison with IR model rats. Pretreatment with Black cumin 0.5% led to a non-significantly increase in the renal MDA level (Table 3).
Histopathological findings
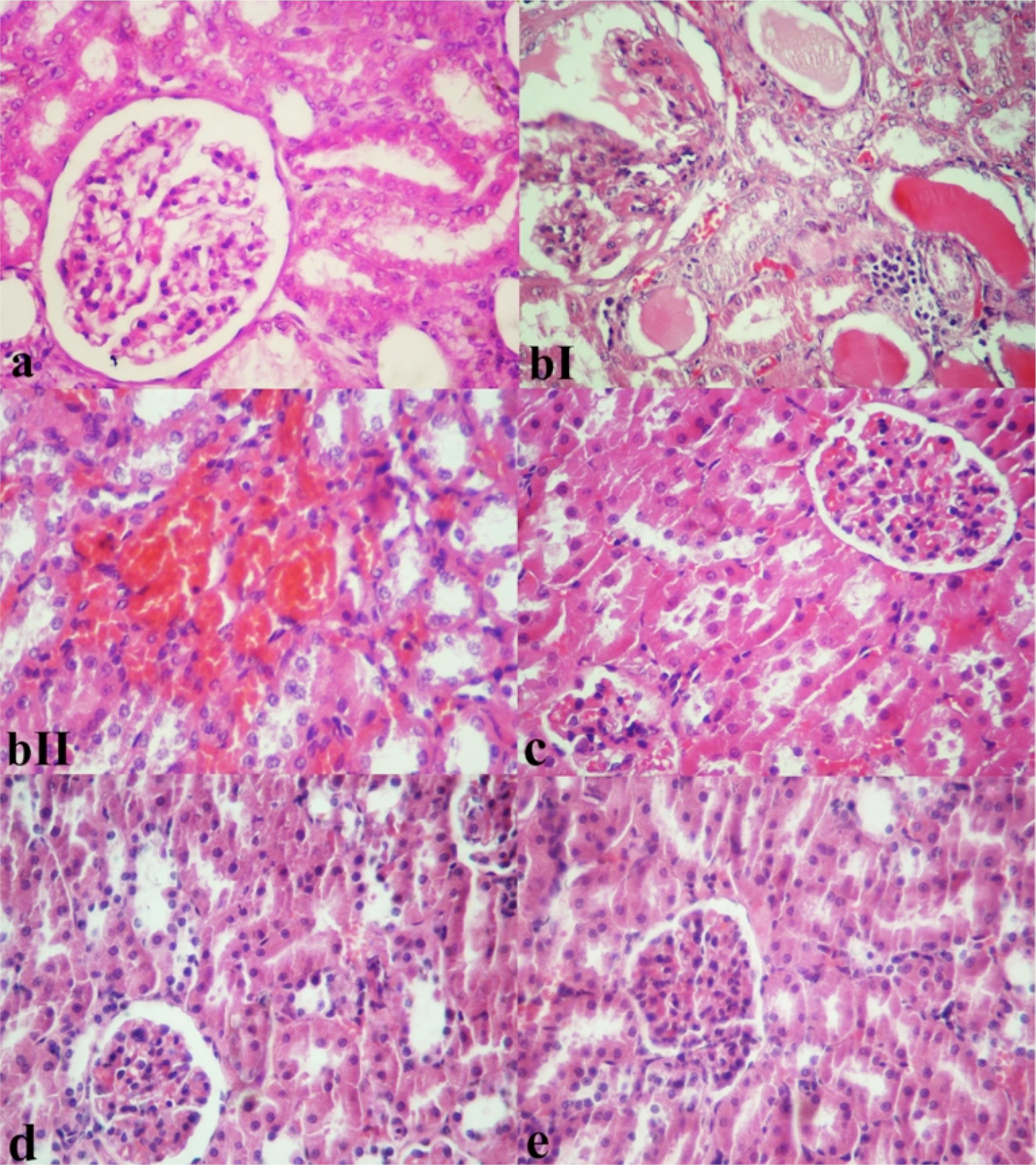
Figure 1 shows the result of histopathological changes of kidney in sham operated, IR model and IR rats pretreated with Black cumin (0.5, 1 and 2%). In Sham group, the kidney tissue showed regular morphology of renal parenchyma with well-defined glomeruli and tubuli (Figure 1a). In renal tissue of IR rats, Tubular epithelial degeneration, acute tubular lytic necrosis and detachment of the cells from the basal membrane as well as interstitial lymphocytic infiltration, hyperemia, oedema and wide spread hemorrhages were observed. Dilation of urinary space with hyaline proteinaceouse droplets, adhesiveness of visceral and parietal bowmann's capsule, mesenchial moderate hypercellularity with hyperemia and some time the necrosis of squamous cell in parietal wall of bowmann's capsule were also prominent. In addition, hyaline casts in some time with obstruction and dilation of tubules were visible (Figure 1 bI and bII). In contrast, pretreatment with Black cumin 0.5% caused considerable reversion of pathological changes. In this group, low pathological injuries consisting of petty hyperemia and slight edema in tubular cells were distinguished. Mild infiltration of lymphocytes in interstitial areas was distinguishable (Figure 1c). Sections of the kidneys of the rats treated with Black cumin 1% showed architectural and cytological preservation of structure except for slight vacuolation of tubular cells (Figure 1d). Pretreatment with Black cumin 2% retrieved renal histopathological injuries to near normal and remarkably histological changes were not observed (Figure 1e). Pathologic grading of kidney with ischemia/reperfusion injury from experimental groups is listed in Table 4.
Microscopic appearance from kidney tissues of the experimental rats (H and E, x250). a) Sham operation group rat kidney showing normal renal structure. bI; bII) I/R model rat kidney shows sever interstitial edema and hemorrhage associated with tubular degeneration and necrosis as well as irregular glomerular morphology. I/R model rat kidney. There are hyaline casts occluding the tubules with some tubular dilatation. c) I/R+ Back cumin 0.5%-treated kidney shows mild glomerular and interstitial hyperemia and apparent glomerular and tubular epithelial cells degeneration. d) I/R+ Back cumin 1%-treated kidney shows normal appearance except for moderate vacuolar degeneration. e) I/R+ Back cumin 2%-treated kidney shows normalcy of renal structure and pathologic changes are no longer present.
Discussion
The results of our study indicated that pre-treatment with Black cumin had preventive effects on renal reperfusion injury (I/R) of the kidney, as evidenced by functional parameters and histological examination. In this study, animals with reperfusion injury displayed renal tissue injury that was characterized by deterioration in kidney function, increase of serum urea, uric acid and creatinine. These changes were in accordance with extensive histopathological damages such as cellular vacuolation, interstitial edema, hyperemia and hemorrhage, tubular necrosis and glomerular changes. However, with Black cumin pre-treatment in the reperfusion rats, functional and histopathological changes were reversed as biochemical observations were supported by histopathological test of kidney sections.
Serum creatinine (Scr), blood urea nitrogen (BUN) and uric acid are markers of glomerular filtration rate3939. Karimi G, Ramezani M, Tahoonian Z. Cisplatin nephrotoxicity and protection by milk thistle extract in rats. Evid Based Complement. Alternat Med. 2005 Sep;2(3):383-6. PMID: 16136217.. In this study, Scr, BUN and uric acid levels in I/R rats were significantly higher than those in sham operated rats. This indicates that renal dysfunction occurred after I/R operation. Our results showed that pre-treatment with Black cumin decreased the rise of Scr, BUN and uric acid induced by ischemia reperfusion operation. This demonstrates that pre-treatment with Black cumin was helpful in preventing IR-induced renal dysfunction in a dose dependent manner.
The data of our study also revealed that the ratio of kidney weight to body weight significantly increased and urine production significantly decreased. It is likely that interstitial edema after renal reperfusion results in increase of the ratio of kidney weight to body weight, and pre-treatment with Black cumin improves edema. Distinguishably, acute tubular necrosis induces oliguria by one of several mechanisms. These include the leakage of urine from damaged tubules across disrupted basement membranes into the renal interstitium or intratubular obstruction due to loss of necrotic epithelium. In addition, because of activation of the rennin-angiotensin system, particularly affecting outer cortical nephrons, glomerular blood flow and filtration are decreased, resulting in reduced formation of urine3939. Karimi G, Ramezani M, Tahoonian Z. Cisplatin nephrotoxicity and protection by milk thistle extract in rats. Evid Based Complement. Alternat Med. 2005 Sep;2(3):383-6. PMID: 16136217..
Acute renal failure created by ischemia and reperfusion is histopathologically characterized by inflammation of renal tissue, extensive tubular damage, tubular cell necrosis, glomerular injury, and tubular obstruction with cell debris22. Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007 Oct;376:1-43. doi: 10.1007/s00210-007-0183-5.
https://doi.org/10.1007/s00210-007-0183-...
. Much of this tubular and glomerular dysfunction has been postulated to occur during the reperfusion period following anoxia, and generation of ROS (reactive oxygen species) has been postulated as one of the major factors contributing to this reperfusion injury.
In renal I/R injury, ROS are responsible for lipid peroxidation of biological membranes, which it will ultimately results in cell death4040. Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000 Aug;58(2):658-73. doi: 10.1046/j.1523-1755.2000.00212.x.
https://doi.org/10.1046/j.1523-1755.2000...
, 4141. Singh D, Chander V, Chopra K. The effect of quercetin, a bioflavonoid on ischemia/reperfusion induced renal injury in rats. Arch Med Res. 2004 Nov-Dec;35(6):484-94. doi: 10.1016/j.arcmed.2004.10.004.
https://doi.org/10.1016/j.arcmed.2004.10...
. The protection provided by free radical scavengers against ROS produced during I/R supports the hypothesis that free radical species are involved in the cellular pathogenesis of I/R4242. Joo JD, Kim M, D'Agati VD, Lee HT. Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol. 2006 Nov;17(11):3115-23. doi: 10.1681/ASN.2006050424.
https://doi.org/10.1681/ASN.2006050424...
. A main event in the induction of kidney injury during I/R is the generation of reactive oxygen species (ROS)4343. Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001 Nov;281(5):F948-57. doi: 10.1152/ajprenal.0071.2001.
https://doi.org/10.1152/ajprenal.0071.20...
, 4444. Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006 Jun;17(6):1503-20. doi: 10.1681/ASN.2006010017.
https://doi.org/10.1681/ASN.2006010017...
. ROS, which are produced during renal reperfusion, have diverse cytotoxic effects, including DNA damage, protein oxidation and nitrosylation, lipid peroxidation, and induction of apoptosis66. Rodrigo R, Bosco C. Oxidative stress and protective effects of polyphenols: Comparative studies in human and rodent kidney. A review. Comp Biochem Physiol C Toxicol Pharmacol. 2006 Mar-Apr;142(3-4):317-27. doi:10.1016/j.cbpc.2005.11.002.
https://doi.org/10.1016/j.cbpc.2005.11.0...
.
We assessed the potential of Black cumin by studying its effect on lipid peroxidation, which was measured in terms of MDA, a stable metabolite of the free radical-mediated lipid peroxidation cascade. In our study, animals subjected to renal I/R demonstrated an increase in the renal MDA and attenuated antioxidant enzymes pool. Black cumin reversed the increase of MDA levels to a considerable extent, thereby confirming its antioxidant role in I/R, indicating that Black cumin prevented lipid peroxidation and protein oxidation in the renal I/R process.
In our study, GSH which is known to be depleted following an ischemic insult4545. Scaduto RC Jr, Gattone VH, Grotyohann LW, Wertz J, Martin LF. Effect of an altered glutathione content on renal ischemic injury. Am J Physiol. 1988 Nov;255(5 Pt 2):F911-21. PMID: 3189564. was decreased with renal ischemia reperfusion process. Black cumin-pretreated rats exhibited higher GSH contents than their respective controls, indicating that Black cumin helped in replenishing the GSH pool.
The fact that Black cumin causes a significant increase in CAT, GSH-Px and SOD activities in comparison with I/R group, suggesting that it might have an antioxidant effect through the increase in SOD, GSH-Px and CAT enzyme activities. Altogether, the mechanism of the protective effect of Black cumin on renal I/R injury can be explained by its antioxidant activity. The rennin-angiotensin system plays a pivotal role in regulation of blood pressure. Renin acts on angiotensinogen to form angiotensin-I, which is converted to angiotensin-II with the help of angiotensin-converting enzyme4646. Gavras HP, Salerno CM. The angiotensin II type 1 receptor blocker losartan in clinical practice: a review. Clin Ther. 1996 Nov-Dec;18(6):1058-67. doi: 10.1016/S0149-2918(96)80061-0.
https://doi.org/10.1016/S0149-2918(96)80...
.
Accumulating evidence suggests that angiotensin-II stimulates intracellular formation of ROS such as superoxide anion and hydrogen peroxide that leads to kidney damage4747. Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007 Sep;18(9):2439-46. doi: 10.1681/ASN.2007020149.
https://doi.org/10.1681/ASN.2007020149...
. Generation of ROS has been postulated as one of the major factors contributing to this reperfusion injury. Oxidative stress can result from increased ROS production, and/or from decreased ROS scavenging capability. The ROS attach to the polyunsaturated fatty acids in the membrane lipids and result in peroxidation, which may lead to disorganization of cell structure and function. After reperfusion and reoxygenation, the imbalance between restoration of oxygen supply and mitochondrial respiratory function results in massive generation of superoxide anion in mitochondria4848. Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000 Jan;35(1 Pt 2):193-201. doi: 10.1161/01.HYP.35.1.193.
https://doi.org/10.1161/01.HYP.35.1.193...
, 4949. Ozyurt H, Irmak MK, Akyol O, Sogut S. Caffeic acid phenethyl ester changes the indices of oxidative stress in serum of rats with renal ischaemia-reperfusion injury. Cell Biochem Funct. 2001 Dec;19(4):259-63. doi: 10.1002/cbf.923.
https://doi.org/10.1002/cbf.923...
. Under these conditions, the defensive system, which is known as antioxidant or antioxidant enzymes, cannot prevent the escape of ROS, especially in mitochondria, and their effects on other intracellular sites. This cascade of events is known as reperfusion injury4949. Ozyurt H, Irmak MK, Akyol O, Sogut S. Caffeic acid phenethyl ester changes the indices of oxidative stress in serum of rats with renal ischaemia-reperfusion injury. Cell Biochem Funct. 2001 Dec;19(4):259-63. doi: 10.1002/cbf.923.
https://doi.org/10.1002/cbf.923...
.
In this study, renal I/R increased oxidative stress products including tissue MDA and depleted the antioxidant enzymes pool, as is evident from the declined activity of superoxide dismutase, catalase, glutathione peroxidase and reduced glutathione. It can be speculated that pretreatment with Black cumin prevented renal I/R-induced lipid peroxidation and protected the kidneys from severe increasing of ROS products and depletion of superoxide dismutase catalase, glutathione peroxidase and reduced glutathione in rats exposed to the renal I/R.
Taken in all, the use of this plant in renal ischemia/reperfusion injury is then supported but the precise active substance(s) of Black cumin, site(s), cellular and molecular mechanism(s) of its pharmacological effect and possible toxicity and interaction with other drugs are still to be determined. Also, because of prolonged duration of giving black cumin, 3 weeks before ischemia, which could delay treatment of renal tumors and stones, be impossible for kidney harvesting from cadaveric donors and for patients with kidney trauma, the different fractions of Black cumin seeds in different doses should be tested for their rapid therapeutic potential through animal modeling. However, our data support a role for Black cumin in attenuation of kidney damage after ischemia/reperfusion injury of the kidneys in an animal model, therefore, future studies and trials to establish efficacy and optimum dosage of this plant in human bodies are essential.
Conclusion
Pretreatment with antioxidant Black cumin significantly protect renal ischemia/reperfusion injury in rats.
References
-
1Radhakrishnan J, Kiryluk K. Acute renal failure outcomes in children and adults. Kidney Int. 2006 Jan;69(1):17-9. doi: 10.1038/sj.ki.5000094.
» https://doi.org/10.1038/sj.ki.5000094 -
2Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007 Oct;376:1-43. doi: 10.1007/s00210-007-0183-5.
» https://doi.org/10.1007/s00210-007-0183-5 -
3Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007 Oct;123(1):7-13. doi: 10.1016/j.clim.2006.09.008.
» https://doi.org/10.1016/j.clim.2006.09.008 -
4Legrand M, Mik EG, Johannes T, Payen D, Ince C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med. 2008 Jul-Aug;14(7-8):502-16. doi: 10.2119/2008-00006.
» https://doi.org/10.2119/2008-00006 -
5Nafar M, Parvin M, Sadeghi P, Ghoraishian M, Soleimani M, Tabibi A, Nouralizadeh A, Amirkhanlou S, Barzi F, Alipour B. Effects of stem cells and granulocyte colony stimulating factor in reperfusion injury. Iran J Kidney Dis. 2010 Jul;4(3):207-13. PMID: 20622308.
-
6Rodrigo R, Bosco C. Oxidative stress and protective effects of polyphenols: Comparative studies in human and rodent kidney. A review. Comp Biochem Physiol C Toxicol Pharmacol. 2006 Mar-Apr;142(3-4):317-27. doi:10.1016/j.cbpc.2005.11.002.
» https://doi.org/10.1016/j.cbpc.2005.11.002 -
7Senturk H, Kabay S, Bayramoglu G, Ozden H, Yaylak F, Yucel M, Olgun EG, Kutlu A. Silymarin attenuates the renal ischemia/reperfusion injury-induced morphological changes in the rat kidney. World J Urol. 2008 Aug;26(4):401-7. doi: 10.1007/s00345-008-0256-1.
» https://doi.org/10.1007/s00345-008-0256-1 -
8Amersi F, Nelson SK, Shen XD, Kato H, Melinek J, Kupiec-Weglinski JW, Horwitz LD, Busuttil RW, Horwitz MA. Bucillamine, a thiol antioxidant, prevents transplantation-associated reperfusion injury. Proc Natl Acad Sci USA. 2002 Jun;99(13):8915-20. doi: 10.1073/pnas.132026099.
» https://doi.org/10.1073/pnas.132026099 -
9Seo MY, Lee SM. Protective effect of low dose of ascorbic acid on hepatobiliary function in hepatic ischemia/reperfusion in rats. J Hepatol. 2002 Jan;36(1):72-7. doi: 10.1016/S0168-8278(01)00236-7.
» https://doi.org/10.1016/S0168-8278(01)00236-7 -
10Burns AT, Davies DR, McLaren AJ, Cerundolo L, Morris PJ, Fuggle SV. Apoptosis in ischemia/reperfusion injury of human renal allografts. Transplantation. 1998 Oct;66(7):872-6. PMID: 9798696.
-
11Witenberg B, Kalir HH, Raviv Z, Kletter Y, Kravtsov V, Fabian I. Inhibition by ascorbic acid of apoptosis induced by oxidative stress in HL-60 myeloid leukemia cells. Biochem Pharmacol. 1999 Apr;57(7):823-32. doi: 10.1016/S0006-2952(98)00351-7.
» https://doi.org/10.1016/S0006-2952(98)00351-7 -
12Hajhashemi V, Ghannadi A, Jafarabadi H. Black cumin seed essential oil, as a potent analgesic and anti-inflammatory drug. Phytother Res. 2004 Mar;18(3):195-9. doi: 10.1002/ptr.1390.
» https://doi.org/10.1002/ptr.1390 -
13Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000 Aug;14(5):323-8. PMID: 10925395.
-
14Padhye S, Banerjee S, Ahmad A, Mohammad R, Sarkar FH. From here to eternity-the secret of Pharaohs: Therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008 Sep;6(b):495-510. PMID: 19018291.
-
15Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003 Apr;17(4):299-305. doi: 10.1002/ptr.1309.
» https://doi.org/10.1002/ptr.1309 -
16Khattab MM, Nagi MN. Thymoquinone supplementation attenuates hypertension and renal damage in nitric oxide deficient hypertensive rats. Phytother Res. 2007 May;21(5):410-4. doi: 10.1002/ptr.2083.
» https://doi.org/10.1002/ptr.2083 -
17Dehkordi FR, Kamkhah AF. Antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam Clin Pharmacol. 2008 Aug;22(4):447-52. doi: 10.1111/j.1472-8206.2008.00607.
» https://doi.org/10.1111/j.1472-8206.2008.00607 -
18Kaleem M, Kirmani D, Asif M, Ahmed Q, Bano B. Biochemical effects of Nigella sativa L seeds in diabetic rats. Indian J Exp Biol. 2006 Sep;44(9):745-8. PMID: 16999030.
-
19Meddah B, Ducroc R, El Abbes Faouzi M, Eto B, Mahraoui L, Benhaddou-Andaloussi A, Martineau LC, Cherrah Y, Haddad PS. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol. 2009 Jan;121(3):419-24. doi: 10.1016/j.jep.2008.10.040.
» https://doi.org/10.1016/j.jep.2008.10.040 -
20Bamosa AO, Ali BA, al-Hawsawi ZA. The effect of thymoquinone on blood lipids in rats. Indian J Physiol Pharmacol. 2002 Apr;46(2):195-201. PMID: 12500494.
-
21Uz E, Bayrak O, Uz E, Kaya A, Bayrak R, Uz B, Turgut FH, Bavbek N, Kanbay M, Akcay A. Nigella sativa oil for prevention of chronic cyclosporine nephrotoxicity: an experimental model. Am J Nephrol. 2008 Apr;28(3):517-22. doi: 10.1159/000114004.
» https://doi.org/10.1159/000114004 -
22Kanter M, Coskum O, Uysal H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch Toxicol. 2006 Apr;80(4):217-24. doi: 10.1007/s00204-005-0037-1.
» https://doi.org/10.1007/s00204-005-0037-1 -
23Al-Ghamdi MS. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol. 2001 Jun;76(1):45-8. doi: 10.1016/S0378-8741(01)00216-1.
» https://doi.org/10.1016/S0378-8741(01)00216-1 -
24Ait Mbarek L, Ait Mouse H, Elabbadi N, Bensalah M, Gamouh A, Aboufatima R, Benharref A, Chait A, Kamal M, Dalal A, Zyad A. Anti tumor properties of blackseed (Nigella sativa L.) extracts. Braz J Med Biol Res. 2007 Jun;40(6):839-47. doi: 10.1590/S0100-879X2006005000108.
» https://doi.org/10.1590/S0100-879X2006005000108 -
25Al-Johar D, Shinwari N, Arif J, Al-Sanea N, Jabbar AA, El-Sayed R, Mashhour A, Billedo G, El-Doush I, Al-Saleh I. Role of Nigella sativa and a number of its antioxidant constituents towards azoxymethane-induced genotoxic effects and colon cancer in rats. Phytother Res. 2008 Oct;22(10):1311-23. doi: 10.1002/ptr.2487.
» https://doi.org/10.1002/ptr.2487 -
26Machmudah S, Shiramizu Y, Goto M, Sasaki M, Hirose T. Extraction of Nigella sativa L. using supercritical Co2: A study of antioxidant activity of the extract. Sep Sci Technol. 2005 Jan;40(6):1267-75. doi: 10.1081/SS-200053005.
» https://doi.org/10.1081/SS-200053005 -
27Famadan MF. Oxidative stability of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.) and niger (Guizotia abyssinica cass.) Crude seed oils upon stripping. Eur J Lipid Sci Technol. 2004 Jan;106(1):35-43. doi: 10.1002/ejlt.200300895.
» https://doi.org/10.1002/ejlt.200300895 -
28Tauseef Sultan M, Masood Sadiq B, Rabia Shabeer A, Rizwana B, Ambreen N, Hafiz Ansar RS. Supplementation of powdered black cumin (Nigella sativa) seeds reduces the risk of hypercholesterolemia. Funct Foods Health Dis. 2011 Dec;12(1):516-24.
-
29Dollah MA, Parhizkar S, Latiff LA, Hafanizam Bin Hassan M. Toxicity effect of Nigella Sativa on the liver function of rats. Adv Pharm Bull. 2013 Feb;3(1):97-102. doi: 10.5681/apb.2013.016.
» https://doi.org/10.5681/apb.2013.016 -
30Bhalodia Y, Kanzariya N, Patel R, Patel N, Vaghasiya J, Jivani N, Raval H. renoprotective activity of benincasa cerifera fruit extract on ischemia/reperfusion-induced renal damage in rat. Iran J Kidney Dis. 2009 Apr;3(2):80-5. PMID: 19395782.
-
31Bradford MM. A rapid and sensitive method of the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976 May;72:248-54. PMID: 942051.
-
32Esterbauer H, Cheesman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407-21. doi: 10.1016/0076-6879(90)86134-H.
» https://doi.org/10.1016/0076-6879(90)86134-H -
33Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun. 1972 Jan;46(2):849-54. PMID: 4400444.
-
34Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984 Apr;21(2):130-2. PMID: 6490072.
-
35Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973 Feb;179(73):588-90. doi:10.1126/science.179.4073.588.
» https://doi.org/10.1126/science.179.4073.588 -
36Thiemermann C, Patel NS, Kvale EO, Cockerill GW, Brown PA, Stewart KN, Cuzzocrea S, Britti D, Mota-Filipe H, Chatterjee PK. High density lipoprotein (HDL) reduces renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003 Jul;14(7):1833-43. PMID: 12819243.
-
37Singh D, Chander V, Chopra K. Protective effect of catechin on ischemia-reperfusion-induced renal injury in rats. Pharmacol Rep. 2005 Jan-Feb;57(1):70-6. PMID: 15849379.
-
38Chen H, Xing B, Liu X, Zhan B, Zhou J, Zhu H, Chen Z. Ozone oxidative preconditioning inhibits inflammation and apoptosis in a rat model of renal ischemia/reperfusion injury. Eur J Pharmacol. 2008 Mar;581(3):306-14. PMID: 18093583.
-
39Karimi G, Ramezani M, Tahoonian Z. Cisplatin nephrotoxicity and protection by milk thistle extract in rats. Evid Based Complement. Alternat Med. 2005 Sep;2(3):383-6. PMID: 16136217.
-
40Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000 Aug;58(2):658-73. doi: 10.1046/j.1523-1755.2000.00212.x.
» https://doi.org/10.1046/j.1523-1755.2000.00212.x -
41Singh D, Chander V, Chopra K. The effect of quercetin, a bioflavonoid on ischemia/reperfusion induced renal injury in rats. Arch Med Res. 2004 Nov-Dec;35(6):484-94. doi: 10.1016/j.arcmed.2004.10.004.
» https://doi.org/10.1016/j.arcmed.2004.10.004 -
42Joo JD, Kim M, D'Agati VD, Lee HT. Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol. 2006 Nov;17(11):3115-23. doi: 10.1681/ASN.2006050424.
» https://doi.org/10.1681/ASN.2006050424 -
43Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001 Nov;281(5):F948-57. doi: 10.1152/ajprenal.0071.2001.
» https://doi.org/10.1152/ajprenal.0071.2001 -
44Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006 Jun;17(6):1503-20. doi: 10.1681/ASN.2006010017.
» https://doi.org/10.1681/ASN.2006010017 -
45Scaduto RC Jr, Gattone VH, Grotyohann LW, Wertz J, Martin LF. Effect of an altered glutathione content on renal ischemic injury. Am J Physiol. 1988 Nov;255(5 Pt 2):F911-21. PMID: 3189564.
-
46Gavras HP, Salerno CM. The angiotensin II type 1 receptor blocker losartan in clinical practice: a review. Clin Ther. 1996 Nov-Dec;18(6):1058-67. doi: 10.1016/S0149-2918(96)80061-0.
» https://doi.org/10.1016/S0149-2918(96)80061-0 -
47Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007 Sep;18(9):2439-46. doi: 10.1681/ASN.2007020149.
» https://doi.org/10.1681/ASN.2007020149 -
48Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000 Jan;35(1 Pt 2):193-201. doi: 10.1161/01.HYP.35.1.193.
» https://doi.org/10.1161/01.HYP.35.1.193 -
49Ozyurt H, Irmak MK, Akyol O, Sogut S. Caffeic acid phenethyl ester changes the indices of oxidative stress in serum of rats with renal ischaemia-reperfusion injury. Cell Biochem Funct. 2001 Dec;19(4):259-63. doi: 10.1002/cbf.923.
» https://doi.org/10.1002/cbf.923
-
Financial source: Tabriz Branch, Islamic Azad University
-
1
Reseach performed at Surgery and Radiology Section, Department of Clinical Sciences, College of Veterinary Medicine, Tabriz Branch, Islamic Azad University, Tabriz, Iran.
Publication Dates
-
Publication in this collection
Aug 2015
History
-
Received
21 Apr 2015 -
Reviewed
18 June 2015 -
Accepted
24 July 2015