Abstract
PURPOSE:
To determine the effectiveness of low-intensity therapeutic ultrasound (LITUS) on wound healing in rats with third-degree burns.
METHODS:
Twenty rats were divided into the Control Group that comprised four rats without third-degree burns that did not undergo LITUS, the Burned Group (BG), comprising eight rats with third-degree burns that did not undergo LITUS, and the Burned with Treatment Group (BTG), comprising eight rats with third-degree burns that were administered LITUS. LITUS began 24 h after injury and involved daily applications for 8 min at 0.1 W/cm2 for 14 days.
RESULTS:
The BTG lost less weight than the BG (Q=2.75; p<0.05). No visible differences were apparent among the groups' lesions on day 4. By the end of treatment, wound healing was more evident in the BTG. No statistically significant differences were found between the BG and the BTG in relation to the parameters measured using the histological changes in burn wound healing scoring system.
CONCLUSION:
The LITUS protocol applied to the animals with third-degree burns accelerated the formation of fibrin-leukocyte crusts and significantly reduced weight loss. However, burn wound healing was not accelerated.
Physical therapy; Burn; Rats; Wound Healing; Ultrasonic Therapy
Introduction
Burns are a serious public health problem11. Popescu FC, Busuioc CJ, Mogoşanu GD, Pop OT, Pârvănescu H, Lascăr I, Nicolae CI, Mogoantă L. Pericytes and myofibroblasts reaction in experimental thermal third degree skin burns. Rom J Morphol Embryol. 2011;52(3 Suppl):1011-7. PMID: 22119818.,22. Mock C. WHO joins forces with International Society for Burn Injuries to confront global burden of burns. Inj Prev. 2007 Oct;13(5):303.. They represent one of the major causes of trauma in all age groups, and they lead to significant sequelae or even death. Indeed, 4.5 deaths/100.000 people per year occur in low and middle income countries as a consequence of burns22. Mock C. WHO joins forces with International Society for Burn Injuries to confront global burden of burns. Inj Prev. 2007 Oct;13(5):303..
Large burns are one of the most aggressive causes of skin integrity losses. Burns are generally caused by flammable substances and overheated liquids22. Mock C. WHO joins forces with International Society for Burn Injuries to confront global burden of burns. Inj Prev. 2007 Oct;13(5):303.. Depending on the severity of the injury, epidermal necrosis can occur, which affects the dermis and the underlying tissues, and can lead to systemic changes that compromise other organs and tissues that are remote from the burnt area33. Oliveira Fd, Bevilacqua LR, Anaruma CA, Boldrini Sde C, Liberti EA. Morphological changes in distant muscle fibers following thermal injury in Wistar rats. Acta Cir Bras. 2010 Nov/Dec;25(6):525-8. PMID: 21120285.. In addition, hypovolemic shock and infectious or inflammatory diseases can occur, which are among the most challenging situations that patients with burns and their care teams have to confront.
The skin's recovery process is long, and it causes permanent changes that compromise the esthetic appearances of patients who have been burnt22. Mock C. WHO joins forces with International Society for Burn Injuries to confront global burden of burns. Inj Prev. 2007 Oct;13(5):303.,44. Grishkevich VM. Ankle dorsiflexion postburn scar contractures: anatomy and reconstructive techniques. Burns. 2012 Sep;38(6):882-8. PMID: 22325850.. These changes cause contractures55. Dematte MF, Gemperli R, Salles AG, Dolhnikoff M, Lanças T, Saldiva PH, Ferreira MC. Mechanical evaluation of the resistance and elastance of post-burn scars after topical treatment with tretinoin. Clinics (Sao Paulo). 2011;66(11):1949-54. PMID: 22086527. and limit motion, which, in addition to the esthetic issues, can affect individuals socially, economically, and emotionally22. Mock C. WHO joins forces with International Society for Burn Injuries to confront global burden of burns. Inj Prev. 2007 Oct;13(5):303.,66. Medeiros ADC, Ramos AMDO, Dantas Filho AM, Azevedo RDCFD, Araújo FLFB. Tratamento tópico de queimaduras do dorso de ratos com ácido hialurônico. Acta Cir Bras. 1999 Oct/Dec;14(4):203-7. doi: 10.1590/S0102-86501999000400010.
https://doi.org/10.1590/S0102-8650199900...
.
The treatment of burns is challenging. Several clinical and surgical approaches are being developed to reduce wound healing times and minimize the sequelae22. Mock C. WHO joins forces with International Society for Burn Injuries to confront global burden of burns. Inj Prev. 2007 Oct;13(5):303.,77. Ferreira AS, Barbieri CH, Mazzer N, Campos AD, Mendonça AC. Measurement of healing area using planimetry after applying low intensity ultrasound to the skin of rats. Rev Bras Fisioter. 2008 Sept/Oct;12(5):351-8. doi: 10.1590/S1413-35552008000500003.
https://doi.org/10.1590/S1413-3555200800...
. Physical therapy offers resources, including low-intensity therapeutic ultrasound (LITUS), which can help to accelerate the burn wound-healing process88. Cambier DC, Vanderstraeten GG. Failure of therapeutic ultrasound in healing burn injuries. Burns. 1997 May;23(3):248-9. PMID: 9232286. and can prevent or reduce adhesions. LITUS is a commonly used therapeutic tool that enhances healing processes in a range of injuries99. Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng. 2004;6:229-48. PMID: 15255769. by accelerating the inflammatory phase and stimulating neovascularization1010. Hogan RD, Burke KM, Franklin TD. The effect of ultrasound on microvascular hemodynamics in skeletal muscle: effects during ischemia. Microvasc Res. 1982 May;23(3):370-9. PMID: 7099026., collagen synthesis, and fibroblast proliferation99. Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng. 2004;6:229-48. PMID: 15255769.,1111. Freitas TP, Gomes M, Fraga DB, Freitas LS, Rezin GT, Santos PM, Silveira PC, Paula MM, Pinho RA, Streck EL. Effect of therapeutic pulsed ultrasound on lipoperoxidation and fibrogenesis in an animal model of wound healing. J Surg Res. 2010 Jun;161(1):168-71. PMID: 19577772.. Accordingly, LITUS can hasten healing by encouraging the formation of a functional and esthetically satisfactory scar, and, as such, it provides non-invasive99. Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng. 2004;6:229-48. PMID: 15255769. and low-cost therapy.
Research into large burns has gained greater attention from researchers because of the importance of the skin's physiological function. However, few studies have been published that have investigated the effect of conventional LITUS on the healing of third-degree burns. However, treatment with LITUS can have negative effects, because the equipment's parameters are not standardized, and the treatment itself might be inadequate99. Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng. 2004;6:229-48. PMID: 15255769.,1212. Steiss JE, Adams CC. Effect of coat on rate of temperature increase in muscle during ultrasound treatment of dogs. Am J Vet Res. 1999 Jan;60(1):76-80. PMID: 19577772.. Accordingly, new studies investigating the use of LITUS in the treatment of burns are required. Therefore, this study determined the effectiveness of LITUS that is commonly utilized in physical therapy clinics on the wound healing and tissue repair processes of third-degree scalding burns in rats.
Methods
This prospective, longitudinal, and descriptive study analyzed the application of LITUS to rats that had been subjected to third-degree burns.
Twenty male adult Wistar rats (Rattus norvegicus ), aged between 90 days and 120 days and weighing 230-300g, were used for the experiment. During the experiment, the rats were kept in a room in which the humidity, noise, and temperature (22ºC) levels were controlled, and in a cycle comprising 12h dark and 12h light. The rats were randomly assigned to six cages, with each cage containing between two and four animals. The cages were covered with clean straw, and the animals had access to filtered water and food ad libitum .
All of the experimental procedures were performed at the Laboratory of Morphophysiopathology Applied to Health/CCBS of Pará State University (UEPA). The study was conducted after receiving approval from the Ethics Committee on Animal Use (Comitê de Ética em Uso de Animais ) at UEPA (protocol number 09/2012), and it complied with the Guide for the Care and Use of Laboratory Animals1313. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals.Washington (DC): The National Academies Press; 2011..
Experimental groups
The animals were divided into three groups, namely, the Control Group (CG) that did not receive third-degree burns or LITUS (n = 4), the Burned Group (BG) that received third-degree burns and was not administered LITUS (n = 8), and the Burned with Treatment Group (BTG) that received third-degree burns and was treated with LITUS (n = 8), which was applied 24 h after the injuries and daily for 8 min for 14 days.
Burn protocol
Scalding water at 100ºC was used to burn 30% of the animals' body surfaces. To ensure that the burnt area characterized a large burn, a template was made (Figures 1A and 1B) that was based on that developed by Walker and Mason1414. Walker HL, Mason AD Jr. A standard animal burn. J Trauma. 1968 Nov;8(6):1049-51. PMID: 5722120., and it incorporated modifications that were created by the Assistive Technology Laboratory at UEPA. A galvanic sheet was used for the burn template that was 7 cm wide and 28 cm long. Its interior was coated with insulating rubber and ethylene-vinyl acetate; hence, the template thermally insulated the parts of the body that were not to be exposed to scalding. The central region of the burn template was open and corresponded to the location and area of the thermal injury.
The total body surface area A (Figure 1C) relative to the animal's body mass was calculated using the following Equation 11414. Walker HL, Mason AD Jr. A standard animal burn. J Trauma. 1968 Nov;8(6):1049-51. PMID: 5722120.:
A = K ⋅W2 /3 Equation 1
where,
A = total body surface area in mm2;
K = 10 (constant ratio); and
W = weight in grams.
The thermal injury corresponded to 30% of the rat's body surface area A.
- A. The burn template. c: ethylene-vinyl acetate, f: wood support, g: galvanized sheet. B. The burn template. a: surface area (30% of the rat's surface area), b: insulating rubber, d: insulating vest. C. The area of the burn on the animal's back.
Before being burnt, the animals were given analgesia in the form of manipulated Tramadol(r) (5 mg/kg) that was administered orally via a gavage tube. Then, the animals were anesthetized using intraperitoneally injected Ketamina(r) (50-70 mg/kg) and Xilazina(r) (5-10 mg/kg). After being anesthetized, the animals' backs were shaved, and they were immobilized and placed on the template in the supine position. Then, the template containing the animal was immersed for 10 s into water at 100ºC. This procedure resulted in non-lethal third-degree burns1515. Barbosa RCC, Guimarães SB, Vasconcelos PRCD, Chaves CR, Vasconcelos PRLD. Metabolic effects of glutamine in rats subjected to scald burn. Acta Cir Bras. 2003 Nov/Dec;18(6): 537-41. doi: 10.1590/S0102-86502003000600008.
https://doi.org/10.1590/S0102-8650200300...
. After being burnt, the animals, including those in the CG, were given analgesia orally using Tramadol(r) (5 mg/kg), which was administered via a gavage tube every 12 h for 5 consecutive days.
Animal preparation for LITUS treatment
After being burnt, the animals were kept at room temperature until the anesthesia wore off to avoid hypothermia and death. Subsequently, the animals were placed individually in acrylic rat cages where they had access to filtered water and food ad libitum . To prevent the contamination of the injuries, the cages were covered with clean straw. The animals' weights were measured on the day of the injury, then on days 4, 7, 10, 13, and 15 when they were euthanized.
Treatment protocol
LITUS began 24h after the burn injuries, and comprised daily applications of LITUS for 14 consecutive days using an Avatar III TUS0203 (KLD Biosistemas Equipamentos Eletrônicos Ltda., Amparo, SP, Brazil). To resemble clinical practice, pulsed LITUS at 3 MHz was applied to the contours of the wounds using a spatial average temporal average (SATA) intensity of 0.1 W/cm2, a 20% loading cycle, 32 Hz modulation, and a 2.5 cm2 effective radiating area, and LITUS was applied for 8 min in a circular motion.
To apply the LITUS, the animals were manually restrained with the burnt back region accessible to the treatment. The lesion was cleaned with saline and covered with a polyvinyl chloride (PVC) film that was 10.5 mm thick. Hydrogel was subsequently placed on the PVC film to couple the ultrasound transducer.
Histological analysis
The animals were euthanized by decapitation on day 15. The injured and surrounding areas of skin were removed with a scalpel. The skin removed comprised three zones, namely, the wound area (burnt skin), the area surrounding the wound, representing the transition from the burnt skin to the adjacent skin, and the adjacent skin. The excised skin was fixed in 10% buffered formalin for 48 h in preparation for histological processing. The tissue sections were stained using hematoxylin and eosin1616. Galeano M, Altavilla D, Bitto A, Minutoli L, Calò M, Lo Cascio PL, Polito F, Giugliano G, Squadrito G, Mioni C, Giuliani D, Venuti FS, Squadrito F. Recombinant human erythropoietin improves angiogenesis and wound healing in experimental burn wounds. Crit Care Med. 2006 Apr;34(4):1139-46. PMID: 16484928.. The histological analyses were performed using a trinocular microscope equipped with an Axio Scope A1 image-capturing system (Carl Zeiss Microscopy LLC, Thornwood, NY, USA) that consisted of an HRC AxioCam camera and AxioVision Release 4.7.2 software. Two evaluators, comprising a dermatologist and a pathologist, used the histological changes in burn wound healing scoring system1616. Galeano M, Altavilla D, Bitto A, Minutoli L, Calò M, Lo Cascio PL, Polito F, Giugliano G, Squadrito G, Mioni C, Giuliani D, Venuti FS, Squadrito F. Recombinant human erythropoietin improves angiogenesis and wound healing in experimental burn wounds. Crit Care Med. 2006 Apr;34(4):1139-46. PMID: 16484928. to perform blind histological evaluations of the reepithelization, granulation, inflammation, and angiogenesis.
Statistical analyses
The sample size was determined based on a value of α = 0.05, three groups, including one control group, and a test power of 80%. To examine differences in the animals' weights and histological scores, the Kolmogorov-Smirnov normality test and the analysis of variance (ANOVA) were used [F(model degrees of freedom, residual degrees of freedom)]. The Kruskal-Wallis one-way ANOVA by ranks [H(degrees of freedom)] was used for weight-loss and quantitative histological analysis, then Dunn's post hoc test [Q] was applied, as appropriate. A significance level of α = 0.05 was used. SigmaStat software, version 3.5 (Systat Software Inc., San Jose, CA, USA) was used for the statistical analyses.
Results
A weight-loss analysis along the 15 days for each group was performed. The weight-loss variations in CG [F(5,18) = 1.74; p = 0.177] and BTG [H(5) = 8.42; p = 0.135] showed no statistically significant difference. On the other side, the weight-loss variation for the BG group was statistically significant [F(5,40) = 3.20; p = 0.016].
When comparing the groups together, there was a statistically significant difference among them in relation to weight loss [H(2) = 7.98; p = 0.018] (Figure 2) and the post-test findings revealed that this difference was only between the BG and BTG [Q = 2.75; p < 0.05]. The analysis of the coefficients of variation (CV) demonstrated that the BTG (CV = 0.06) had sustained less weight variation during the study compared with the BG (CV = 0.11) and the CG (CV = 0.07).
- Weight variations within the Control Group (CG), Burned Group (BG), and the Burned with Treatment Group (BTG) during the experimental period. Data presented are the means ± standard deviations. *: post-test revealed difference only between groups BG and BTG [Q = 2.75; p < 0.05].
Regarding the macroscopic appearances of the burns, there were no differences between the groups from day 2 to day 4 following the injuries. On day 5, the animals in the BG had large unhealed wounds and the animals in the BTG were at a more advanced stage of healing, because the wounds were covered with fibrin-leukocyte crusts. On day 14, healing was more evident in the BTG compared with the BG, which was inferred from the presence of darker and visibly larger crusts (Figure 3).
- Macroscopic appearance of the burns on the animals from the burned group (BG) and the burned with treatment group (BTG) on days 2, 5, and 14 after the scalding injury.
The qualitative histological analysis showed the presence of typically irregular and dense arrangements of collagen fibers (Figures 4A and 4B) within the reticular dermis of the skin from the animals in the CG. The skin from the animals in the BG showed that the superficial layers, namely, the epidermis and a major part of the dermis, had been destroyed, and that eschar and a dense inflammatory infiltrate were present between the remaining collagen fibers. The arrangement of the collagen fibers had changed compared with the CG (Figures 4C and 4D). In the BTG, the inflammatory cell infiltrate was not as dispersed among the collagen fibers, but, instead, the cells were clustered. The appearance of the collagen fibers in the skin from the BTG was similar to that in the skin from the CG, because they comprised thick fibers that were arranged in different directions and they had an undulating appearance with many eosinophilic fibers, which is the typical appearance of normal dense connective tissue (Figures 4E and 4F).
- The collagen fibers (asterisk) within the skin from the study animals. A. Control Group (CG) (hematoxylin and eosin [H&E], ×5). B. CG (H&E, ×10). C. An inflammatory cell infiltrate (arrow ) is evident among the remaining collagen fibers in the Burned Group (BG) (H&E, ×5). D. The arrangement of the collagen fibers in the BG (H&E, ×10). E. Inflammatory cells (arrow ) within the Burned with Treatment Group (BTG) (H&E, ×5). F. The collagen fibers in the skin from the BTG have a thicker appearance (asterisk ) and the inflammatory cells (arrow ) appear to be in streaks (H&E, ×10).
The coagulation areas that included the presence of eschar and inflammatory cell infiltrates, were similar in the skin from the animals in the BG and BTG (Figures 5A and 5B, respectively). However, in the areas of hyperemia, the thickness of the epidermis differed between the BG (Figure 5C) and BTG (Figure 5D). The epithelial tissue within the skin of the animals from the BTG was thicker, particularly within the basal layer region. In the areas of stasis, the skin from the animals in the BG had thinner epidermal layers (Figure 5E) compared with the skin from the animals in the BTG (Figure 5F). The epidermis of the skin from the BTG animals showed intense proliferative activity within the basal layer, which is characteristic of reepithelization (Figures 5D and 5F).
The muscle fibers within the skin from the CG animals were well circumscribed with narrow spaces between them (Figure 5G). The muscle fibers within the skin from the BG animals had irregular outlines and there was more space between the fibers, with loosely arranged muscle fascicles, thick endomysia, and many fibroblasts present (Figure 5H). Compared with the BG, the skin from the BTG had loosely arranged muscle fascicles and the arrangement of the muscle fibers was more orderly (Figure 5I). The hypodermis was disorganized in the skin from the animals in the BG (Figure 5H) and the BTG (Figure 5I) compared with the skin from the animals in the CG (Figure 5G).
- Coagulation areas in the skin from the A. Burned Group (BG) (hematoxylin and eosin [H&E], ×5) and the B. Burned with Treatment Group (BTG) (H&E, ×5) showing crusts (s) and inflammatory cell infiltrates (arrows ). The hyperemia area in the C. skin from the BG has a thinner epidermis (arrow ) and dermis (d) (H&E, ×10), and the hyperemia area in the D. skin from the BTG has a thicker epidermis (arrow ), especially on the basal layer, and dermis (d) (H&E, ×10). The stasis area in the E. skin from the BG shows the presence of a crust (s) and a thinner epidermis (arrow ) (H&E, ×10), and the stasis area in the F. skin from the BTG shows the presence of a crust (s) and a thicker epidermis (arrow ) (H&E, ×10). Muscle tissue (m), fibroblasts (arrow ), and the hypodermis (t) in skin from the G. Control Group (CG) (H&E, ×10), H. BG (H&E, ×10), and I. BTG (H&E, ×10).
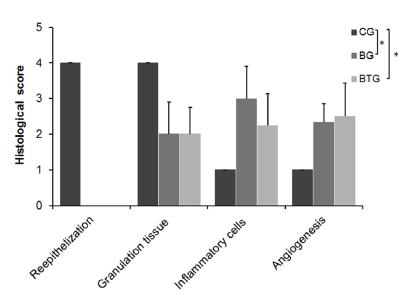
The quantitative histological analysis conducted using the histological changes in burn wound healing scoring system determined statistically significant differences among the groups with respect to reepithelization [H(2) = 17.00; p ≤ 0.001], granulation tissue [H(2) = 9.48; p = 0.009], inflammation [H(2) = 8.62; p = 0.013], angiogenesis [H(2) = 7.71; p = 0.021]. To discover which groups are responsible for such differences, the post-test was applied. It was found that the groups BG and BTG present statistical differences when compared to the CG, nevertheless, they presented no statistical differences between each other (Figure 6).
- Quantitative histological analysis of the control group (CG), Burned Group (BG), and the Burned with Treatment Group (BTG), based on the reepithelialization, granulation, inflammation, and angiogenesis criteria of the histological changes in burn wound healing scoring system. *post-test revealed differences between CG and BTG and between CG and BG.
Discussion
A severely burnt individual's weight declines as a consequence of the high level of energy consumption, which subsequently leads to muscle tissue loss and malnutrition1717. Wu X, Wolf SE, Walters TJ. Muscle contractile properties in severely burned rats. Burns. 2010 Sep;36(6):905-11. PMID: 20381255.. Hence, it is necessary to accelerate the healing process to minimize tissue loss and malnutrition. In this study, the burns covered 30% of the body surface and can be considered as extensive and with systemic commitment1515. Barbosa RCC, Guimarães SB, Vasconcelos PRCD, Chaves CR, Vasconcelos PRLD. Metabolic effects of glutamine in rats subjected to scald burn. Acta Cir Bras. 2003 Nov/Dec;18(6): 537-41. doi: 10.1590/S0102-86502003000600008.
https://doi.org/10.1590/S0102-8650200300...
,1818. de Oliveira BCC, de Oliveira F, Martini DT, Prisco CRD, da Silva Riguetti MM, Liberti EA, de Campos Boldrini S. The relative effects of severe burn injury and pre-and post-natal protein deprivation on mandibular condyle morphology. Histol Histopathol. 2010; 25(1): 45-54. PMID:19924640.. The BG group was the only one to significantly lost weight along the 15 days. Additionally, this weight loss was also significant when compared to the BTG group, for the same period. In the BG, two animals died, which may have been related to complications associated with the burns and the animals' generally inferior physical condition compared with the BTG animals, and this concurs with previously published reports1717. Wu X, Wolf SE, Walters TJ. Muscle contractile properties in severely burned rats. Burns. 2010 Sep;36(6):905-11. PMID: 20381255..
The BG animals showed higher amounts of weight loss after day 7. This finding is consistent with research previously conducted by Wu et al .1717. Wu X, Wolf SE, Walters TJ. Muscle contractile properties in severely burned rats. Burns. 2010 Sep;36(6):905-11. PMID: 20381255. who evaluated severely burnt rats, and found that they continuously lost weight, that the weight loss intensified between days 7 and 14 after the injuries, and that it was accompanied by decreases in muscle mass.
After 14 days, the BTG animals had developed larger fibrin-leukocyte crusts than the BG animals. This is the most characteristic part of the healing process, because it is indicative of the new tissue that is growing to fill in the defect. The crust originates from the fibrin coagulum and inflammatory exudate that serves as a provisional matrix for the next stage of healing1919. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004 Jan;9:283-9. PMID: 14766366.. Ferreira et al .77. Ferreira AS, Barbieri CH, Mazzer N, Campos AD, Mendonça AC. Measurement of healing area using planimetry after applying low intensity ultrasound to the skin of rats. Rev Bras Fisioter. 2008 Sept/Oct;12(5):351-8. doi: 10.1590/S1413-35552008000500003.
https://doi.org/10.1590/S1413-3555200800...
found similar outcomes after 14 days in Wistar rats that had sustained cutaneous injuries and were treated with conventional LITUS at 1.5 MHz and administered at a SATA intensity of 30 mW/cm2. Emesen2020. Emsen IM. The effect of ultrasound on flap survival: An experimental study in rats. Burns. 2007 May;33(3):369-71. doi: 10.1016/j.burns.2006.08.007.
https://doi.org/10.1016/j.burns.2006.08....
applied pulsed ultrasound at 0.75 MHz and 3MHz at intensities of 0.1 W/cm2 and 0.18 W/cm2. They suggest that ultrasound therapy is useful in accelerating the inflammatory and early proliferative stages of repair.
In the current study, LITUS administered at a SATA intensity of 0.1 W/cm² was applied for 8 min each day for 14 days. A SATA intensity of 0.1 W/cm² was used because this reflects the SATA intensity that is available within physical therapists' ultrasound equipment, which differs from the more expensive equipment used in research into healing. Hence, we ensured that any potential use of such a therapeutic modality would be less costly and more accessible. In addition, a SATA intensity of 0.1 W/cm2appeared to accelerate the inflammatory processes and cellular proliferation2121. Young SR, Dyson M. Effect of therapeutic ultrasound on the healing of full-thickness excised skin lesions. Ultrasonics. 1990 May;28(3):175-80. PMID: 2339476.. Lower LITUS intensities, like those used in this study, reduce cellular damage by using pulsed acoustic energy, which induces fibroblast proliferation and promotes tissue repair in other types of injuries99. Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng. 2004;6:229-48. PMID: 15255769.,2222. Fontes-Pereira AJ, Teixeira RDC, Oliveira AJBD, Pontes RWF, Barros RSMD, Negrão JNC. The effect of low-intensity therapeutic ultrasound in induced fracture of rat tibiae. Acta Ortop Bras. 2013 Jan/Feb;21(1):18-22. doi: 10.1590/S1413-78522013000100003.
https://doi.org/10.1590/S1413-7852201300...
. The duration of the treatment was chosen to enable us to determine the effects of LITUS within a short timeframe.
The similarities between the BTG and BG with regard to the levels of inflammatory cell infiltration when the tissues were analyzed qualitatively and quantitatively using the histological changes in burn wound healing scoring system, concurs with the findings from the study by Piedade et al.2323. Piedade MC, Galhardo MS, Battlehner CN, Ferreira MA, Caldini EG, de Toledo OM. Effect of ultrasound therapy on the repair of gastrocnemius muscle injury in rats. Ultrasonics. 2008 Sep;48(5):403-11. PMID: 18384832.. These investigators analyzed the effects of LITUS (1 MHz pulsed ultrasound (50%) at 0.57 W/cm2 for 5 min) on lacerated muscle healing in rats, and they observed a reduction in the inflammatory infiltrate in the group that was treated immediately; however, 24 days later, both the BTG and the BG had developed similar inflammatory infiltrates. Freitas et al .1111. Freitas TP, Gomes M, Fraga DB, Freitas LS, Rezin GT, Santos PM, Silveira PC, Paula MM, Pinho RA, Streck EL. Effect of therapeutic pulsed ultrasound on lipoperoxidation and fibrogenesis in an animal model of wound healing. J Surg Res. 2010 Jun;161(1):168-71. PMID: 19577772. asserted that the purpose of using pulsed LITUS (0.4, 0.6, and 0.8 W/cm2) in wound healing is to accelerate the inflammatory phase, that is, the migration and proliferation of the fibroblasts that are fundamental to the healing process. The inflammatory response plays a fundamental role in the supply of growth factors and in the cytokine signaling that are responsible for cell and tissue movements and are important for the subsequent stages of wound repair and contraction2424. Huet E, Vallée B, Szul D, Verrecchia F, Mourah S, Jester JV, Hoang-Xuan T, Menashi S, Gabison EE. Extracellular matrix metalloproteinase inducer/CD147 promotes myofibroblast differentiation by inducing alpha-smooth muscle actin expression and collagen gel contraction: implications in tissue remodeling. FASEB J. 2008 Apr;22(4):1144-54. PMID: 17965264.,2525. Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007 Mar;127(3):514-25. PMID: 17299434..
The histological analysis of the skin's structure in the areas of hyperemia and injury stasis revealed the changes in the epidermis, and it had increased in thickness in the BTG compared with the BG. These observations are similar to those made by Bem et al.2626. Bem DMD, Maciel CD, Zuanon JA, Neto CB, Parizotto NA. Análise histológica em tecido epitelial sadio de ratos Wistar (in vivo) irradiados com diferentes intensidades do ultrassom. Rev Bras Fisioter. 2010 Mar/Apr;14(2):114-20. doi: 10.1590/S1413-35552010005000010.
https://doi.org/10.1590/S1413-3555201000...
who suggested that epidermal thickening had occurred because the cells within the basal layer of the epidermis had been stimulated to undergo mitosis. The basal layer contains keratinocytes that are essential to reepithelization, and distinct stem cells are present in the basal layer that migrate throughout the contour of the wound to fill in the injured area2727. Gantwerker EA, Hom DB. Skin: histology and physiology of wound healing. Clin Plast Surg. 2012 Jan;39(1):85-97. PMID: 22099852..
Ferreira et al .77. Ferreira AS, Barbieri CH, Mazzer N, Campos AD, Mendonça AC. Measurement of healing area using planimetry after applying low intensity ultrasound to the skin of rats. Rev Bras Fisioter. 2008 Sept/Oct;12(5):351-8. doi: 10.1590/S1413-35552008000500003.
https://doi.org/10.1590/S1413-3555200800...
applied LITUS for 10 min on alternate days, and treatment began immediately after the injuries during the acute inflammatory phase. Planimetric measurements enabled these investigators to verify that the animals treated with LITUS had smaller injuries compared with the group that did not receive treatment, and they concluded that LITUS stimulated healing after 14 days of treatment.
In contrast to this study, Cambier and Vanderstraeten88. Cambier DC, Vanderstraeten GG. Failure of therapeutic ultrasound in healing burn injuries. Burns. 1997 May;23(3):248-9. PMID: 9232286. evaluated the effect of pulsed LITUS at 3 MHz that was administered at a SATA intensity of 0.25 W/cm2, a loading cycle of 20%, and a frequency of 100 Hz. They applied LITUS to 20 female Fisher rats with third-degree burns in 5-min treatment sessions over the course of 6 weeks, but the histological data failed to reach statistical significance. The investigators concluded that LITUS had no effect on healing burns.
In the BTG animals, the centripetal proliferation of the basal layer was suggestive of an attempt at reepithelization. Reepithelization plays a major role in reorganizing the function of the epidermis, which is responsible for providing mechanical protection, local temperature regulation, and protection against external agents.
The collagen fibers in the skin from the BTG animals had an appearance that was comparable with that of the collagen fibers in the skin from the CG animals, and their collagen fibers were better organized than those of the BG animals. When skin sustains an injury, it requires collagen to repair and restore its anatomical structure and function. However, when there are considerable levels of disorganized collagen deposits within the injured area, the skin loses its normal morphological structure and its function is compromised, which leads to fibrosis2727. Gantwerker EA, Hom DB. Skin: histology and physiology of wound healing. Clin Plast Surg. 2012 Jan;39(1):85-97. PMID: 22099852.. There were no substantial differences between the BTG and BG with regard to vascularization, thereby indicating that angiogenesis, which is associated with an organism's response to the presence of ischemic tissues, that is, tissues that are growing rapidly and have increased rates of metabolic activity, was not accelerated by the LITUS protocol, which suggests that LITUS had no effect on the injuries.
Further studies are needed to broaden our knowledge about the effects of LITUS on people who have sustained burn injuries. Accordingly, we propose that other LITUS treatment protocols should be tested, including different treatment times and doses. Adopting non-invasive methods to monitor the entire healing process, including magnetic resonance imaging and quantitative ultrasound2828. Fontes-Pereira A, Matusin DP, Rosa P, Schanaider A, von Krüger MA, Pereira WCA. Ultrasound method applied to characterize healthy femoral diaphysis of Wistar rats in vivo. Braz J Med Biol Res. 2014 May;47(5):403-10. doi: 10.1590/1414-431X20143443.
https://doi.org/10.1590/1414-431X2014344...
, would enable in vivo observations of the effects of LITUS to be undertaken. It would also be interesting to determine and analyze the levels of oxidative stress, since it is present during the healing of large burn injuries2929. Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns. 2008 Feb;34(1):6-17. PMID: 17905515.. We suspect that LITUS accelerates the regeneration process mainly by enhancing the cellular permeability which allows the arriving of macrophages to the damaged area. Those macrophages will boost the inflammatory process, releasing the growth factors and starting the regenerative process as well as diminishing the oxidative stress. So this cascade of events reduces the systemic inflammation and nutritional deficit, thus controlling the treated rats' weight loss and the death risk1111. Freitas TP, Gomes M, Fraga DB, Freitas LS, Rezin GT, Santos PM, Silveira PC, Paula MM, Pinho RA, Streck EL. Effect of therapeutic pulsed ultrasound on lipoperoxidation and fibrogenesis in an animal model of wound healing. J Surg Res. 2010 Jun;161(1):168-71. PMID: 19577772..
Conclusions
The low-intensity therapeutic ultrasound protocol applied to the animals with third-degree burns in this study accelerated the formation of fibrin-leukocyte crusts and it reduced weight loss. After 14 days of treatment, there were no statistically significant differences between the BG and BTG animals with respect to the reepithelization, granulation, inflammation, and angiogenesis criteria. Thus, the findings from this study suggest that within the parameters used in this research, the effects of LITUS are more closely associated with reducing weight loss than accelerating wound healing, and that it is potentially useful as an additional low-cost tool for the treatment of burn injuries.
Acknowledgements
To Dr Sérgio H. Hirata and Dr Nilceo S. Michalany for applying the scoring system of the histological changes in burn wound healing to this work, and Prof. Jorge Lopes Rodrigues Júnior for constructing the burn template.
References
-
1Popescu FC, Busuioc CJ, Mogoşanu GD, Pop OT, Pârvănescu H, Lascăr I, Nicolae CI, Mogoantă L. Pericytes and myofibroblasts reaction in experimental thermal third degree skin burns. Rom J Morphol Embryol. 2011;52(3 Suppl):1011-7. PMID: 22119818.
-
2Mock C. WHO joins forces with International Society for Burn Injuries to confront global burden of burns. Inj Prev. 2007 Oct;13(5):303.
-
3Oliveira Fd, Bevilacqua LR, Anaruma CA, Boldrini Sde C, Liberti EA. Morphological changes in distant muscle fibers following thermal injury in Wistar rats. Acta Cir Bras. 2010 Nov/Dec;25(6):525-8. PMID: 21120285.
-
4Grishkevich VM. Ankle dorsiflexion postburn scar contractures: anatomy and reconstructive techniques. Burns. 2012 Sep;38(6):882-8. PMID: 22325850.
-
5Dematte MF, Gemperli R, Salles AG, Dolhnikoff M, Lanças T, Saldiva PH, Ferreira MC. Mechanical evaluation of the resistance and elastance of post-burn scars after topical treatment with tretinoin. Clinics (Sao Paulo). 2011;66(11):1949-54. PMID: 22086527.
-
6Medeiros ADC, Ramos AMDO, Dantas Filho AM, Azevedo RDCFD, Araújo FLFB. Tratamento tópico de queimaduras do dorso de ratos com ácido hialurônico. Acta Cir Bras. 1999 Oct/Dec;14(4):203-7. doi: 10.1590/S0102-86501999000400010.
» https://doi.org/10.1590/S0102-86501999000400010 -
7Ferreira AS, Barbieri CH, Mazzer N, Campos AD, Mendonça AC. Measurement of healing area using planimetry after applying low intensity ultrasound to the skin of rats. Rev Bras Fisioter. 2008 Sept/Oct;12(5):351-8. doi: 10.1590/S1413-35552008000500003.
» https://doi.org/10.1590/S1413-35552008000500003 -
8Cambier DC, Vanderstraeten GG. Failure of therapeutic ultrasound in healing burn injuries. Burns. 1997 May;23(3):248-9. PMID: 9232286.
-
9Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng. 2004;6:229-48. PMID: 15255769.
-
10Hogan RD, Burke KM, Franklin TD. The effect of ultrasound on microvascular hemodynamics in skeletal muscle: effects during ischemia. Microvasc Res. 1982 May;23(3):370-9. PMID: 7099026.
-
11Freitas TP, Gomes M, Fraga DB, Freitas LS, Rezin GT, Santos PM, Silveira PC, Paula MM, Pinho RA, Streck EL. Effect of therapeutic pulsed ultrasound on lipoperoxidation and fibrogenesis in an animal model of wound healing. J Surg Res. 2010 Jun;161(1):168-71. PMID: 19577772.
-
12Steiss JE, Adams CC. Effect of coat on rate of temperature increase in muscle during ultrasound treatment of dogs. Am J Vet Res. 1999 Jan;60(1):76-80. PMID: 19577772.
-
13National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals.Washington (DC): The National Academies Press; 2011.
-
14Walker HL, Mason AD Jr. A standard animal burn. J Trauma. 1968 Nov;8(6):1049-51. PMID: 5722120.
-
15Barbosa RCC, Guimarães SB, Vasconcelos PRCD, Chaves CR, Vasconcelos PRLD. Metabolic effects of glutamine in rats subjected to scald burn. Acta Cir Bras. 2003 Nov/Dec;18(6): 537-41. doi: 10.1590/S0102-86502003000600008.
» https://doi.org/10.1590/S0102-86502003000600008 -
16Galeano M, Altavilla D, Bitto A, Minutoli L, Calò M, Lo Cascio PL, Polito F, Giugliano G, Squadrito G, Mioni C, Giuliani D, Venuti FS, Squadrito F. Recombinant human erythropoietin improves angiogenesis and wound healing in experimental burn wounds. Crit Care Med. 2006 Apr;34(4):1139-46. PMID: 16484928.
-
17Wu X, Wolf SE, Walters TJ. Muscle contractile properties in severely burned rats. Burns. 2010 Sep;36(6):905-11. PMID: 20381255.
-
18de Oliveira BCC, de Oliveira F, Martini DT, Prisco CRD, da Silva Riguetti MM, Liberti EA, de Campos Boldrini S. The relative effects of severe burn injury and pre-and post-natal protein deprivation on mandibular condyle morphology. Histol Histopathol. 2010; 25(1): 45-54. PMID:19924640.
-
19Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004 Jan;9:283-9. PMID: 14766366.
-
20Emsen IM. The effect of ultrasound on flap survival: An experimental study in rats. Burns. 2007 May;33(3):369-71. doi: 10.1016/j.burns.2006.08.007.
» https://doi.org/10.1016/j.burns.2006.08.007 -
21Young SR, Dyson M. Effect of therapeutic ultrasound on the healing of full-thickness excised skin lesions. Ultrasonics. 1990 May;28(3):175-80. PMID: 2339476.
-
22Fontes-Pereira AJ, Teixeira RDC, Oliveira AJBD, Pontes RWF, Barros RSMD, Negrão JNC. The effect of low-intensity therapeutic ultrasound in induced fracture of rat tibiae. Acta Ortop Bras. 2013 Jan/Feb;21(1):18-22. doi: 10.1590/S1413-78522013000100003.
» https://doi.org/10.1590/S1413-78522013000100003 -
23Piedade MC, Galhardo MS, Battlehner CN, Ferreira MA, Caldini EG, de Toledo OM. Effect of ultrasound therapy on the repair of gastrocnemius muscle injury in rats. Ultrasonics. 2008 Sep;48(5):403-11. PMID: 18384832.
-
24Huet E, Vallée B, Szul D, Verrecchia F, Mourah S, Jester JV, Hoang-Xuan T, Menashi S, Gabison EE. Extracellular matrix metalloproteinase inducer/CD147 promotes myofibroblast differentiation by inducing alpha-smooth muscle actin expression and collagen gel contraction: implications in tissue remodeling. FASEB J. 2008 Apr;22(4):1144-54. PMID: 17965264.
-
25Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007 Mar;127(3):514-25. PMID: 17299434.
-
26Bem DMD, Maciel CD, Zuanon JA, Neto CB, Parizotto NA. Análise histológica em tecido epitelial sadio de ratos Wistar (in vivo) irradiados com diferentes intensidades do ultrassom. Rev Bras Fisioter. 2010 Mar/Apr;14(2):114-20. doi: 10.1590/S1413-35552010005000010.
» https://doi.org/10.1590/S1413-35552010005000010 -
27Gantwerker EA, Hom DB. Skin: histology and physiology of wound healing. Clin Plast Surg. 2012 Jan;39(1):85-97. PMID: 22099852.
-
28Fontes-Pereira A, Matusin DP, Rosa P, Schanaider A, von Krüger MA, Pereira WCA. Ultrasound method applied to characterize healthy femoral diaphysis of Wistar rats in vivo. Braz J Med Biol Res. 2014 May;47(5):403-10. doi: 10.1590/1414-431X20143443.
» https://doi.org/10.1590/1414-431X20143443 -
29Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns. 2008 Feb;34(1):6-17. PMID: 17905515.
-
Financial sources: CNPq, CAPES and FAPERJ
-
1
Research performed at Laboratory of Morphophysiopathology Applied to Health/CCBS, Universidade do Estado do Pará (UEPA), Brazil.
Publication Dates
-
Publication in this collection
Jan 2016
History
-
Received
20 Sept 2015 -
Reviewed
15 Nov 2015 -
Accepted
17 Dec 2015