Abstract
PURPOSE:
To evaluated the potential antioxidant agent Legalon
(r)
SIL (silibinin-C-2',3-bis(hydrogensuccinat)) in the skeletal muscle of rats.
METHODS:
IRI was achieved via tourniquet application in Wistar-albino rats. Experimental groups were chosen as (i) sham control, (ii) IRI (3+2 h), (iii) IRI and Legalon
(r)
SIL-50 (50 mg/kg/i.p.), (iv) IRI and Legalon
(r)
SIL-100 (100 mg/kg/i.p.), and (v) IRI and Legalon
(r)
SIL-200 (200 mg/kg/ i.p.). Muscle viability (evaluated by triphenyltetrazolium chloride dye method), malondialdehyde, superoxide dismutase, catalase, and glutathione peroxidase were assessed in muscle samples using a spectrophotometer.
RESULTS:
Although viability of the injured limb non-significantly declined in the IRI group, administration of Legalon
(r)
SIL did not prevent injury. However, dramatic increase observed in malondialdehyde levels in the IRI group was prohibited by Legalon
(r)
SIL in a statistically significant manner. In comparison with the sham-control group, IRI and Legalon
(r)
SIL administration did not cause any significant alterations in the levels of superoxide dismutase, catalase, and glutathione peroxidase.
CONCLUSION:
Although Legalon
(r)
SIL was not sufficient to prevent muscle injury in terms of viability, it is found to be an effective option to reduce reactive oxygen species-induced cell injury.
Ischemia; Reperfusion Injury; Oxidative Stress; Muscle, Skeletal; Rats
Introduction
Ischemia-reperfusion injury (IRI) of skeletal muscle appears in various types of clinical conditions such as thrombolytic therapy, aortic cross-clamping during repair of abdominal aortic aneurysms, replantation, free tissue transfer, composite tissue allotransplantation, time-consuming reconstructive operations, tourniquet application, and crush injury11. Khanna A, Cowled PA, Fitridge RA. Nitric oxide and skeletal muscle reperfusion injury: current controversies (research review). J Surg Res. 2005 Sep;128(1):98-107. PMID: 15961106.,22. Wang WZ, Baynosa RC, Zamboni WA. Update on ischemia-reperfusion injury for the plastic surgeon: 2011. Plast Reconstr Surg. 2011 Dec;128(6):685e-92e. PMID: 22094770.. Among several mechanisms suggested to be responsible for IRI, reactive oxygen species (ROS)-induced cell injury has been indicated to have a prominent role in the etiology of this type of injury33. Cotran RS, Kumar V, Robbins SL. Cellular injury and cellular death. In: Schoen FJ, eds. Robbins-pathologic basis of disease. Philadelphia: W.B. Saunders; 1994. p.1-34.. In harmony, ROS generation in response to reperfusion of ischemic skeletal muscle has been shown to account for the etiology of IRI44. Lindsay T, Romaschin A, Walker PM. Free radical mediated damage in skeletal muscle. Microcirc Endothelium Lymphatics. 1989 Jun-Oct;5(3-5):157-70. PMID: 2700374.. In this context, a rational approach has been the use of agents with antioxidant potential in order to protect the tissue from oxidative stress-induced damage. Several antioxidant substances, thus, have been used to mitigate the injury, though with no established clinical benefit to date22. Wang WZ, Baynosa RC, Zamboni WA. Update on ischemia-reperfusion injury for the plastic surgeon: 2011. Plast Reconstr Surg. 2011 Dec;128(6):685e-92e. PMID: 22094770.,55. Gute DC, Ishida T, Yarimizu K, Korthuis RJ. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Mol Cell Biochem. 1998 Feb;179(1-2):169-87. PMID: 9543359.. For this reason, there is still a need to discover novel molecules to be used in IRI.
Silymarin, an extract of Silybum marianum (milk thistle), has been applied in order to alleviate suffering from various liver diseases66. Luper S. A review of plants used in the treatment of liver disease: part 1. Altern Med Rev. 1998 Dec;3(6):410-21. PMID: 9855566.. Constituting approximately 50% of silymarin77. Zholobenko A, Modriansky M. Silymarin and its constituents in cardiac preconditioning. Fitoterapia. 2014 Sep;97:122-32. PMID: 24879900., silibinin has been shown to have anti-oxidant and anti-inflammatory properties66. Luper S. A review of plants used in the treatment of liver disease: part 1. Altern Med Rev. 1998 Dec;3(6):410-21. PMID: 9855566.,88. de Groot H, Rauen U. Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam Clin Pharmacol. 1998;12(3):249-55. PMID: 9646056.. Indeed, silymarin and silibinin have been found to exert beneficial effects in various IRI models established in different tissues, including the liver, the brain, the kidneys, the heart, and the intestine99. Valenzuela A, Barría T, Guerra R, Garrido A. Inhibitory effect of the flavonoid silymarin on the erythrocyte hemolysis induced by phenylhydrazine. Biochem Biophys Res Commun. 1985 Jan 31;126(2):712-8. PMID: 3977887.
10. Valenzuela A, Guerra R. Protective effect of the flavonoid silybin dihemisuccinate on the toxicity of phenylhydrazine on rat liver. FEBS Lett. 1985 Feb 25;181(2):291-4. PMID: 3972111.
11. Wu CG, Chamuleau RA, Bosch KS, Frederiks WM. Protective effect of silymarin on rat liver injury induced by ischemia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64(5):259-63. PMID: 8287122.
12. Oliveira CP, Lopasso FP, Laurindo FR, Leitão RM, Laudanna AA. Protection against liver ischemia-reperfusion injury in rats by silymarin or verapamil. Transplant Proc. 2001 Sep;33(6):3010-4. PMID: 11543828.
13. Rao PR, Viswanath RK. Cardioprotective activity of silymarin in ischemia-reperfusion-induced myocardial infarction in albino rats. Exp Clin Cardiol. 2007 Winter;12(4):179-87. PMID: 18651002.
14. Ligeret H, Brault A, Vallerand D, Haddad Y, Haddad PS. Antioxidant and mitochondrial protective effects of silibinin in cold preservation-warm reperfusion liver injury. J Ethnopharmacol. 2008 Feb 12;115(3):507-14. PMID: 18061382.
15. Senturk H, Kabay S, Bayramoglu G, Ozden H, Yaylak F, Yucel M, Olgun EG, Kutlu A. Silymarin attenuates the renal ischemia/reperfusion injury-induced morphological changes in the rat kidney. World J Urol. 2008 Aug;26(4):401-7. PMID: 18408933.
16. Hou YC, Liou KT, Chern CM, Wang YH, Liao JF, Chang S, Chou YH, Shen YC. Preventive effect of silymarin in cerebral ischemia-reperfusion-induced brain injury in rats possibly through impairing NF-?B and STAT-1 activation. Phytomedicine. 2010 Oct;17(12):963-73. PMID: 20833521.-1717. Demir M, Amanvermez R, Kamali Polat A, Karabiçak I, Cinar H, Kesicioglu T, Polat C. The effect of silymarin on mesenteric ischemia-reperfusion injury. Med Princ Pract. 2014;23(2):140-4. PMID: 24356575.. As there had been no prior investigations exploring the potential effect of silibinin on IRI of skeletal muscle, we previously made an attempt to investigate its effects in a rodent model1818. Ergün Y, Kurutas EB, Atalay F, Alici T. Effects of silibinin and ethanol on skeletal muscle ischemia-reperfusion injury. Acta Cir Bras. 2013 Mar;28(3):179-84. PMID: 23503858.. Since silibinin is not water-soluble, we used ethanol to dissolve the substance. Unexpectedly, however, ethanol, as a vehicle, showed protective effects on IRI, thereby preventing a proper testing of the effects of silibinin due to masking1818. Ergün Y, Kurutas EB, Atalay F, Alici T. Effects of silibinin and ethanol on skeletal muscle ischemia-reperfusion injury. Acta Cir Bras. 2013 Mar;28(3):179-84. PMID: 23503858.. For this reason, we, in the present study, selected a water soluble form of silibinin, Legalon(r) SIL (silibinin-C-2',3-bis (hydrogensuccinat)), to properly explore the exact effect in a tourniquet-induced IRI rat model.
Methods
The experiments were conducted in adherence with the European Communities Council Directive (86/609/EEC) and the Council for International Organization of Medical Sciences-CIOMS. It was approved by the Kahramanmaraş Sütçü İmam University Animal Ethics Committee (2012/5-4).
Animal preparation and IRI model
Adult male Wistar-albino rats were kept in a room with a temperature of 22±2°C and a humidity of 50-60% in a 12 h day/night cycle, and fed with laboratory chow and water ad libitum. Intraperitoneal urethane (1000 mg/kg/IP, Sigma-Aldrich) was administered before the experiment to attain general anesthesia throughout the procedure. Ischaemia was achieved by the application of an elastic rubber band as high as possible around the right thigh of the rat, and ischaemic period was selected to be 3 h1919. Zhang B, Knight KR, Dowsing B, Guida E, Phan LH, Hickey MJ, Morrison WA, Stewart AG. Timing of administration of dexamethasone or the nitric oxide synthase inhibitor, nitro-L-arginine methyl ester, is critical for effective treatment of ischaemia-reperfusion injury to rat skeletal muscle. Clin Sci (Lond). 1997 Aug;93(2):167-74. PMID: 9301432.. After the rats had been fixed on a pad, a 60 W household light bulb was placed above the animals and powered on in order to keep body temperatures constant at 36±1°C, measured using a rectal thermometer. Heads of the rats were shielded by aluminum foil to avoid overheating and dehydration. After the tourniquet had been released, the limb was allowed to perfuse for 2 h. Ischemia and reperfusion of the limbs were confirmed by observation of changes in the color of the paws. At the end of the perfusion period, muscle samples were collected directly from right and left gastrocnemius muscles in succession. The animals were then killed by cervical dislocation under general anesthesia.
Experimental protocols
The rats were randomly allocated to one of the five groups (n=6): sham control group, where rats solely received urethane anesthesia (Group I), IRI group, where rats were subject to tourniquet-based IRI (Group II), and Legalon(r) SIL-50, Legalon(r) SIL-100, Legalon(r) SIL-200 groups, where rats received Legalon(r) SIL (50, 100, and 200 mg/kg/i.p., respectively) in addition to IRI (Group III, IV, V). Legalon(r) SIL (Madaus GmbH, Cologne, Germany (Rottapharm/Madaus Group)) was dissolved in saline (12.5 ml/kg) and administered 30 min prior to the reperfusion period. The doses were selected according to the literature2020. Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Invest. 2002;22:51-65..
Tissue viability for gastrocnemius muscle by the triphenyltetrazolium chloride method
At the end of the ischaemia-reperfusion period, gastrocnemius muscles were excised from both sides of the hind limbs and were rinsed in ice-cold Ringer's lactate solution. Muscle samples were dissected free of blood vessels, nerves, and fascia. The samples were stored at -80°C until the assessment day. Viability was then evaluated by the triphenyltetrazolium chloride method, which assesses mitochondrial oxidative enzyme activity and is an indicator of irreversible cellular infarction2121. Belkin M, Brown RD, Wright JG, LaMorte WW, Hobson RW 2nd. A new quantitative spectrophotometric assay of ischemia-reperfusion injury in skeletal muscle. Am J Surg. 1988 Aug;156(2):83-6. PMID: 3400818.. Briefly, gastrocnemius muscles were weighed and homogenized in 3 ml of 0.25 M sucrose. Additional sucrose was then added to make a 20 percent homogenate by weight. The homogenate was filtered through a fine stainless steel mesh to remove any remaining fragments of fascia. Protein content of the homogenate was determined by the method of Lowry et al.2222. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265-75. PMID: 14907713.. A 1 ml aliquot of the homogenate was then mixed with 1 ml of 0.15 percent triphenyltetrazolium chloride (Sigma, St.Louis, MO) in 0.033 M phosphate buffer (dibasic sodium phosphate, pH 7.4). Reactions were performed in triplicate. The reaction mixture was stirred on a Vortex mixer and incubated at 39°C in a shaking water bath for 1 h. After the reaction had been stopped, the mixture was diluted with 4 ml of acetone, centrifuged for 10 min at 1.500 rpm, and absorbance of clear red formazan dye was measured at 485 nm in a spectrophotometer (Shimadzu, Japan). Absorbance per mg protein was calculated for each limb, and the activity of each ischaemic limb was compared with the contralateral control limb to express the ischaemic limb activity as a percentage of that of the control limb.
Biochemical measurements
Tissues were weighed, blotted on filter paper, and homogenized with three volumes of ice-cold 1.15% KCI. The activities of antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX), and the level of malondialdehyde (MDA) were measured in the supernatants obtained from centrifugation at 14.000 rpm.
MDA levels, reflecting lipid peroxidation rate in tissue samples, were measured according to procedure of Ohkawa et al.2323. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351-8. PMID: 36810.. The reaction mixture contained 0.1 ml tissue sample, 0.2 ml of 8.1% sodium dodecyl sulphate (SDS), 1.5 ml of 20% acetic acid, and 1.5 ml of 0.8 % aqueous solution of TBA. The pH of the mixture was then adjusted 3.5, the volume was made up to 4.0 ml with distilled water, and a 5.0 ml mixture of n-butanol and pyridine (15:1,v/v) was added. The mixture was shaken vigorously. After centrifugation at 4.000 rpm for 10 minutes, the absorbance of the organic layer was measured at 532 nm. The protein concentration of tissue samples was measured by a Spectronic-UV 120 spectrophotometer using the method of Lowry et al.2222. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265-75. PMID: 14907713.. MDA levels in tissue samples were expressed as nmol/mg protein.
SOD activity was measured according to the method described by Fridovich22. Wang WZ, Baynosa RC, Zamboni WA. Update on ischemia-reperfusion injury for the plastic surgeon: 2011. Plast Reconstr Surg. 2011 Dec;128(6):685e-92e. PMID: 22094770.. This method employs xanthine and xanthine oxidase to generate superoxide radicals which react with p-iodonitrotetrazlium violet (INT) to form a red formazan dye, which was measured at 505 nm. Assay medium consisted of the 0.01 M phosphate buffer, 3-cyclohexilamino-1-propanesulfonicacid (CAPS) buffer solution (50 mM CAPS, 0.94 mM EDTA, saturated NaOH) with pH 10.2, solution of substrate (0.05 nM xanthine, 0.025 mM INT), and 80 UL xanthine oxidase. SOD activity in tissue samples was expressed as U/mg protein.
CAT activities were determined by measuring the decrease in hydrogen peroxide concentration at 230 nm using the method of Beutler2525. Beutler E. Red cell metabolism. 2ed. New York: Grune and Stratton Company; 1975.. Assay medium consisted of 1 M Tris HCI, 5 mM Na2EDTA buffer solution (pH 8.0), 1 M phosphate buffer solution (pH 7.0), and 10 mM H2O2. CAT activities in tissue samples were expressed as U/mg protein.
GPX in tissue samples was assayed in a 1-ml system containing potassium phosphate buffer (0.1 M, pH 7.0), NADPH (0.2 mM), glutathione reductase (1 i.u), GSH (4 mM), EDTA (4 mM), sodium azide (4 mM), and appropriate amount of enzyme glutathione peroxidase (0.02 ml). The reaction mixture was incubated at 37°C for 10 minutes after 10 µL of 10 mM had been added to start the reaction. No t-butyl hydroperoxide was added to the blank cuvette. The rate of reaction was measured at 37°C by following the decrease in absorbance at 340 nm using a spectrophotometer2626. Awasthi YC, Beutler E, Srivastava SK. Purification and properties of human erythrocyte glutathione peroxidase. J Biol Chem. 1975 Jul 10;250(13):5144-9. PMID: 807573.. Activity in tissue samples was expressed in units per milligram of protein.
Statistics
Data were expressed as means±SEM. When normal distribution and homogeneity of variances were lacking in groups, non-parametric tests, i.e., Kruskal-Wallis test and Mann-Whitney U test, were performed. However, data were, generally, analyzed by one way analysis of variance (ANOVA), with the significance of individual comparisons assessed by Bonferroni test. P values less than 0.05 were accepted as significant.
Results
The effect of Legalon(r) SIL on tissue viability in skeletal muscle
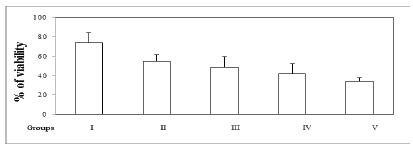
The principle of triphenyltetrazolium chloride method in exploring IRI is that viable mitochondrial enzymes of the redox cytochrome chain convert a tetrazolium compound to a coloured dye, formazan. Formation of this dye is thus used to measure mitochondrial oxidase enzyme activity which reflects the viability of muscle cells2121. Belkin M, Brown RD, Wright JG, LaMorte WW, Hobson RW 2nd. A new quantitative spectrophotometric assay of ischemia-reperfusion injury in skeletal muscle. Am J Surg. 1988 Aug;156(2):83-6. PMID: 3400818.. The viability of the ischaemic-reperfused limb, reported as a percentage of the value obtained for the normal contralateral muscle, can be used for an accurate comparison among experimental groups. In the present study, as the Shapiro-Wilk test of normality had indicated normal distribution of data regarding all groups (P=0.664, P=0.963, P=0.723, P=0.376, P=0.606 for Groups I through V, respectively) and the test of homogeneity of variances had showed a P value above 0.05 (P=0.190), statistics were calculated using parametric tests. Statistical analysis with respect to all groups indicated no difference (ANOVA, F=2.342, P=0.088). However, viabilities of ischaemic limbs were 74±10% and 55±7% in sham control and IRI groups, respectively (Figure 1). Although statistically not significant, a decline in viability was seen in the latter (Figure 1). Furthermore, Legalon(r) SIL was not able to prevent this decline caused by IRI, since values obtained from Legalon(r) SIL-50, Legalon(r) SIL-100, and Legalon(r) SIL-200 groups were similar to that from the IRI group (Figure 1).
- Viability of ischaemic hindlimb as a percentage of the contralateral control muscle. Group I: sham control, group II: IRI (3+2 h), group III: IRI and Legalon(r) SIL-50 (50 mg/kg/IP), group IV: IRI and Legalon(r) SIL-100 (100 mg/kg/IP), group V: IRI and Legalon(r) SIL-200 (200 mg/kg/IP) (n=6 per group). Data were expressed as means±SEM and one way analysis variance (ANOVA) with Bonferroni test was performed, significance was accepted P<0.05, * vs. group I.
The effect of Legalon(r) SIL on MDA, CAT, SOD and GPX in skeletal muscle
Since the Shapiro-Wilk test had indicated lack of normal distribution for MDA data from groups III and V (P=0.015, P=0.001), further statistical analyses were performed using non-parametric tests. Kruskal-Wallis test indicated statistically significant differences between the experimental groups (X22. Wang WZ, Baynosa RC, Zamboni WA. Update on ischemia-reperfusion injury for the plastic surgeon: 2011. Plast Reconstr Surg. 2011 Dec;128(6):685e-92e. PMID: 22094770.=10.017, P=0.040). Post-hoc comparisons showed that MDA level, an indicator of oxidative stress, increased significantly in the IRI group in comparison to the sham control group (Mann-Whitney U test, Z=-2.242, P=0.025), indicating oxidative-stress induced injury within the muscle (Table 1). Although there was no gradual dose-response relationship, Legalon(r) SIL, with three distinct doses applied, prevented the observed increase in MDA levels (Table 1). Regarding SOD, data from all groups were normally distributed according to the Shapiro-Wilk test (P=0.350, P=0.747, P=0.969, P=0.180, P=0.655). Since the test of homogeneity of variances had indicated a P value of 0.073, ANOVA was performed and no differences were detected between the groups (F=2.592, P=0.061, Table 1). P values for the Shapiro-Wilk test and the test of homogeneity of variances regarding CAT data were >0.05 and 0.700, respectively. ANOVA had hence been applied and demonstrated no difference between groups (F=1.173, P=0.347, Table 1). Although P values were above 0.05 for Groups II-V, it was 0.014 for Group I regarding GPX data. Therefore, non-parametric Kruskal-Wallis test had been selected as the appropriate method, which then indicated no difference between the groups (X22. Wang WZ, Baynosa RC, Zamboni WA. Update on ischemia-reperfusion injury for the plastic surgeon: 2011. Plast Reconstr Surg. 2011 Dec;128(6):685e-92e. PMID: 22094770.=1.742, P=0.783, Table 1).
Discussion
The present study showed that Legalon(r) SIL was insufficient in preventing cellular infarction, which was assessed by triphenyltetrazolium chloride method. However, it was found to have the capacity to prevent ROS-induced lipid peroxidation in a skeletal muscle IRI model of rat.
Although a statistically significant difference could not be detected between the sham control and IRI groups, tissue viability of the latter (54±7%) seemed to be less than that of the former (74±10%). This may suggest that ischaemia-reperfusion insult brings about a slight but considerable degree of cellular infarction in skeletal muscle of rats. However, none of the doses of Legalon(r) SIL were able to prevent cellular infarction. Although not statistically significant, 200 mg/kg dose of the drug was observed to indeed worsen the injury (34±4%). This may imply that Legalon(r) SIL, particularly at the highest dose, may facilitate cellular infarction in the current experimental setup. In fact, in a previous study performed in our laboratory, administration of 200 mg/kg silibinin had not been tolerated by the mice (i.e. almost all died probably due to lung edema), and the dose was reduced to an effective but not harmful level (50 mg/kg)1818. Ergün Y, Kurutas EB, Atalay F, Alici T. Effects of silibinin and ethanol on skeletal muscle ischemia-reperfusion injury. Acta Cir Bras. 2013 Mar;28(3):179-84. PMID: 23503858.. Nevertheless, the probability of the highest dose of Legalon(r) SIL being toxic is less than it simply being ineffective against cellular infarction in the present study. According to the Preclinical Safety Data of the manufacturer, the drug has proved to be virtually non-toxic and the LD50 levels after intravenous injection had been classified as > 1000 mg/kg in both male and female rats and mice. These results are in harmony with the toxicity profile of silymarin, for which LD50 regarding acute toxicity after intravenous infusion has been detected to be 385 mg/kg in rats2020. Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Invest. 2002;22:51-65.. In our previous study in mice, ethanol, the compound used to solve silibinin, may have contributed to the toxicity observed. Finally, as a medicinal product registered for mushroom poisoning in Germany, Legalon(r) SIL comprises chemicals other than silibinin, such that 528.5 mg silibinine-C-2',3-bis(hydrogensuccinate) disodium salt is equivalent to 350 mg silibinin, rendering active constituent exposure of rats to less than 200 mg/kg.
In the present study MDA, a marker of ROS-induced lipid peroxidation, increased in a statistically significant manner in IRI group in comparison with the sham control group. In contrast to the relative ineffectiveness of Legalon(r) SIL on cellular infarction of rat skeletal muscle, the increase observed in MDA levels was profoundly prevented by the drug. Microvascular dysfunction, edema formation, muscle dysfunction, and cellular infarction are the different manifestations of IRI and are associated with various etiological factors, including ROS generation, elaboration of proinflammatory mediators, infiltration of leukocytes, Ca++ overload, phospholipid peroxidation and depletion, impaired nitric oxide metabolism, and reduced ATP production2727. Rubin BB, Romaschin A, Walker PM, Gute DC, Korthuis RJ. Mechanisms of postischemic injury in skeletal muscle: intervention strategies. J Appl Physiol (1985). 1996 Feb;80(2):369-87. PMID: 8929572.. Accordingly, ROS-induced lipid peroxidation (represented by MDA levels) can be accepted to be one of the important causes of cellular infarction (determined by the use of triphenyltetrazolium chloride method). As an antioxidant agent, Legalon(r) SIL was only able to negate one of the etiological factors (ROS-induced lipid peroxidation) responsible for cellular infarction and it is obvious that this effect was not enough to exert an overall reduction in muscle necrosis. Alternatively, the lack of protective effect of Legalon(r) SIL regarding cellular infarction would be due to the relatively late administration time of the drug. According to the data obtained by Belkin et al.2121. Belkin M, Brown RD, Wright JG, LaMorte WW, Hobson RW 2nd. A new quantitative spectrophotometric assay of ischemia-reperfusion injury in skeletal muscle. Am J Surg. 1988 Aug;156(2):83-6. PMID: 3400818., triphenyltetrazolium chloride method is an indicator of irreversible injury and 3 h of ischemic period is a threshold for the damage to appear. We, in the present study, applied the drug 2.5 hours after the start of ischemia and therefore it is possible that disposition of the drug to the site of injury may occur after the critical time regarding irreversibility. Obviously the lack of different time points regarding drug administration is the weakness of the present study. In support of our results, several lines of evidence of distinct origin indicate the beneficial effects of silibinin in IRI1111. Wu CG, Chamuleau RA, Bosch KS, Frederiks WM. Protective effect of silymarin on rat liver injury induced by ischemia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64(5):259-63. PMID: 8287122.
12. Oliveira CP, Lopasso FP, Laurindo FR, Leitão RM, Laudanna AA. Protection against liver ischemia-reperfusion injury in rats by silymarin or verapamil. Transplant Proc. 2001 Sep;33(6):3010-4. PMID: 11543828.
13. Rao PR, Viswanath RK. Cardioprotective activity of silymarin in ischemia-reperfusion-induced myocardial infarction in albino rats. Exp Clin Cardiol. 2007 Winter;12(4):179-87. PMID: 18651002.
14. Ligeret H, Brault A, Vallerand D, Haddad Y, Haddad PS. Antioxidant and mitochondrial protective effects of silibinin in cold preservation-warm reperfusion liver injury. J Ethnopharmacol. 2008 Feb 12;115(3):507-14. PMID: 18061382.
15. Senturk H, Kabay S, Bayramoglu G, Ozden H, Yaylak F, Yucel M, Olgun EG, Kutlu A. Silymarin attenuates the renal ischemia/reperfusion injury-induced morphological changes in the rat kidney. World J Urol. 2008 Aug;26(4):401-7. PMID: 18408933.-1616. Hou YC, Liou KT, Chern CM, Wang YH, Liao JF, Chang S, Chou YH, Shen YC. Preventive effect of silymarin in cerebral ischemia-reperfusion-induced brain injury in rats possibly through impairing NF-?B and STAT-1 activation. Phytomedicine. 2010 Oct;17(12):963-73. PMID: 20833521.. Anti-oxidant and anti-inflammatory properties of silibinin have indeed been accepted to account for its beneficial effects66. Luper S. A review of plants used in the treatment of liver disease: part 1. Altern Med Rev. 1998 Dec;3(6):410-21. PMID: 9855566.,88. de Groot H, Rauen U. Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam Clin Pharmacol. 1998;12(3):249-55. PMID: 9646056..
Despite the elevated levels of MDA in the IRI group, it was interesting to detect the lack of alterations in the activities of main endogenous antioxidant enzymes SOD, CAT and GPX, which stimulate the breakdown of superoxide anion to less toxic products33. Cotran RS, Kumar V, Robbins SL. Cellular injury and cellular death. In: Schoen FJ, eds. Robbins-pathologic basis of disease. Philadelphia: W.B. Saunders; 1994. p.1-34.,2828. Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35-97. PMID: 4371571.,2929. Ogawa T, Mimura Y. Antioxidant effect of zinc on acute renal failure induced by ischemia-reperfusion injury in rats. Am J Nephrol. 1999;19(5):609-14. PMID: 10575193.. A loss in enzyme activity due to a decrease in the amount of intact cells caused by tissue injury is generally the expected outcome. However, based on the principle of enzyme-substrate kinetics, over-produced ROS may stimulate enzyme activities in the remaining intact cells, counterbalancing a reduction that would otherwise be detected. Alternatively, the potential compensation mechanisms resulting from up-regulation of relevant genes to prevent a decrease in enzyme activity can be another rational explanation. The main factor specifying the direction of enzyme activity may be the amount of ROS produced. Perhaps above a critical level of ROS concentration, the first scenario takes place and enzyme activity decreases due to dramatic decrease in intact cells. On the other hand, below this critical level, the opposite scenario occurs and enzyme activity increases. In the present study, ROS concentration might have been restricted in a level around above-mentioned critical level so that opposite mechanisms had balanced each other and no alteration was seen.
Overall, the only noteworthy effect extracted from the present results with respect to Legalon(r) SIL is the antioxidant effect of this compound. The proposed mechanisms for the antioxidant effect of silymarin/silibinin derived from studies conducted in tissues other than skeletal muscle are as follows: (i) scavenging ROS, (ii) increasing the content of glutathione, (iii) stimulation of the expression of SOD, (iv) prevention of the effects of tumour necrosis factor-α (TNF-α), which markedly increases lipid peroxidation, (v) inhibition of neutrophil migration, and (vi) inhibition of CYP450, some of which generate ROS2020. Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Invest. 2002;22:51-65.. In one study, silymarin has been shown to reduce the levels of TNF-α in parallel with those of heat shock protein (HSP)-70, SOD, and thiobarbituric acid-reactive substance (TBARS) in a rat model of mesenteric IRI model, indicating the role of TNF-α in the antioxidant effect of silymarin1717. Demir M, Amanvermez R, Kamali Polat A, Karabiçak I, Cinar H, Kesicioglu T, Polat C. The effect of silymarin on mesenteric ischemia-reperfusion injury. Med Princ Pract. 2014;23(2):140-4. PMID: 24356575.. Similarly, silymarin prescribed to patients undergoing coronary artery bypass grafting surgery decreased both TNF-α and MDA levels in comparison with control patients3030. Altaei T. Protective effect of silymarin during coronary artery bypass grafting surgery. Exp Clin Cardiol. 2012 Spring;17(1):34-8. PMID: 23204899.. Further investigations are needed to determine with certainty which of these mechanisms are responsible for IRI in rat skeletal muscle.
Conclusions
Therapeutic effect of Legalon(r) SIL was shown to be limited due to its inefficiency on tissue viability, it may be utilized as an adjuvant therapy rather than used solely. The advantage of Legalon(r) SIL over other antioxidant substances is that it is currently used in several countries for the treatment of mushroom poisoning to prevent liver damage and is under clinical trial in the US for this indication. This is important because time-consuming animal and some part of human studies on safety have been completed which may enable Legalon(r) SIL to, more rapidly, become available for any new indication. However, further studies are needed to prove the efficacy of Legalon(r) SIL in the skeletal muscle IRI in combination with other beneficial substances.
Acknowledgement
To Madaus GmbH, Cologne, Germany (Rottapharm/Madaus Group) for sending us Legalon(r) SIL as a gift.
References
-
1Khanna A, Cowled PA, Fitridge RA. Nitric oxide and skeletal muscle reperfusion injury: current controversies (research review). J Surg Res. 2005 Sep;128(1):98-107. PMID: 15961106.
-
2Wang WZ, Baynosa RC, Zamboni WA. Update on ischemia-reperfusion injury for the plastic surgeon: 2011. Plast Reconstr Surg. 2011 Dec;128(6):685e-92e. PMID: 22094770.
-
3Cotran RS, Kumar V, Robbins SL. Cellular injury and cellular death. In: Schoen FJ, eds. Robbins-pathologic basis of disease. Philadelphia: W.B. Saunders; 1994. p.1-34.
-
4Lindsay T, Romaschin A, Walker PM. Free radical mediated damage in skeletal muscle. Microcirc Endothelium Lymphatics. 1989 Jun-Oct;5(3-5):157-70. PMID: 2700374.
-
5Gute DC, Ishida T, Yarimizu K, Korthuis RJ. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Mol Cell Biochem. 1998 Feb;179(1-2):169-87. PMID: 9543359.
-
6Luper S. A review of plants used in the treatment of liver disease: part 1. Altern Med Rev. 1998 Dec;3(6):410-21. PMID: 9855566.
-
7Zholobenko A, Modriansky M. Silymarin and its constituents in cardiac preconditioning. Fitoterapia. 2014 Sep;97:122-32. PMID: 24879900.
-
8de Groot H, Rauen U. Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam Clin Pharmacol. 1998;12(3):249-55. PMID: 9646056.
-
9Valenzuela A, Barría T, Guerra R, Garrido A. Inhibitory effect of the flavonoid silymarin on the erythrocyte hemolysis induced by phenylhydrazine. Biochem Biophys Res Commun. 1985 Jan 31;126(2):712-8. PMID: 3977887.
-
10Valenzuela A, Guerra R. Protective effect of the flavonoid silybin dihemisuccinate on the toxicity of phenylhydrazine on rat liver. FEBS Lett. 1985 Feb 25;181(2):291-4. PMID: 3972111.
-
11Wu CG, Chamuleau RA, Bosch KS, Frederiks WM. Protective effect of silymarin on rat liver injury induced by ischemia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64(5):259-63. PMID: 8287122.
-
12Oliveira CP, Lopasso FP, Laurindo FR, Leitão RM, Laudanna AA. Protection against liver ischemia-reperfusion injury in rats by silymarin or verapamil. Transplant Proc. 2001 Sep;33(6):3010-4. PMID: 11543828.
-
13Rao PR, Viswanath RK. Cardioprotective activity of silymarin in ischemia-reperfusion-induced myocardial infarction in albino rats. Exp Clin Cardiol. 2007 Winter;12(4):179-87. PMID: 18651002.
-
14Ligeret H, Brault A, Vallerand D, Haddad Y, Haddad PS. Antioxidant and mitochondrial protective effects of silibinin in cold preservation-warm reperfusion liver injury. J Ethnopharmacol. 2008 Feb 12;115(3):507-14. PMID: 18061382.
-
15Senturk H, Kabay S, Bayramoglu G, Ozden H, Yaylak F, Yucel M, Olgun EG, Kutlu A. Silymarin attenuates the renal ischemia/reperfusion injury-induced morphological changes in the rat kidney. World J Urol. 2008 Aug;26(4):401-7. PMID: 18408933.
-
16Hou YC, Liou KT, Chern CM, Wang YH, Liao JF, Chang S, Chou YH, Shen YC. Preventive effect of silymarin in cerebral ischemia-reperfusion-induced brain injury in rats possibly through impairing NF-?B and STAT-1 activation. Phytomedicine. 2010 Oct;17(12):963-73. PMID: 20833521.
-
17Demir M, Amanvermez R, Kamali Polat A, Karabiçak I, Cinar H, Kesicioglu T, Polat C. The effect of silymarin on mesenteric ischemia-reperfusion injury. Med Princ Pract. 2014;23(2):140-4. PMID: 24356575.
-
18Ergün Y, Kurutas EB, Atalay F, Alici T. Effects of silibinin and ethanol on skeletal muscle ischemia-reperfusion injury. Acta Cir Bras. 2013 Mar;28(3):179-84. PMID: 23503858.
-
19Zhang B, Knight KR, Dowsing B, Guida E, Phan LH, Hickey MJ, Morrison WA, Stewart AG. Timing of administration of dexamethasone or the nitric oxide synthase inhibitor, nitro-L-arginine methyl ester, is critical for effective treatment of ischaemia-reperfusion injury to rat skeletal muscle. Clin Sci (Lond). 1997 Aug;93(2):167-74. PMID: 9301432.
-
20Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Invest. 2002;22:51-65.
-
21Belkin M, Brown RD, Wright JG, LaMorte WW, Hobson RW 2nd. A new quantitative spectrophotometric assay of ischemia-reperfusion injury in skeletal muscle. Am J Surg. 1988 Aug;156(2):83-6. PMID: 3400818.
-
22Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265-75. PMID: 14907713.
-
23Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351-8. PMID: 36810.
-
24Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875-80. PMID: 210504.
-
25Beutler E. Red cell metabolism. 2ed. New York: Grune and Stratton Company; 1975.
-
26Awasthi YC, Beutler E, Srivastava SK. Purification and properties of human erythrocyte glutathione peroxidase. J Biol Chem. 1975 Jul 10;250(13):5144-9. PMID: 807573.
-
27Rubin BB, Romaschin A, Walker PM, Gute DC, Korthuis RJ. Mechanisms of postischemic injury in skeletal muscle: intervention strategies. J Appl Physiol (1985). 1996 Feb;80(2):369-87. PMID: 8929572.
-
28Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35-97. PMID: 4371571.
-
29Ogawa T, Mimura Y. Antioxidant effect of zinc on acute renal failure induced by ischemia-reperfusion injury in rats. Am J Nephrol. 1999;19(5):609-14. PMID: 10575193.
-
30Altaei T. Protective effect of silymarin during coronary artery bypass grafting surgery. Exp Clin Cardiol. 2012 Spring;17(1):34-8. PMID: 23204899.
-
Financial source: Scientific Research Projects, Kahramanmaraş Sütçü İmam University
-
1
Research performed at Departments of Pharmacology and Biochemistry, School of Medicine, Kahramanmaraş Sütçü İmam University, Turkey.
Publication Dates
-
Publication in this collection
Apr 2016
History
-
Received
15 Dec 2015 -
Reviewed
18 Feb 2016 -
Accepted
12 Mar 2016