ABSTRACT
PURPOSE :
To compare ileal anastomoses in the immediate postoperative healing period after meloxicam use.
METHODS:
Forty two male Wistar rats were randomly divided into two groups of 21, COX and control group. To COX meloxicam in combination with morphine was given in 3 days period. Control group received only morphine during the same period. Each group was divided into three sub-groups of 7, which were euthanized at 5, 10, and 21 days postoperatively. Comparison was based in histological evaluation of collagen type I and III using sirius red, immunohistochemical through vascular endothelial growth factor and matrix metalloproteinase-9.
RESULTS:
Healing process in scheduled periods did not show significant differences (p>0.05) between the COX and control groups during any of the periods.
CONCLUSION:
The use of meloxicam in the postoperative period following ileal anastomosis did not affect healing.
Key words:
Wound Healing. Intestine; Small. Anti-Inflammatory Agents. Rats.
Introduction
The effects of non-steroid anti-inflammatory drugs on intestinal healing are still poorly understood and controversial. Some authors have reported that non-steroid anti-inflammatory drugs promote intestinal healing, while others have shown their deleterious effects on intestinal healing by increasing the rate of anastomotic dehiscence which consequently leads to a higher mortality rate11. Tognini JRF, Fagundes DJ, Novo NF, Juliano Y. Biomechanical and morphological study in rats' abdominal wall healing under meloxicam action. Acta Cir Bras. 2000;15(3). doi: 10.1590/S0102-86502000000300003.
2. Cahill RA, Sheehan KM, Scanlon RW, Murray FE, Kay EW, Redmond HP. Effects of a selective cyclo-oxygenase 2 inhibitor on colonic anastomotic and skin wound integrity. Br J Surg. 2004;91(12):1613-8. PMID: 15505871.
3. Lima AF, Lourenço LG, Matos D, Rodrigues CF. Effect of the celexoxib in microscopic changes of the esophageal mucosal of rats induced by esofagojejunostomy. Rev Col Bras Cir. 2014 May-Jun;41(3):193-7. PMID: 25140651.-44. Costa FL, Tiussi LD, Nascimento MS, Corrêa AC, Yasojima EY, Pires CA. Diclofenac topical gel in excisional wounds maintain heal quality and reduce phlogistic signals. Acta Cir Bras . 2014 May;29(5):328-33. PMID: 24863321.. Also herbal medicines from Amazon region in Brasil had demonstrated some influence in wound healing55. Araújo LA, Mrué F, Neves RA, Alves MM, Silva-Júnior NJ, Silva MS, Melo-Reis PR. Effects of topical treatment with euphorbia tirucalli latex on the survival and intestinal adhesions in rats with experimental peritonitis. Arq Bras Cir Dig. 2015 Dec;28(4):243-6. doi: 10.1590/S0102-6720201500040006.
6. Castilho TJ, Campos AC, Mello EV. Effect of Omega-3 fatty acid in the healing process of colonic anastomosis in rats. Arq Bras Cir Dig. 2015 Dec;28(4):258-61. doi: 10.1590/S0102-6720201500040010.
7. Yasojima EY, Teixeira RK, Houat AP, Costa FL, Yamaki VN, Feitosa-Junior DJ, Silva CA, Brito MV. Copaiba oil influences ventral hernia repair with Vicryl(r) mesh? Arq Bras Cir Dig. 2015 Jul-Sep;28(3):186-9. doi: 10.1590/S0102-67202015000300010.
8. Silva CE1, Santos OJ2, Ribas-Filho JM1, Tabushi FI1, Kume MH1, Jukonis LB1, Cella IF1. Effect of Carapa guianensis Aublet (Andiroba) and Orbignya phalerata (Babassu) in colonic healing in rats. Rev Col Bras Cir. 2015 Dec;42(6):399-406. doi: 10.1590/0100-69912015006009.
9. Vizzotto Junior AO, Campos AC, Mello EV, Castilho TJ. Influence of preoperative supplementation of omega-3 fatty acid in the healing of colonic anastomoses in malnourished rats receiving paclitaxel. Rev Col Bras Cir. 2015 Mar-Apr;42(2):116-23. doi: 10.1590/0100-69912015002009.
10. Comelli Júnior E, Skinovski J, Sigwalt MF, Branco AB, Luz SR, Baulé Cde P. Rupture point analysis of intestinal anastomotic healing in rats under the action of pure Copaíba (Copaifera Iangsdorfii) oil. Acta Cir Bras . 2010 Aug;25(4):362-7. PMID: 20676496.-1111. Passarini Junior JR1, Gaspi FO, Neves LM, Esquisatto MA, Santos GM, Mendonça FA. Application of Jatropha curcas L. seed oil (Euphorbiaceae) and microcurrent on the healing of experimental wounds in Wistar rats. Acta Cir Bras . 2012 Jul;27(7):441-7. PMID: 22760827..
The process of gastrointestinal healing, which is comprised by the stages of inflammation, deposition, and maturation of collagen, resembles the process in other wounds but is influenced by the peritoneum, which reacts to different trauma with peritonitis and/or formation of adhesions, as well as by the multiple layers of the gastric or intestinal wall1212. Berenguer B, Alarcón de la Lastra C, Moreno FJ, Martín MJ. Chronic gastric ulcer healing in rats subjected to selective and non-selective cyclooxygenase-2 inhibitors. Eur J Pharmacol. 2002 3;442(1-2):125-35. PMID: 12020690.,1313. Rieder F, Brenmoehl J, Leeb S, Schölmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007;56(1):130-9. doi: 10.1136/gut.2006.090456.. The gain in strength during healing from gastric and intestinal sutures under normal conditions is faster than in sutures placed in skin and tendons; the production of collagen from smooth muscle fibers, observed in the gastrointestinal tract, does not occur in the dermis, and regulation of collagen synthesis in the gastrointestinal tract is different from that observed in the skin1414. Kiyama T, Onda M, Tokunaga A, Efron DT, Barbul A. Effect of matrix metalloproteinase inhibition on colonic anastomotic healing in rats. J Gastrointest Surg. 2001;5(3):303-11. doi: 10.1016/S1091-255X(01)80052-4.. The inflammatory phase is intense in the intestine and longer in the large intestine than in the small intestine1515. Goldman R. Growth factors and chronic wound healing: past, present, and future. Adv Skin Wound Care. 2004;17(1):24-35. PMID: 14752324..
Collagen is the main component of the extracellular matrix of the tissue. It is structured into a dense and dynamic web, as a result of its constant deposition and reabsorption. Glandular tissues in the digestive tract seem to concentrate more collagen compared to non-glandular in wounds evaluated up to 40 days after surgery. The amount of collagen changes in the gut during the period after anastomosis, especially in the peri-anastomotic region1616. Pantaroto M, Lopes Filho GJ, Pinto CA, Antico Filho A. Comparative study of collagen deposition in the colon wall of patients operated for sigmoid diverticular disease. Acta Cir Bras . 2015 Oct;30(10):715-9. doi: 10.1590/S0102-865020150100000010.. This variation is the result of the interaction between its synthesis, uptake, and degradation in the scar tissue1717. Inan A, Koca C, Sen M. Effects of diclofenac sodium on bursting pressures of anastomoses and hydroxyproline contents of perianastomotic tissues in a laboratory study. Int J Surg. 2006;4(4):222-7. PMID: 17462355.. Three subtypes of collagen (I, III, and V) have been identified during the healing process in the small intestine. Collagen type I and III reach more significant proportions, and the relationship between them has greater importance in the study of anastomotic healing1818. Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9-18. PMID: 17276196.
19. Lee JL, Mukhtar H, Bickers DR, Kopelovich L, Athar M. Cyclooxygenases in the skin: pharmacological and toxicological implications. Toxicol Appl Pharmacol. 2003;192(3):294-306. PMID: 14575647.-2020. Wilgus TA, Vodovotz Y, Vittadini E, Clubbs EA, Oberyszyn TM. Reduction of scar formation in full-thickness wounds with topical celecoxib treatment. Wound Repair Regen. 2003;11(1):25-34. PMID: 12581424..
Degradation of the collagen in the extracellular matrix is obtained by a family of metalloproteinases which separate intact fibers into smaller fragments that can be phagocytosed and subsequently degraded by lysosomal enzymes into their constituent amino acids1818. Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9-18. PMID: 17276196.,2121. Clark TP. The clinical pharmacology of cyclooxygenase-2-selective and dual inhibitors. Vet Clin North Am Small Anim Pract. 2006;36(5):1061-85. PMID: 16984827..
Chai et al.2222. Chai J, Jones MK, Tarnawski AS. Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. FASEB J. 2004;18(11):1264-6. PMID: 15180964. demonstrated that both cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) are important in regulating angiogenesis, and that non-steroidal anti-inflammatory drugs which are selective or preferential for COX-2 inhibit the effects of angiogenesis in a similar manner to non-selective or non-preferential anti-inflammatory drugs.
Other studies have also suggested that both COX-1 and COX-2 are important in the formation of collagen during wound healing, and that their deficiency may lead to alterations in the development of granulation tissue and re-epithelialization by inhibiting the proliferation of keratinocytes1212. Berenguer B, Alarcón de la Lastra C, Moreno FJ, Martín MJ. Chronic gastric ulcer healing in rats subjected to selective and non-selective cyclooxygenase-2 inhibitors. Eur J Pharmacol. 2002 3;442(1-2):125-35. PMID: 12020690.,1919. Lee JL, Mukhtar H, Bickers DR, Kopelovich L, Athar M. Cyclooxygenases in the skin: pharmacological and toxicological implications. Toxicol Appl Pharmacol. 2003;192(3):294-306. PMID: 14575647.,2020. Wilgus TA, Vodovotz Y, Vittadini E, Clubbs EA, Oberyszyn TM. Reduction of scar formation in full-thickness wounds with topical celecoxib treatment. Wound Repair Regen. 2003;11(1):25-34. PMID: 12581424.,2323. Zhang YP, Hao XQ, Zhang LM, Tian YT. Enhanced cyclooxygenase-2 activity leads to intestinal dysmotility following hemorrhagic shock. Acta Cir Bras . 2015 Dec;30(12):838-43. doi: 10.1590/S0102-865020150120000008..
Meloxicam is a modern derivative of oxicam which develops a preferential inhibitory activity on COX-2 in the biosynthetic prostaglandin cascade, and is commercially available in oral or injectable formulation. Intravenous or subcutaneous pre-operative administration of meloxicam in dogs at a dose 0.2 mg/kg has been shown to provide effective postoperative analgesia for up to 24 hours after application2121. Clark TP. The clinical pharmacology of cyclooxygenase-2-selective and dual inhibitors. Vet Clin North Am Small Anim Pract. 2006;36(5):1061-85. PMID: 16984827..
Angiogenesis is an essential process for the formation of new blood vessels from pre-existing capillaries2222. Chai J, Jones MK, Tarnawski AS. Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. FASEB J. 2004;18(11):1264-6. PMID: 15180964.,2424. Campos ACL, Borges-Branco A, Groth AK. Cicatrização de feridas. Arq Bras Cir Dig. 2007;20(1):51-8. doi: 10.1590/S0102-67202007000100010.. It is an important event in various physiological phenomena such as embryonic development, chronic inflammation, and tissue repair. Considering these conditions, angiogenesis is highly regulated; it begins over short periods (days), and then is completely inhibited. Angiogenesis involves the proliferation and migration of endothelial cells, remodeling of the extracellular matrix, tubular formation, recruitment of adjacent structures to support the neovessels, anastomoses, and basal membrane development2424. Campos ACL, Borges-Branco A, Groth AK. Cicatrização de feridas. Arq Bras Cir Dig. 2007;20(1):51-8. doi: 10.1590/S0102-67202007000100010.,2525. Ramsden JD. Angiogenesis in the thyroid gland. J Endocrinol. 2000 Sep;166(3):475-80. PMID: 11029748.. Vascular endothelial growth factor (VEGF) is a mitogen specific to endothelial cells which is produced by various cell types such as keratinocytes, fibroblasts, and macrophages1818. Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9-18. PMID: 17276196.,2525. Ramsden JD. Angiogenesis in the thyroid gland. J Endocrinol. 2000 Sep;166(3):475-80. PMID: 11029748.,2626. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581-611. PMID: 15294883.. It is also known to increase vascular permeability and to be involved in the occurrence and progression of the inflammatory process. It is a chemical mediator of the healing process and its receptors are classified as VEGFR-1, VEGFR-2, and VEGFR-32727. Kaka U, Hui Cheng C, Meng GY, Fakurazi S, Kaka A, Behan AA, Ebrahimi M. Electroencephalographic changes associated with antinociceptive actions of lidocaine, ketamine, meloxicam, and morphine administration in minimally anaesthetized dogs. Biomed Res Int. 2015;2015:305367. doi: 10.1155/2015/305367.
28. Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential taget for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368-80. PMID: 12409337.
29. Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005 10;23(5):1011-27. PMID: 15585754.
30. Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006 30;39(5):469-78. PMID: 17002866.-3131. Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005 15;65(3):550-63. PMID: 15664381..
Angiogenesis cannot be discussed without citing the importance of metalloproteinases in cellular events such as cell migration, invasion, proliferation, and apoptosis. They are responsible for the regulation of processes such as morphogenesis, angiogenesis, healing, and the degradation of the extracellular matrix2222. Chai J, Jones MK, Tarnawski AS. Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. FASEB J. 2004;18(11):1264-6. PMID: 15180964.,3232. Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9(2):267-85. PMID: 15963249.. Endothelial cells synthesize several metalloproteinases3333. Marques e Silva S, Jerônimo MS, Silva-Pereira Id, Tavares AH, Bocca AL, Sousa JB. Effects of metoclopramide on the expression of metalloproteinases and interleukins in left colonic anastomoses. An experimental study. Acta Cir Bras . 2015 Nov;30(11):762-9. doi: 10.1590/S0102-865020150110000007.; among these, MMP-9 plays an important role in angiogenesis3232. Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9(2):267-85. PMID: 15963249..
The objective of this study was to evaluate the influence of meloxicam on the ileal enteroanastomosis cicatrization by performing histological, as well as immunohistochemical assessment.
Methods
The study was approved in advance by the Positivo University Research Ethics Committee and registered under process 016/2008. It was conducted in the Experimental Surgery Laboratory at the vivarium of Universidade Positivo, Curitiba PR, Brazil.
Forty two male Wistar rats (Rattus norvegicus albinus) were used with an average weight of 250 g. Environmental conditions were controlled electronically, with a temperature of 22oC and a 12-hour light-dark cycle. The animals were identified and kept individually in metabolic stainless steel cages. During a three-day acclimatization period before the start of the experiment, the rats had drinking water and rat chow ad libitum. After the acclimatization period they were weighed and randomly divided into two groups, each containing 21 animals, according to the type of treatment: control group (CTL) without anti-inflammatory and COX group (COX) with meloxicam. Each group was divided into three subgroups of 7 animals each, which were euthanized at 5, 10, and 21 days postoperatively, periods which took into account angiogenesis, the deposition and stabilization of collagen2424. Campos ACL, Borges-Branco A, Groth AK. Cicatrização de feridas. Arq Bras Cir Dig. 2007;20(1):51-8. doi: 10.1590/S0102-67202007000100010..
Surgical and anesthetic procedure
The animals were clinically evaluated to observe the processes of dehydration, wounds, and physical condition. They received general anesthesia via intramuscular injection of butorphanol tartrate followed by maintenance inhalation of 100% isofluorane combined with 100% oxygen3434. Felzemburgh VA, Cettolin QC, Machado KM, Campos JH. Comparison between the anesthetic induction times in the first and second surgery in rabbits. Acta Cir Bras . 2012 Jul;27(7):482-6. PMID: 22760834.. Each anesthetized animal was positioned on the operating table in dorsal decubitus. Next they were shaved and the abdomen received antiseptic treatment using chlorhexidine gluconate 2%. Subsequently, the surgical fields were established and a 4 cm incision was made along the midline of the abdomen to access the abdominal cavity. After inspection of the cavity, the distal segment of the ileum was located and sectioned around its circumference (enterotomy). Next, terminoterminal anastomosis was performed using one suture line, totaling 8 simple separated stitches using monofilament nylon 6-03535. Costa MA, Campos AC, Coelho JC, de Barros AM, Matsumoto HM. Oral glutamine and the healing of colonic anastomoses in rats. JPEN J Parenter Enteral Nutr. 2003;27(3):182-5. PMID: 12757111..
Clinical and experimental handling
Immediately after the surgery, the COX animals received 0.5 mg/kg of subcutaneous meloxicam, which was maintained every 24 hours for three days. Four hours after the surgery, all animals received 2 mg/kg of subcutaneous morphine3636. Guimarães Alves IP, Montoro Nicácio G, Diniz MS, Alves Rocha TL, Prada Kanashiro G, Navarro Cassu R. Analgesic comparison of systemic lidocaine, morphine or lidocaine plus morphine infusion in dogs undergoing fracture repair. Acta Cir Bras . 2014 Apr;29(4):245-51. PMID: 24760025., which was maintained every 12 hours for three days. During the postoperative period, the animals were clinically evaluated to identify pain and signs of infection. No antibiotics was used, and no postoperative complications were seen.
At 5, 10, and 21 days after surgery the animals were euthanized using a lethal dose of anesthetic, corresponding to four times the value of the induction dose. Immediately after verification of death, each rat underwent median laparotomy and careful inspection of the cavity. After lysis of adhesions, the anastomoses were sectioned with margins of 3 cm on each side to produce a 6 cm segment of intestine. This sample was stretched over a piece of filter paper and stored in a flask containing formalin 10% for subsequent pathological and immunohistochemical assays2424. Campos ACL, Borges-Branco A, Groth AK. Cicatrização de feridas. Arq Bras Cir Dig. 2007;20(1):51-8. doi: 10.1590/S0102-67202007000100010.,3535. Costa MA, Campos AC, Coelho JC, de Barros AM, Matsumoto HM. Oral glutamine and the healing of colonic anastomoses in rats. JPEN J Parenter Enteral Nutr. 2003;27(3):182-5. PMID: 12757111..
Histological and immunohistochemical evaluation
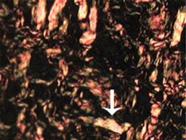
The samples were prepared for histological evaluation and five samples were obtained from the same anastomosis through a single 4µm thick microtome slice The slides were stained using three different methods: hematoxylin-eosin, Masson trichrome, and picrosirius red F3BA (Figure 1). The remaining two slides were used for immunohistochemical (Figure 2) marking with antibodies to metalloproteinase-9 and VEGFr (recipient of the vascular endothelial growth factor).
Meloxicam average. Ileum anastomosis, 21 days after surgery. Type-II collagen. Picrosirius-red. Obj. 40x84x63mm (300 x 300DPI).
Meloxicam average. Ileum anastomosis, 21 days after surgery. A) Gray colour of cells with VEGFr receptors. B) Brown colour of metallopreoteinase antibody. Obj. 40x80x28mm (300 x 300 DPI)
The assays in the area of the anastomosis were completed using data obtained from the mean quantitative expression of collagen in five sample areas from ileal wall of each rat. Five microscopic fields representative of the anastomotic area with total x200 increase were captured using the computerized Image Pro Plus(r) system version 6.0 and an Olympus(r) BX-40 microscope with a light polarizer and a Sony(r) camera. The type I and III collagen was identified and quantified by the program and expressed as a percentage3535. Costa MA, Campos AC, Coelho JC, de Barros AM, Matsumoto HM. Oral glutamine and the healing of colonic anastomoses in rats. JPEN J Parenter Enteral Nutr. 2003;27(3):182-5. PMID: 12757111. (Figures 1 and 2).
To quantify the expressions of metalloproteinase-9 and VEGFr-1, the samples were treated with an Advance kit (Dako(r)) at the Experimental Pathology Laboratory of the Pontifical Catholic University of Paraná, Curitiba, PR, Brasil. The positive controls proving the efficiency of the antibody were samples of human placenta for metalloproteinase-9 and an injured artery for VEGFr. The sections were dewaxed after blocking the endogenous peroxidase with a solution of hydrogen peroxide and methanol 5%. The antigenic recovery was done in Immuno Retriever (Dako(r)) device. The area of the section was demarcated using a Dako hydrophobic pen (Dako(r)). Aliquots of the antibodies to metalloproteinase-9 (MMP-9) and VEGFr were pipetted onto the samples. The sections were washed and bathed for 15 minutes in pH 7.3 tris-buffered saline. Next, the sections received the Advance link (Dako(r)). After 30 minutes they were rinsed in the buffered solution and treated with the Advance enzyme (Dako(r)). After 30 minutes, the sections were washed in the buffer, dried, and exposed to chromogen DAB at 1:1 dilution in buffer solution until a brown color was visible. The sections were then immediately washed in distilled water to interrupt the precipitation reaction. The sections were then counterstained with Harris hematoxylin for five minutes, and subsequently washed in running water. Dehydration with absolute ethyl alcohol was followed by diaphonization in xylene.
Three microscopic fields representative of the anastomotic area with a total x200 increase were captured using the computerized Image Pro Plus(r) system version 6.0 (Media Cybernetics, California, USA) and an Olympus(r) BX-51 microscope with a light polarizer. This system allowed quantification of the area occupied by each complex type, in other words, the expression of MMP-9 and VEGF-1 in the anastomotic scar3535. Costa MA, Campos AC, Coelho JC, de Barros AM, Matsumoto HM. Oral glutamine and the healing of colonic anastomoses in rats. JPEN J Parenter Enteral Nutr. 2003;27(3):182-5. PMID: 12757111..
Statistical analysis
For statistical analysis the nonparametric Mann-Whitney test was used. The level of significance adopted was 5% (α=0.05). The calculations were carried out using the statistical software Graph Pad Prism Version 3.0 for Windows (San Diego, California, USA). The data obtained by quantifying the elements were compared between the two groups at days 5, 10, and 21 after surgery.
Results
This study demonstrated that the use of meloxicam during the postoperative period after ileal anastomoses in Wistar rats did not significantly affect (p>0.05) the time of collagen fiber synthesis by activated fibroblasts. Immunohistochemical assessment of MMP-9 and vascular endothelial growth factor also demonstrated that meloxicam did not have significantly impact (p>0.05) in angiogenesis and fibroblastic phase of the repair process.
According to the data obtained, no differences were observed (p>0.05) between the control group (CTL) and the meloxicam group (COX) during the proliferative phase of healing in the ileum anastomosis.
When comparing the results of the evaluations throughout the entire post-operative period in relation to the behavior of type I and III collagen fibers, there was no significant difference (p>0.05) between the CTL and COX groups.
Evaluation of the samples on days 5, 10, and 21 post-procedure in both groups showed no significant differences (p>0.05) in the repair process(Tables 1 and 2).
Averages and standard deviation of the sums related to areas occupied by the fibers of collagen type I and III of the five fields captured in the anastomotic scar \r\n84x34mm (300 x 300 DPI). 84x34mm (300 x 300 DPI)
Averages and standard deviation of the sums related to areas occupied by metalloproteinases 9 and cells with receptors for VEGF, respectively, of the five fields captured in the anastomotic scar 77x27mm (300 x 300 DPI).
There was no significant difference (p>0.05) in the assessment of the elements that represent the proliferative phase of wound healing with meloxicam and those who did not receive this drug.
In this study, according to the histological and immunohistochemical assays used to assess the composition of collagen in the scar and angiogenesis factors, preferential inhibition of COX-2 by meloxicam did not affect healing.
Discussion
The participation of arachidonate derivatives is evident from the beginning of the repair process, when thromboxanes signal platelets, promote vasoconstriction, and act as chemotactic gradients in the extracellular matrix; this is followed by the overt involvement of prostaglandins in maintaining the chemotactic gradient, as inhibition of prostaglandins can affect related processes3737. Ikeuchi D, Onodera H, Aung T, Kan S, Kawamoto K, Imamura M, Maetani S. Correlation of tensile strength with bursting pressure in the evaluation of intestinal anastomosis. Dig Surg. 1999;16(6):478-85. doi: 10.1590/S0102-86502013000600008.. This study demonstrates that the use of meloxicam did not have significantly impact (p>0.05) at the time of synthesis of collagen fibers by the activated fibroblasts. They were preserved by the matrix through the chemotactic gradient, which is largely the result of prostaglandins derived from macrophages, and of course by the action of cicloxigenases. In order for these activated fibroblasts to synthesize collagen fibers, this must occur alongside angiogenesis, which is promoted by growth factors, low oxygen tension in the tissue, and metalloproteinases which participate in vessel channeling. Through the immunohistochemical assessment of MMP-9 and vascular endothelial growth factor, this study also showed that meloxicam did not have significant impact (p>0.05) in angiogenesis and fibroplasia phases of healing in the ileal anastomosis.
Evidence of neovascularization between the second and third days after enterectomies was cited by Jiborn et al. in 1980, when these authors also confirmed that in the first 12 hours fibroblasts reached the damaged region and began to multiply, accounting for the total production of intestinal collagen. That interference by anti-inflammatory drugs used in the immediate postoperative period may alter this scenario. Comparison between the two groups in this study demonstrated that meloxicam had no influence on the ileal anastomoses in rats.
According to Minossi et al.3939. Minossi JG, Leite CVS, Naresse LE, Rodrigues MAM, Curi PR, Kobayasi S. Ação do diclofenaco de sódio em anastomoses realizadas no intestino delgado de ratos. Acta Cir Bras . 1998;13(1):37-43. doi: 10.1590/S0102-86501998000100006., meloxicam could lead to delayed intestinal healing by two mechanisms: first, by inhibiting the formation of prostaglandins, and consequently slowing cell regeneration and revascularization of the scar. The second mechanism occurs through a direct process of tissue injury at the mitochondrial level of the enterocytes, permitting an increase in the inflammatory process through bacterial proliferation and translocation. Therefore, we produced a study in which was evaluated the intestinal healing process by quantification and qualification of the collagen and by the immunohistochemistry of factors related to neovascularization. This study model showed that selective or preferential inhibition of prostaglandin synthesis by meloxicam did not affect the repair process after intestinal anastomosis, when the behavior of the type I and type III collagen fibers were assessed, nor did it impact the expression of MMP-9 and endothelial cells with receptors for VEGF. It is possible that inflammatory modulation and patient comfort during the immediate postoperative period justifies the results we obtained and confirms those obtained in the studies by Enberg et al.3838. Enberg TB, Braun LD, Kuzma AB. Gastrointestinal perforation in five dogs associated with the administration of meloxicam. J Vet Emer Crit Care. 2006;16:34-43. doi: 10.1111/j.1476-4431.2005.00157.x..
When comparing the results during all postoperative periods with regard to the behavior of the collagen I and III, no difference was seen (p>0.05) in the CTL and COX groups, demonstrating that the proliferative phase of wound healing is not affected by meloxicam to the point of altering the synthesis and maturation of these fibers. Similar results were obtained by Tognini et al.11. Tognini JRF, Fagundes DJ, Novo NF, Juliano Y. Biomechanical and morphological study in rats' abdominal wall healing under meloxicam action. Acta Cir Bras. 2000;15(3). doi: 10.1590/S0102-86502000000300003..
Non-steroid anti-inflammatory drugs may diminish the second wave of chemical mediators, which are byproducts of the breakdown of arachidonic acid and impact the healing process3434. Felzemburgh VA, Cettolin QC, Machado KM, Campos JH. Comparison between the anesthetic induction times in the first and second surgery in rabbits. Acta Cir Bras . 2012 Jul;27(7):482-6. PMID: 22760834.,4040. Greca FH, Noronha L, Bendhack M, Feres A, Soccol A, Duda JR. Use of small intestine submucosa as ureteral allograft in pigs. Int Braz J Urol. 2004;30(4):327-35. PMID: 15679971.. The impact of meloxicam on the inflammatory stage in this study was not significant, perhaps because it is preferential to the inhibition of cyclooxygenase-2, which is believed to be inductive in tissue injuries and at the same time does not inhibit the cicloxigenases, behaving only like a modulating drug1212. Berenguer B, Alarcón de la Lastra C, Moreno FJ, Martín MJ. Chronic gastric ulcer healing in rats subjected to selective and non-selective cyclooxygenase-2 inhibitors. Eur J Pharmacol. 2002 3;442(1-2):125-35. PMID: 12020690.,3838. Enberg TB, Braun LD, Kuzma AB. Gastrointestinal perforation in five dogs associated with the administration of meloxicam. J Vet Emer Crit Care. 2006;16:34-43. doi: 10.1111/j.1476-4431.2005.00157.x.,4141. Deneuche AJ, Dufayet C, Goby L, Fayolle P, Desbois C. Analgesic comparison of meloxicam or ketoprofen for orthopedic surgery in dogs. Vet Surg. 2004;33(6):650-60. PMID: 15659022.,4242. Barbuto RC, Araujo ID, Bonomi DO, Tafuri LS, Calvão Neto A, Malinowski R3, Bardin VS3, Leite MD4, Duarte IG5. Use of the amniotic membrane to cover the peritoneal cavity in the reconstruction of the abdominal wall with polypropylene mesh in rats. Rev Col Bras Cir. 2015 Jan-Feb;42(1):49-55. doi: 10.1590/0100-69912015001010..
Chai et al.2222. Chai J, Jones MK, Tarnawski AS. Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. FASEB J. 2004;18(11):1264-6. PMID: 15180964. have shown that selective and preferential inhibitors for COX-2 inhibit the effects of angiogenesis. Other studies have also suggested that both COX-1 and COX-2 are important in the formation of collagen during wound healing, and that their deficiency may lead to alterations in the development of granulation tissue and re-epithelialization by inhibiting the proliferation of keratinocytes1212. Berenguer B, Alarcón de la Lastra C, Moreno FJ, Martín MJ. Chronic gastric ulcer healing in rats subjected to selective and non-selective cyclooxygenase-2 inhibitors. Eur J Pharmacol. 2002 3;442(1-2):125-35. PMID: 12020690.,1818. Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9-18. PMID: 17276196.,1919. Lee JL, Mukhtar H, Bickers DR, Kopelovich L, Athar M. Cyclooxygenases in the skin: pharmacological and toxicological implications. Toxicol Appl Pharmacol. 2003;192(3):294-306. PMID: 14575647.. According to the histological and immunochemical assessments used in this study to evaluate the composition of collagen in the scar and factors that participate in angiogenesis, the preferential COX-2 inhibitor meloxicam did not demonstrate any changes in healing when the two groups were compared.
While Clark2121. Clark TP. The clinical pharmacology of cyclooxygenase-2-selective and dual inhibitors. Vet Clin North Am Small Anim Pract. 2006;36(5):1061-85. PMID: 16984827. reported reduced byproducts of the degradation of arachidonic acid and negative impacts on healing resulting from the use of non-steroid anti-inflammatory drugs, the groups compared in this study did not show significant differences in behavior and did not confirm this damage.
The metalloproteinases collaborate in inactivating the endothelial cells with receptors for VEGF as a way of keeping vascular proliferation in balance. There is homeostasis between the heightened presence of metalloproteinases and the activation of endothelial cells with receptors for VEGF. It is synthesized by macrophages and the MMP-9 comes from neutrophils; in other words, both are of inflammatory origin and released in response to inflammatory mediators, which may be reduced in the presence of anti-inflammatory substances3131. Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005 15;65(3):550-63. PMID: 15664381.,3232. Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9(2):267-85. PMID: 15963249.. The group which used meloxicam in this study was able to modulate inflammation without altering angiogenesis in ileal anastomosis in rats.
Conclusion
The use of meloxicam in the postoperative period following ileal anastomosis did not affect healing.
References
-
1Tognini JRF, Fagundes DJ, Novo NF, Juliano Y. Biomechanical and morphological study in rats' abdominal wall healing under meloxicam action. Acta Cir Bras. 2000;15(3). doi: 10.1590/S0102-86502000000300003.
-
2Cahill RA, Sheehan KM, Scanlon RW, Murray FE, Kay EW, Redmond HP. Effects of a selective cyclo-oxygenase 2 inhibitor on colonic anastomotic and skin wound integrity. Br J Surg. 2004;91(12):1613-8. PMID: 15505871.
-
3Lima AF, Lourenço LG, Matos D, Rodrigues CF. Effect of the celexoxib in microscopic changes of the esophageal mucosal of rats induced by esofagojejunostomy. Rev Col Bras Cir. 2014 May-Jun;41(3):193-7. PMID: 25140651.
-
4Costa FL, Tiussi LD, Nascimento MS, Corrêa AC, Yasojima EY, Pires CA. Diclofenac topical gel in excisional wounds maintain heal quality and reduce phlogistic signals. Acta Cir Bras . 2014 May;29(5):328-33. PMID: 24863321.
-
5Araújo LA, Mrué F, Neves RA, Alves MM, Silva-Júnior NJ, Silva MS, Melo-Reis PR. Effects of topical treatment with euphorbia tirucalli latex on the survival and intestinal adhesions in rats with experimental peritonitis. Arq Bras Cir Dig. 2015 Dec;28(4):243-6. doi: 10.1590/S0102-6720201500040006.
-
6Castilho TJ, Campos AC, Mello EV. Effect of Omega-3 fatty acid in the healing process of colonic anastomosis in rats. Arq Bras Cir Dig. 2015 Dec;28(4):258-61. doi: 10.1590/S0102-6720201500040010.
-
7Yasojima EY, Teixeira RK, Houat AP, Costa FL, Yamaki VN, Feitosa-Junior DJ, Silva CA, Brito MV. Copaiba oil influences ventral hernia repair with Vicryl(r) mesh? Arq Bras Cir Dig. 2015 Jul-Sep;28(3):186-9. doi: 10.1590/S0102-67202015000300010.
-
8Silva CE1, Santos OJ2, Ribas-Filho JM1, Tabushi FI1, Kume MH1, Jukonis LB1, Cella IF1. Effect of Carapa guianensis Aublet (Andiroba) and Orbignya phalerata (Babassu) in colonic healing in rats. Rev Col Bras Cir. 2015 Dec;42(6):399-406. doi: 10.1590/0100-69912015006009.
-
9Vizzotto Junior AO, Campos AC, Mello EV, Castilho TJ. Influence of preoperative supplementation of omega-3 fatty acid in the healing of colonic anastomoses in malnourished rats receiving paclitaxel. Rev Col Bras Cir. 2015 Mar-Apr;42(2):116-23. doi: 10.1590/0100-69912015002009.
-
10Comelli Júnior E, Skinovski J, Sigwalt MF, Branco AB, Luz SR, Baulé Cde P. Rupture point analysis of intestinal anastomotic healing in rats under the action of pure Copaíba (Copaifera Iangsdorfii) oil. Acta Cir Bras . 2010 Aug;25(4):362-7. PMID: 20676496.
-
11Passarini Junior JR1, Gaspi FO, Neves LM, Esquisatto MA, Santos GM, Mendonça FA. Application of Jatropha curcas L. seed oil (Euphorbiaceae) and microcurrent on the healing of experimental wounds in Wistar rats. Acta Cir Bras . 2012 Jul;27(7):441-7. PMID: 22760827.
-
12Berenguer B, Alarcón de la Lastra C, Moreno FJ, Martín MJ. Chronic gastric ulcer healing in rats subjected to selective and non-selective cyclooxygenase-2 inhibitors. Eur J Pharmacol. 2002 3;442(1-2):125-35. PMID: 12020690.
-
13Rieder F, Brenmoehl J, Leeb S, Schölmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007;56(1):130-9. doi: 10.1136/gut.2006.090456.
-
14Kiyama T, Onda M, Tokunaga A, Efron DT, Barbul A. Effect of matrix metalloproteinase inhibition on colonic anastomotic healing in rats. J Gastrointest Surg. 2001;5(3):303-11. doi: 10.1016/S1091-255X(01)80052-4.
-
15Goldman R. Growth factors and chronic wound healing: past, present, and future. Adv Skin Wound Care. 2004;17(1):24-35. PMID: 14752324.
-
16Pantaroto M, Lopes Filho GJ, Pinto CA, Antico Filho A. Comparative study of collagen deposition in the colon wall of patients operated for sigmoid diverticular disease. Acta Cir Bras . 2015 Oct;30(10):715-9. doi: 10.1590/S0102-865020150100000010.
-
17Inan A, Koca C, Sen M. Effects of diclofenac sodium on bursting pressures of anastomoses and hydroxyproline contents of perianastomotic tissues in a laboratory study. Int J Surg. 2006;4(4):222-7. PMID: 17462355.
-
18Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9-18. PMID: 17276196.
-
19Lee JL, Mukhtar H, Bickers DR, Kopelovich L, Athar M. Cyclooxygenases in the skin: pharmacological and toxicological implications. Toxicol Appl Pharmacol. 2003;192(3):294-306. PMID: 14575647.
-
20Wilgus TA, Vodovotz Y, Vittadini E, Clubbs EA, Oberyszyn TM. Reduction of scar formation in full-thickness wounds with topical celecoxib treatment. Wound Repair Regen. 2003;11(1):25-34. PMID: 12581424.
-
21Clark TP. The clinical pharmacology of cyclooxygenase-2-selective and dual inhibitors. Vet Clin North Am Small Anim Pract. 2006;36(5):1061-85. PMID: 16984827.
-
22Chai J, Jones MK, Tarnawski AS. Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. FASEB J. 2004;18(11):1264-6. PMID: 15180964.
-
23Zhang YP, Hao XQ, Zhang LM, Tian YT. Enhanced cyclooxygenase-2 activity leads to intestinal dysmotility following hemorrhagic shock. Acta Cir Bras . 2015 Dec;30(12):838-43. doi: 10.1590/S0102-865020150120000008.
-
24Campos ACL, Borges-Branco A, Groth AK. Cicatrização de feridas. Arq Bras Cir Dig. 2007;20(1):51-8. doi: 10.1590/S0102-67202007000100010.
-
25Ramsden JD. Angiogenesis in the thyroid gland. J Endocrinol. 2000 Sep;166(3):475-80. PMID: 11029748.
-
26Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581-611. PMID: 15294883.
-
27Kaka U, Hui Cheng C, Meng GY, Fakurazi S, Kaka A, Behan AA, Ebrahimi M. Electroencephalographic changes associated with antinociceptive actions of lidocaine, ketamine, meloxicam, and morphine administration in minimally anaesthetized dogs. Biomed Res Int. 2015;2015:305367. doi: 10.1155/2015/305367.
-
28Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential taget for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368-80. PMID: 12409337.
-
29Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005 10;23(5):1011-27. PMID: 15585754.
-
30Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006 30;39(5):469-78. PMID: 17002866.
-
31Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005 15;65(3):550-63. PMID: 15664381.
-
32Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9(2):267-85. PMID: 15963249.
-
33Marques e Silva S, Jerônimo MS, Silva-Pereira Id, Tavares AH, Bocca AL, Sousa JB. Effects of metoclopramide on the expression of metalloproteinases and interleukins in left colonic anastomoses. An experimental study. Acta Cir Bras . 2015 Nov;30(11):762-9. doi: 10.1590/S0102-865020150110000007.
-
34Felzemburgh VA, Cettolin QC, Machado KM, Campos JH. Comparison between the anesthetic induction times in the first and second surgery in rabbits. Acta Cir Bras . 2012 Jul;27(7):482-6. PMID: 22760834.
-
35Costa MA, Campos AC, Coelho JC, de Barros AM, Matsumoto HM. Oral glutamine and the healing of colonic anastomoses in rats. JPEN J Parenter Enteral Nutr. 2003;27(3):182-5. PMID: 12757111.
-
36Guimarães Alves IP, Montoro Nicácio G, Diniz MS, Alves Rocha TL, Prada Kanashiro G, Navarro Cassu R. Analgesic comparison of systemic lidocaine, morphine or lidocaine plus morphine infusion in dogs undergoing fracture repair. Acta Cir Bras . 2014 Apr;29(4):245-51. PMID: 24760025.
-
37Ikeuchi D, Onodera H, Aung T, Kan S, Kawamoto K, Imamura M, Maetani S. Correlation of tensile strength with bursting pressure in the evaluation of intestinal anastomosis. Dig Surg. 1999;16(6):478-85. doi: 10.1590/S0102-86502013000600008.
-
38Enberg TB, Braun LD, Kuzma AB. Gastrointestinal perforation in five dogs associated with the administration of meloxicam. J Vet Emer Crit Care. 2006;16:34-43. doi: 10.1111/j.1476-4431.2005.00157.x.
-
39Minossi JG, Leite CVS, Naresse LE, Rodrigues MAM, Curi PR, Kobayasi S. Ação do diclofenaco de sódio em anastomoses realizadas no intestino delgado de ratos. Acta Cir Bras . 1998;13(1):37-43. doi: 10.1590/S0102-86501998000100006.
-
40Greca FH, Noronha L, Bendhack M, Feres A, Soccol A, Duda JR. Use of small intestine submucosa as ureteral allograft in pigs. Int Braz J Urol. 2004;30(4):327-35. PMID: 15679971.
-
41Deneuche AJ, Dufayet C, Goby L, Fayolle P, Desbois C. Analgesic comparison of meloxicam or ketoprofen for orthopedic surgery in dogs. Vet Surg. 2004;33(6):650-60. PMID: 15659022.
-
42Barbuto RC, Araujo ID, Bonomi DO, Tafuri LS, Calvão Neto A, Malinowski R3, Bardin VS3, Leite MD4, Duarte IG5. Use of the amniotic membrane to cover the peritoneal cavity in the reconstruction of the abdominal wall with polypropylene mesh in rats. Rev Col Bras Cir. 2015 Jan-Feb;42(1):49-55. doi: 10.1590/0100-69912015001010.
-
Financial source: none
-
1
Research performed at Laboratory of Experimental Surgery, Central Vivariun, Universidade Positivo, Curitiba-PR, Brazil. Part of Master degree thesis, Postgraduate Program in Animal Sciences, Pontificia Universidade Católica do Paraná (PUCPR). Tutor: Antônio Felipe Paulino de Figueiredo Wouk.
Publication Dates
-
Publication in this collection
May 2016
History
-
Received
20 Jan 2016 -
Reviewed
21 Mar 2016 -
Accepted
22 Apr 2016