ABSTRACT
PURPOSE:
To evaluate the usefulness of a knee osteoarthritis model through functional, radiological and microscopic changes of the synovial membrane.
METHODS:
Forty eight rats were divided randomly into two groups. The first received 0.9% saline in the joint and corresponded to the control group. The second was submitted to experimental osteoarthritis of the right knee induced by monosodium iodoacetate and corresponded to the osteoarthritis group. All animals were subjected to comparative tests of forced ambulation and joint movements, inability to articulate and tactile allodynia on day 1 post-experiment by forced ambulation (Roto-rod test), joint assessment of disability (weight bearing test) and assessment of tactile allodynia (Von Frey test). After inflammatory induction they were divided into four sub-groups corresponding to the scheduled death in 7, 14, 21 and 28 days when they were submitted to radiographic examination of the knee, arthrotomy and collection of the synovial membrane.
RESULTS:
The osteoarthritis group showed significant differences compared to control group on days 7 and 14 in Roto-rod, in weight bearing and Von Frey tests in all days, and in radiological evaluation. Microscopic examination of the synovial membrane showed abnormalities of inflammatory character at all stages.
CONCLUSION:
The osteoarthritis induced by intra-articular monosodium iodoacetate in rats knee is a good model to be used in related research, because it provides mensurable changes on joint movements, tactile allodynia, progressive radiological degeneration and microscopic inflammation of the synovial membrane, that represent markers for osteoarthritis evaluation
Key words:
Osteoarthritis; Knee; Models; Animal; Rats
Introduction
Osteoarthritis is the most common chronic disease in elderly individuals11 Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003 Nov;11(11):821-30. PMID: 14609535.,22 Harvey VL, Dickenson AH. Behavioural and electrophysiological characterisation of experimentally induced osteoarthritis and neuropathy in C57Bl/6 mice. Mol Pain. 2009 Apr 20;5:18. doi: 10.1186/1744-8069-5-18.
https://doi.org/10.1186/1744-8069-5-18...
. It is defined by the American College of Rheumatology as a set of heterogeneous changes that induce painful joint symptoms and signs associated with defects in the integrity of articular cartilage and synovial membrane, in association with changes on articular bone margin33 Asada S, Akagi M, Matsushita T, Hashimoto K, Mori S, Hamanishi C. Effects of cartilage remnants of the posterior femoral condyles on femoral component rotation in varus knee osteoarthritis. Knee. 2012 Jun;19(3):185-9. doi: 10.1016/j.knee.2011.02.008.
https://doi.org/10.1016/j.knee.2011.02.0...
,44 Coimbra IB, Pastor EH, Greve JMD, Puccinelli MLC, Fuller R, Cavalcanti FS, Maciel FMB, Honda E. Consenso brasileiro para tratamento da osteoartrite (artrose). Rev Bras Reumatol. 2002;42(6):371-4.. The cartilage - the main target of the degenerative osteoarthritis changes - has two functions: to permit joint motion with minimal friction and to absorb shock preventing bone damage55 Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006 Mar 18;332(7542):639-42. PMID: 16543327.
6 Gleghorn JP, Jones AR, Flannery CR, Bonassar LJ. Boundary mode lubrication of articular cartilage by recombinant human lubricin. J Orthop Res. 2009 Jun;27(6):771-7. doi: 10.1002/jor.20798.
https://doi.org/10.1002/jor.20798...
-77 Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, Jay GD. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):5852-7. doi: 10.1073/pnas.1219289110.
https://doi.org/10.1073/pnas.1219289110...
. At the moment, many palliative drugs as anti-inflammatory, corticosteroids and opioids have been used in the treatment of the disease in humans; however, none of them stopped the evolutionary process of the lesion, which is the main goal of treatment88 Cialdai C, Giuliani S, Valenti C, Tramontana M, Maggi CA. Effect of Intra-articular 4-(S)-amino-5-(4-{4-[2,4-dichloro-3-(2,4-dimethyl-8-quinolyloxymethyl)phenylsulfonamido]-tetrahydro-2H-4-pyranylcarbonyl} piperazino)-5-oxopentyl](trimethyl)ammonium chloride hydrochloride (MEN16132), a kinin B2 receptor antagonist, on nociceptive response in monosodium iodoacetate-induced experimental osteoarthritis in rats. J Pharmacol Exp Ther. 2009 Dec;331(3):1025-32. doi: 10.1124/jpet.109.159657.
https://doi.org/10.1124/jpet.109.159657...
.
Ideal experimental model should reproduce the disease and allow joint study using biomechanical, radiological and microscopic evaluations of involved tissues. The pathogenesis should also be similar to tissue injury affecting the articulation of the human as well as the therapeutic response99 Little CB, Smith MM. Animal models of osteoarthritis. Curr Rheumatol Rev.2008;4(3):175-82. doi: 10.2174/157339708785133523
https://doi.org/10.2174/1573397087851335...
. Chemical experimental models of osteoarthritis present fast installation results. Currently, the most used is with monosodium iodoacetate inhibitory activity of glyceraldehyde-3-phosphate dehydrogenase glycolysis and induces death of chondrocytes. Intra-articular injection leads to the destruction of chondrocytes in rodents and non-rodents. When it is used in rodent, cartilage lesions with loss of proteoglycan matrix and functional changes with stiffness similar to those observed in human osteoarthritis, are produced1010 Schuelert N, McDougall JJ. Grading of monosodium iodoacetate-induced osteoarthritis reveals a concentration-dependent sensitization of nociceptors in the knee joint of the rat. Neurosci Lett. 2009 Nov 13;465(2):184-8. doi: 10.1016/j.neulet.2009.08.063.
https://doi.org/10.1016/j.neulet.2009.08...
,1111 Combe R, Bramwell S, Field MJ. The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? Neurosci Lett. 2004 Nov 11;370(2-3):236-40. PMID: 15488329.. Given the importance of the synovial membrane and subchondral bone in the pathogenesis of joint pain in osteoarthritis, many experimental studies have been published with monosodium iodoacetate, but most of them only evaluated histopathological and radiological aspects, and not functional ones.
The present study aims to investigate the pain in static and dynamic joint inability, the tactile allodynia, and the correlation of radiological and histopathological changes.
Methods
This study was approved by the Ethics Committee in Research of the Universidade Federal do Maranhão (UFMA) (no. 17315/2011-65) and the procedures were performed in accordance to standards of the Brazilian College of Animal Experimentation1212 Pinheiro BB, Fiorelli AI, Gomes OM. Effects of ischemic postconditioning on left ventricular function of isolated rat hearts. Rev Bras Cir Cardiovasc. 2009 Jan-Mar;24(1):31-7. PMID: 19504016.,1313 Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004 Nov;112(1-2):83-93. PMID: 15494188..
The sample consisted of 48 male Wistar rats (Rattus norvegicus) adult male, weighing between 230-280 g. After the adaptation period they were randomly distributed into two groups of 24: control group (CG) and osteoarthritis group (OG). After a week, they underwent daily training in clinical measurement devices to adapt to them, facilitating motor and sensory capacity assessment after injury.
Handling and joint induction of osteoarthritis
The procedure in CG was intra-articular injection of saline solution 0.9% and in OG intra-articular injection of monosodium iodoacetate. Each group was subdivided into four subgroups. The CG animals were called CG-7, CG-14, CG-21 and CG-28 and the osteoarthritis group OG-7, OG-14, OG-21 and OG-28. This subdivision was made according to the day of the clinical evaluation and euthanasia. Behavioral assessment was performed on the first day before the induction of osteoarthritis and on the 7th, 14th, 21st, and 28th days. Radiographs of the hind limbs and synovial membrane specimens withdrawn were realized in the same periods after euthanasia. The rats were anesthetized with thiopental 40 mg/kg intraperitoneally. The intra-articular puncture was performed with the right knee flexed at 900 through the patellar ligament with 26 G needle into the space between the tibia and femur. The injection site was localized through the palpation of tibial tuberosity; just above it there is a small depression representing the injection site. The needle insertion was performed by touching the intercondylar region of the femur. At this moment, with slight retraction, the substance is injected. In animals of CG was used 50 ml of saline 0.9% in the right and left knees. In OG was introduced in right knee 2 mg of monosodium iodoacetate in maximum volume of 50 ml of 0.9% saline, and in left equal volume of saline 0.9% for itself control to be compared with CG results.
Evaluation of forced ambulation (Roto-rod test)
The animals were placed randomly on a rotating cylinder (Roto-rod, Figure 1) with increasing speed, forcing them to walk continuously to avoid falling. The performance index provided motor learning and use of the affected limb1414 Shiotsuki H, Yoshimi K, Shimo Y, Funayama M, Takamatsu Y, Ikeda K, Takahashi R, Kitazawa S, Hattori N. A rotarod test for evaluation of motor skill learning. J Neurosci Methods. 2010 Jun 15;189(2):180-5. doi: 10.1016/j.jneumeth.2010.03.026.
https://doi.org/10.1016/j.jneumeth.2010....
,1515 Pinto WBVR, Ko GM. Teste de Roto-rod: contribuições no estudo das doenças neuromusculares, das síndromes extrapiramidais e das ataxias cerebelares. RESBCAL. 2012;1(2):202-12.. They were kept on the rotating bar for 5 min to become accustomed to the appliance. Five minutes after the adaptation period, they were again placed on Roto-rod and the rotational speed was increased from 5 to 35 rpm in range of 5 min. The latency to fall was measured automatically by a mechanical sensor at the base of the device. The first results of the assessment of motor activity of the animals occurred on day 1 in all animals, and then sequentially on days 7, 14, 21 and 28 after induction. The forced ambulation was graduated. Was used a numerical scale ranging 5 to 1, where 5 meant normal use of the limb; 4 slight claudication; 3 severe claudication; 2 intermittent disuse of the affected paw and 1 complete disuse of the affected paw1616 Kalff KM, El Mouedden M, van Egmond J, Veening J, Joosten L, Scheffer GJ, Meert T, Vissers K. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur J Pharmacol. 2010 Sep 1;641(2-3):108-13. doi: 10.1016/j.ejphar.2010.05.022.
https://doi.org/10.1016/j.ejphar.2010.05...
.
Joint assessment of disability (weight bearing test)
Changes in weight distribution between the right hind limb (osteoarthritis) and left (without osteoarthritis) in all animals was used as an indicator of joint discomfort caused by handling of the joint. The capacitance test apparatus is shown in Figure 2 (model IITC Life Science, CA, USA)11 Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003 Nov;11(11):821-30. PMID: 14609535.,1616 Kalff KM, El Mouedden M, van Egmond J, Veening J, Joosten L, Scheffer GJ, Meert T, Vissers K. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur J Pharmacol. 2010 Sep 1;641(2-3):108-13. doi: 10.1016/j.ejphar.2010.05.022.
https://doi.org/10.1016/j.ejphar.2010.05...
.
Before measurements were taken, waited up period of 5 min was necessary for rat´s adaptation to the device and the re-start was only done when they had were in correct position, ie both hind limbs on the platforms and the fore limbs on plexiglass ramp. During testing, were observed and recorded the changes in weight distribution between right and left (contralateral control) to assess discomfort index in the right hind limb1313 Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004 Nov;112(1-2):83-93. PMID: 15494188.. The weight exerted on each hind limb (measured in grams) was evaluated over a period of 5 s. The final measurement of the weight distribution was given by the average of three measurements. Changes in the distribution were calculated as follows1616 Kalff KM, El Mouedden M, van Egmond J, Veening J, Joosten L, Scheffer GJ, Meert T, Vissers K. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur J Pharmacol. 2010 Sep 1;641(2-3):108-13. doi: 10.1016/j.ejphar.2010.05.022.
https://doi.org/10.1016/j.ejphar.2010.05...
,1717 Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, Jezierski J, Luginbuhl A, Bilsky EJ. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol Biochem Behav. 2011 Mar;98(1):35-42. doi: 10.1016/j.pbb.2010.12.009.
https://doi.org/10.1016/j.pbb.2010.12.00...
.
Assessment of tactile allodynia (Von Frey test)
Was evaluated by digital analgesymeter (EFF Insight model 302, Sao Paulo, Brazil) calibrated to record maximum force of 150 g, maintaining accuracy of 0.1 g to 80 g force. The contact pressure transducer with hind limb was performed using a disposable tip made of polypropylene with 0.5 mm diameter adapted to it1818 Vivancos GG, Verri WA Jr, Cunha TM, Schivo IR, Parada CA, Cunha FQ, Ferreira SH. An electronic pressure-meter nociception paw test for rats. Braz J Med Biol Res. 2004 Mar;37(3):391-9. PMID: 15060709.. The animals were placed in individual acrylic transparent boxes measuring 12x20x17 cm, which floor consisted of a mesh network of 5 mm2 of non-malleable wire of 1 mm in thickness for 15 min before the experiment in order to adapt to the environment. Mirrors 25 cm below the boxes of experimentation were positioned for easy viewing in the plantar region of the hind limbs. Through the holes of the mesh the researcher applied linearly increasing pressure in the central region of the plantar hind limb until response was jolt ("flinch") stimulation of the pelvic limb (Figure 3). The stimuli were repeated up to six times in the ipsilateral and contralateral hind limbs until the animal presents three similar measures with clear "flinch" response after removal of the pelvic limb1313 Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004 Nov;112(1-2):83-93. PMID: 15494188..
The nociceptive threshold was defined as the percentage of active suspension force to cause the affected ipsilateral hind limb and determined as follows1616 Kalff KM, El Mouedden M, van Egmond J, Veening J, Joosten L, Scheffer GJ, Meert T, Vissers K. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur J Pharmacol. 2010 Sep 1;641(2-3):108-13. doi: 10.1016/j.ejphar.2010.05.022.
https://doi.org/10.1016/j.ejphar.2010.05...
:
Radiographic evaluation
Procedure was performed immediately after euthanasia, before rigor mortis. Were used dental radiography periapical film unit. Radiographs were made in dorsoplantar position at full extension after euthanasia with usual development process. The images were analyzed by a radiologist without knowledge of joint and subgroup of animals evaluated. Were studied the changes of joint space, subchondral sclerosis and osteophyte formation. For radiological rating osteoarthritis scale was used1919 Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957 Dec;16(4):494-502. PMID: 13498604. ranging from 0 to 4, where 0 = no signs of osteoarthritis; 1 = minimal osteophytes of doubtful meaning; 2 = definite osteophytes and joint space preserved; 3 = moderate osteophytes and narrowing joint space; 4 = significant joint space narrowing and subchondral sclerosis.
Collection of synovial membrane and histopathology
After euthanasia and radiographic evaluation, the animals were placed in dorsal recumbency. With Adssen forceps, skin and subcutaneous were freed from patellar tendon and the anterior aspect of the joint was exposed. Patellar tendon and patella were raised proximally from the lower portion of its insertion on the tibial tuberosity. With a scalpel, arthrotomy was made that allowed the identification of the synovial membrane following en-bloc resection.
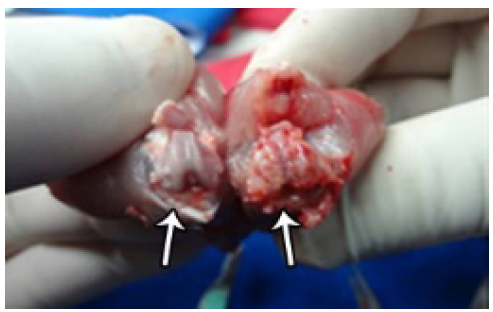
After removal of the capsule the appearance of macroscopic lesions at the articular cartilage were evaluated (Figure 4).
Shows the comparison between two knees after capsule resection where CG can be seen on left side with chondral lesion although with normal aspect and on right side, OG with osteoarthritis.
After removal, the synovial membranes were prepared and stained with hematoxylin-eosin in the usual method. The inflammation was characterized as expansion of the synovial membrane by edema, fluid and fibrin with infiltration of macrophages, neutrophils, lymphocytes and plasma cells. To assess the degree of synovial inflammation the following grading system was used: grade 0 no inflammation; grade 1 minimal inflammation; grade 2 mild inflammation; grade 3 moderate inflammation; and grade 4 severe inflammation2020 Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010 Oct;18 Suppl 3:S24-34. doi: 10.1016/j.joca.2010.05.030.
https://doi.org/10.1016/j.joca.2010.05.0...
. All samples were evaluated by the same pathologist, without knowledge of the animal and knee, to avoid different interpretation, as soon as it was based in qualitative analysis.
Statistical analysis
The comparison of the different experimental groups was performed using the Mann-Whitney test for ordinal qualitative variables and Student t test for numerical variables. p value<0.05 was indicated significance and the data were analyzed using the software Stat(r) GraphpadIn.
Results
Forced ambulation evaluation (Roto-rod test)
All CG rats showed similar evaluation on days 1, 7, 14, 21 and 28, and forced ambulation measured almost in all by the highest degree, 5 (Table 1). After injection of monosodium iodoacetate, the animals of OG showed significant reduction on gait score compared to the CG, with a difference of 1 point in the score maintained in the subsequent analyzes of the 7th to 14th days of the experiment. From the 21st to the 28th, the animals in both groups showed clinically similar march. In the comparison between CG and OG on day 1 there was no change. Statistically significant difference occurred in 7th (p=0.0018) and 14th (p=0.0067) days. In 21st (p= 0.174) and 28th (0.275), there was no difference between groups. The CG was 4.97 and the average OG was 4.53, as shown in Table 1 returning to normal standard.
Joint assessment of disability (weight bearing test)
CG had symmetrical support in both hind limbs from beginning to end of the experiment with the final score of 50.96 showing absence of joint discomfort. After injection of monosodium iodoacetate, OG started showing signs of joint discomfort, distributing the weight predominantly on healthy pelvic limb (left side). In general, the alterations in the distribution of weight on the hind limbs followed biphasic distribution. In the first phase there was a marked reduction in the weight placed on the limb affected, having its maximum effect on the 7th day post-induction (p=0.0001). By the 14th day the OG animals had 40% distribution of weight on the affected leg. In the second phase, which started from the 21th day, the asymmetry in the distribution of weight returned to accentuate with values ranging from 33-35% and was not resolved until the end of the experiment with the final score of 37.45. In the comparison between groups, on day 1 it was unchanged; it was different on day 7 (p<0.0001), 14 (p=0.0165), 21 (p<0.0001) and 28 (p=0.0001), being more significant on the 7th day of the experiment (Table 2).
Assessment of tactile allodynia (Von Frey test)
When stimulated by the tip of Von Frey, CG animals showed similar responses from the first to the last test, demonstrating the absence of tactile allodynia with a final average of 49.80. The injection of monosodium iodoacetate induced tactile allodynia in animals, as demonstrated by the nociceptive paw withdrawal threshold of OG animals, on all days of the study; the final average was 37.66. The biggest change was observed on the 7th day of the experiment (p<0.0001). From the 14th day, the nociceptive withdrawal threshold of the OG paw increased again, but remained below the CG until the end of the experiment. In the comparison between groups, on day 1 was unchanged; difference was observed in 7th (p<0.0001), 14 th (p=0.0046), 21st (p<0.0001) and 28th (p=0.0001) days (Table 3).
Radiographic evaluation
CG animals showed no radiographic changes in the first week of the experiment, but in the last had some slight modifications with final average of 0.5, without significance. In the first week the OG animals showed no signs of joint damage; already on the 14th day were no significant changes remaining until the end of the experiment, obtaining final average of 2.75. In the group comparison, there was no difference on day 7 and yes, significant, at 14 (p=0.0063), 21 (p=0.0049) and 28 (p=0.0063) days (Table 4, Figure 5).
Radiographic aspects: 7th day (A. CG normal and B. GO normal); 14th day (C. CG normal and D. GO with osteoarthritis - arrow); 21st day (E. CG normal and E. GO with osteoarthritis - arrow); 28th day (G. CG early osteoarthritis and H. GO serious injury with destruction of femoral condyles - arrow).
Microscopic analysis
The synovial membrane by microscopy in CG and in the OG left knee served as self control, showed no changes in any animal from beginning to end of the study, final mean zero. The injection of monosodium iodoacetate induced histopathological changes in the synovial membrane on OG from the first to the last week, it was more evident on day 14, final average of 1.3. Comparing groups, there was significant differences in 7 (p=0.0049), 14 (p=0.0025), 21 (p=0.0025) and 28 (p=0.0035) days (Table 5)
Discussion
In the experimental field, pain study in osteoarthritis using animals as models has generated great frustration. Large gains in science knowledge were obtained from animal experiments, but have not led to the development of new effective clinical medication, although the pain in experimental trials are similar to clinical pain. The complexity of the painful phenomenon is still difficult to assess, because various factors such as gender, genotype and media can interfere. Therefore, new models of pain should be pursued2121 Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009 Apr;10(4):283-94. doi: 10.1038/nrn2606.
https://doi.org/10.1038/nrn2606...
. Induced monosodium iodoacetate osteoarthritis shows death and degeneration of chondrocytes, which are responsible for maintaining the integrity of the articular cartilage2222 Kobayashi K, Imaizumi R, Sumichika H, Tanaka H, Goda M, Fukunari A, Komatsu H. Sodium iodoacetate-induced experimental osteoarthritis and associated pain model in rats. J Vet Med Sci. 2003 Nov;65(11):1195-9. PMID: 14665748.. In association with chondral injury, reparative inflammation occurs leading to bone sclerosis, pain decrease in joint space, similar to human osteoarthritis2323 Janusz MJ, Hookfin EB, Heitmeyer SA, Woessner JF, Freemont AJ, Hoyland JA, Brown KK, Hsieh LC, Almstead NG, De B, Natchus MG, Pikul S, Taiwo YO. Moderation of iodoacetate-induced experimental osteoarthritis in rats by matrix metalloproteinase inhibitors. Osteoarthritis Cartilage. 2001 Nov;9(8):751-60. PMID: 11795995.. In addition to inflammation, it can be observed constant bone degeneration and osteophyte formation, seen in the radiographic images shown in this experiment. This model has served as the basis for experimental treatment of osteoarthritis in various ways2424 Cifuentes DJ, Rocha LG, Silva LA, Brito AC, Rueff-Barroso CR, Porto LC, Pinho RA. Decrease in oxidative stress and histological changes induced by physical exercise calibrated in rats with osteoarthritis induced by monosodium iodoacetate. Osteoarthritis Cartilage. 2010 Aug;18(8):1088-95. doi: 10.1016/j.joca.2010.04.004.
https://doi.org/10.1016/j.joca.2010.04.0...
25 Albuquerque PC, Aguiar JL, Pontes Filho NT, Mello RJ, Olbertz CM, Albuquerque PE, Paz ST, Santos AH, Maia CS. A comparative study of the areas of osteochondral defects produced in femoral condyles of rabbits treated with sugar cane biopolymer gel. Acta Cir Bras. 2015 Nov;30(11):770-7. doi: 10.1590/S0102-865020150110000008..
https://doi.org/10.1590/S0102-8650201501...
-2626 Mapp PI, Sagar DR, Ashraf S, Burston JJ, Suri S, Chapman V, Walsh DA. Differences in structural and pain phenotypes in the sodium monoiodoacetate and meniscal transection models of osteoarthritis. Osteoarthritis Cartilage. 2013 Sep;21(9):1336-45. doi: 10.1016/j.joca.2013.06.031.
https://doi.org/10.1016/j.joca.2013.06.0...
.
The Roto-rod, although initially designed for forced ambulation on gait in lesions of the cerebellum1515 Pinto WBVR, Ko GM. Teste de Roto-rod: contribuições no estudo das doenças neuromusculares, das síndromes extrapiramidais e das ataxias cerebelares. RESBCAL. 2012;1(2):202-12., is also used in the grading of joint function in experimental neurological compressions2727 Grava ALS,Ferrari LF,Parada CA,Defino HLA. Modelo experimental para o estudo da hérnia do disco intervertebral. Rev Bras Ortop. 2008 Apr;43(4):116-25. doi: 10.1590/S0102-36162008000300003.
https://doi.org/10.1590/S0102-3616200800...
, and joint changes in experimental arthritis of the knees1616 Kalff KM, El Mouedden M, van Egmond J, Veening J, Joosten L, Scheffer GJ, Meert T, Vissers K. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur J Pharmacol. 2010 Sep 1;641(2-3):108-13. doi: 10.1016/j.ejphar.2010.05.022.
https://doi.org/10.1016/j.ejphar.2010.05...
,2525 Albuquerque PC, Aguiar JL, Pontes Filho NT, Mello RJ, Olbertz CM, Albuquerque PE, Paz ST, Santos AH, Maia CS. A comparative study of the areas of osteochondral defects produced in femoral condyles of rabbits treated with sugar cane biopolymer gel. Acta Cir Bras. 2015 Nov;30(11):770-7. doi: 10.1590/S0102-865020150110000008..
https://doi.org/10.1590/S0102-8650201501...
. This study showed that the greatest difference occurred in 7 and 14 days after induction. Interestingly, after the inflammatory phase of the early days the animals develop motor learning to walk trying to disguise having pain during walking, so that they are not target for predators2828 Roughan JV, Flecknell PA. Evaluation of a short duration behaviour-based post-operative pain scoring system in rats. Eur J Pain. 2003;7(5):397-406. PMID: 12935791.. When subjected to the measurement of articular disability - weight bearing - was observed that all OG animals especially in the first inflammatory phase of the disease had prevalence of less support on the right side, where osteoarthritis was induced. Authors11 Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003 Nov;11(11):821-30. PMID: 14609535.,1717 Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, Jezierski J, Luginbuhl A, Bilsky EJ. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol Biochem Behav. 2011 Mar;98(1):35-42. doi: 10.1016/j.pbb.2010.12.009.
https://doi.org/10.1016/j.pbb.2010.12.00...
,2929 Bajaj P, Bajaj P, Graven-Nielsen T, Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain. 2001 Aug;93(2):107-14. tested monosodium iodoacetate in various dosages, ranging from 0.1 mg to 3 mg, and concluded that higher dosage leads to higher sensitivity to detect the weight bearing joint failure, particularly in the acute phase. Tactile allodynia is defined as painful response to a stimulus that normally is painless1313 Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004 Nov;112(1-2):83-93. PMID: 15494188. and occurs in patients with osteoarthritis3030 Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K, Kawaguchi H. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005 Jul;13(7):632-41. PMID: 15896985.. The present study found biphasic response results more significant in the first phase and inflammatory-like weight bearing. The appearance of radiological signs of osteoarthritis in various animal species can be precocious3030 Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K, Kawaguchi H. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005 Jul;13(7):632-41. PMID: 15896985.. This study in chronic phase showed signs of joint degeneration, narrowing joint space, bone sclerosis, osteophytes in all animals; final assessments identified significant atrophy of the femoral condyles with extensive bone loss. This study supported this hypothesis, both in bone degeneration and in histological changes in cartilage analysis. Comparing the changes of monosodium iodoacetate in rat´s cartilage with human cartilage, similar histological changes were found in literature review, and so this model is an important instrument to study new substances that can be used in mankind.
Conclusion
The osteoarthritis induced by intra-articular monosodium iodoacetate in rats knee is a good model to be used in related research, because it provides mensurable changes on joint movements, tactile allodynia, progressive radiological degeneration and microscopic inflammation of the synovial membrane, that represent markers for osteoarthritis evaluation
References
-
1Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003 Nov;11(11):821-30. PMID: 14609535.
-
2Harvey VL, Dickenson AH. Behavioural and electrophysiological characterisation of experimentally induced osteoarthritis and neuropathy in C57Bl/6 mice. Mol Pain. 2009 Apr 20;5:18. doi: 10.1186/1744-8069-5-18.
» https://doi.org/10.1186/1744-8069-5-18 -
3Asada S, Akagi M, Matsushita T, Hashimoto K, Mori S, Hamanishi C. Effects of cartilage remnants of the posterior femoral condyles on femoral component rotation in varus knee osteoarthritis. Knee. 2012 Jun;19(3):185-9. doi: 10.1016/j.knee.2011.02.008.
» https://doi.org/10.1016/j.knee.2011.02.008 -
4Coimbra IB, Pastor EH, Greve JMD, Puccinelli MLC, Fuller R, Cavalcanti FS, Maciel FMB, Honda E. Consenso brasileiro para tratamento da osteoartrite (artrose). Rev Bras Reumatol. 2002;42(6):371-4.
-
5Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006 Mar 18;332(7542):639-42. PMID: 16543327.
-
6Gleghorn JP, Jones AR, Flannery CR, Bonassar LJ. Boundary mode lubrication of articular cartilage by recombinant human lubricin. J Orthop Res. 2009 Jun;27(6):771-7. doi: 10.1002/jor.20798.
» https://doi.org/10.1002/jor.20798 -
7Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, Jay GD. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):5852-7. doi: 10.1073/pnas.1219289110.
» https://doi.org/10.1073/pnas.1219289110 -
8Cialdai C, Giuliani S, Valenti C, Tramontana M, Maggi CA. Effect of Intra-articular 4-(S)-amino-5-(4-{4-[2,4-dichloro-3-(2,4-dimethyl-8-quinolyloxymethyl)phenylsulfonamido]-tetrahydro-2H-4-pyranylcarbonyl} piperazino)-5-oxopentyl](trimethyl)ammonium chloride hydrochloride (MEN16132), a kinin B2 receptor antagonist, on nociceptive response in monosodium iodoacetate-induced experimental osteoarthritis in rats. J Pharmacol Exp Ther. 2009 Dec;331(3):1025-32. doi: 10.1124/jpet.109.159657.
» https://doi.org/10.1124/jpet.109.159657 -
9Little CB, Smith MM. Animal models of osteoarthritis. Curr Rheumatol Rev.2008;4(3):175-82. doi: 10.2174/157339708785133523
» https://doi.org/10.2174/157339708785133523 -
10Schuelert N, McDougall JJ. Grading of monosodium iodoacetate-induced osteoarthritis reveals a concentration-dependent sensitization of nociceptors in the knee joint of the rat. Neurosci Lett. 2009 Nov 13;465(2):184-8. doi: 10.1016/j.neulet.2009.08.063.
» https://doi.org/10.1016/j.neulet.2009.08.063 -
11Combe R, Bramwell S, Field MJ. The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? Neurosci Lett. 2004 Nov 11;370(2-3):236-40. PMID: 15488329.
-
12Pinheiro BB, Fiorelli AI, Gomes OM. Effects of ischemic postconditioning on left ventricular function of isolated rat hearts. Rev Bras Cir Cardiovasc. 2009 Jan-Mar;24(1):31-7. PMID: 19504016.
-
13Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004 Nov;112(1-2):83-93. PMID: 15494188.
-
14Shiotsuki H, Yoshimi K, Shimo Y, Funayama M, Takamatsu Y, Ikeda K, Takahashi R, Kitazawa S, Hattori N. A rotarod test for evaluation of motor skill learning. J Neurosci Methods. 2010 Jun 15;189(2):180-5. doi: 10.1016/j.jneumeth.2010.03.026.
» https://doi.org/10.1016/j.jneumeth.2010.03.026 -
15Pinto WBVR, Ko GM. Teste de Roto-rod: contribuições no estudo das doenças neuromusculares, das síndromes extrapiramidais e das ataxias cerebelares. RESBCAL. 2012;1(2):202-12.
-
16Kalff KM, El Mouedden M, van Egmond J, Veening J, Joosten L, Scheffer GJ, Meert T, Vissers K. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur J Pharmacol. 2010 Sep 1;641(2-3):108-13. doi: 10.1016/j.ejphar.2010.05.022.
» https://doi.org/10.1016/j.ejphar.2010.05.022 -
17Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, Jezierski J, Luginbuhl A, Bilsky EJ. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol Biochem Behav. 2011 Mar;98(1):35-42. doi: 10.1016/j.pbb.2010.12.009.
» https://doi.org/10.1016/j.pbb.2010.12.009 -
18Vivancos GG, Verri WA Jr, Cunha TM, Schivo IR, Parada CA, Cunha FQ, Ferreira SH. An electronic pressure-meter nociception paw test for rats. Braz J Med Biol Res. 2004 Mar;37(3):391-9. PMID: 15060709.
-
19Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957 Dec;16(4):494-502. PMID: 13498604.
-
20Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010 Oct;18 Suppl 3:S24-34. doi: 10.1016/j.joca.2010.05.030.
» https://doi.org/10.1016/j.joca.2010.05.030 -
21Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009 Apr;10(4):283-94. doi: 10.1038/nrn2606.
» https://doi.org/10.1038/nrn2606 -
22Kobayashi K, Imaizumi R, Sumichika H, Tanaka H, Goda M, Fukunari A, Komatsu H. Sodium iodoacetate-induced experimental osteoarthritis and associated pain model in rats. J Vet Med Sci. 2003 Nov;65(11):1195-9. PMID: 14665748.
-
23Janusz MJ, Hookfin EB, Heitmeyer SA, Woessner JF, Freemont AJ, Hoyland JA, Brown KK, Hsieh LC, Almstead NG, De B, Natchus MG, Pikul S, Taiwo YO. Moderation of iodoacetate-induced experimental osteoarthritis in rats by matrix metalloproteinase inhibitors. Osteoarthritis Cartilage. 2001 Nov;9(8):751-60. PMID: 11795995.
-
24Cifuentes DJ, Rocha LG, Silva LA, Brito AC, Rueff-Barroso CR, Porto LC, Pinho RA. Decrease in oxidative stress and histological changes induced by physical exercise calibrated in rats with osteoarthritis induced by monosodium iodoacetate. Osteoarthritis Cartilage. 2010 Aug;18(8):1088-95. doi: 10.1016/j.joca.2010.04.004.
» https://doi.org/10.1016/j.joca.2010.04.004 -
25Albuquerque PC, Aguiar JL, Pontes Filho NT, Mello RJ, Olbertz CM, Albuquerque PE, Paz ST, Santos AH, Maia CS. A comparative study of the areas of osteochondral defects produced in femoral condyles of rabbits treated with sugar cane biopolymer gel. Acta Cir Bras. 2015 Nov;30(11):770-7. doi: 10.1590/S0102-865020150110000008..
» https://doi.org/10.1590/S0102-865020150110000008. -
26Mapp PI, Sagar DR, Ashraf S, Burston JJ, Suri S, Chapman V, Walsh DA. Differences in structural and pain phenotypes in the sodium monoiodoacetate and meniscal transection models of osteoarthritis. Osteoarthritis Cartilage. 2013 Sep;21(9):1336-45. doi: 10.1016/j.joca.2013.06.031.
» https://doi.org/10.1016/j.joca.2013.06.031 -
27Grava ALS,Ferrari LF,Parada CA,Defino HLA. Modelo experimental para o estudo da hérnia do disco intervertebral. Rev Bras Ortop. 2008 Apr;43(4):116-25. doi: 10.1590/S0102-36162008000300003.
» https://doi.org/10.1590/S0102-36162008000300003 -
28Roughan JV, Flecknell PA. Evaluation of a short duration behaviour-based post-operative pain scoring system in rats. Eur J Pain. 2003;7(5):397-406. PMID: 12935791.
-
29Bajaj P, Bajaj P, Graven-Nielsen T, Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain. 2001 Aug;93(2):107-14.
-
30Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K, Kawaguchi H. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005 Jul;13(7):632-41. PMID: 15896985.
-
Financial source:
none -
1
Research performed at Postgraduate Program in Principles of Surgery, Faculdade Evangélica do Paraná (FEPAR), University Evangelic Hospital of Curitiba, Medical Research Institute, Curitiba-PR, Brazil. Part of Master degree thesis, Postgraduate Program in Principles of Surgery. Tutor: Nicolau Gregori Czeczko.
Publication Dates
-
Publication in this collection
Nov 2016
History
-
Received
14 July 2016 -
Reviewed
15 Sept 2016 -
Accepted
16 Oct 2016