Abstract
Purpose:
To describe a new model of actinic enteritis that does not use radiotherapy machines.
Methods:
Sixteen Wistar rats were divided into four groups, consisting of four animals each: control (group A), two weeks after irradiation (group B), five weeks after irradiation (group C) and eight weeks after irradiation (group D). Animals were given a 10Gy radiation from a Cobalt-60 natural source in a nuclear technology research center. Protections of the surrounding tissues were obtained through the usage of plumb devices with a hole in the center, which served as a collimator. We obtained irradiated and non-irradiated colons from each animal.
Results:
In group B we found an important inflammatory response in the irradiated colon, which appeared in a reduced way in group C and was minimal in group D, in which we found a relevant collagen submucosal deposition/fibrosis. In all groups, the non-irradiated colon had a lower pathological damage in comparison with the irradiated colon.
Conclusion:
We thus described an efficient and feasible technique for obtaining an animal model of actinic enteritis.
Key words:
Radiotherapy; Radiation; Enteritis; Inflammation; Fibrosis; Rats.
Introduction
Radiotherapy still plays a vital role in the management of pelvic malignancies. However, its usage also implies in several injuries in the surrounding organs and this damage accompanies patients throughout their lives. As more patients receive radiotherapy as part of their cancer treatment, and live longer, the incidence of radiotherapy complications continues to increase. Therefore, studies involving radiation and its effects in animals are vast in the literature, mostly trying to determine drugs and means of preventing the damage caused by radiation11 Hogan NM, Kerin MJ, Joyce MR. Gastrointestinal complications of pelvic radiotherapy: medical and surgical management strategies. Curr Probl Surg. 2013;50(9):395-407. PMID: 23930906..
Although the radiation technique is standardized in humans, we observed that there is no consensus in the experimental animal technique in diverse studies and variables such as the dose rate, the total dose, the fractioning scheme and the source unit change according to the researcher's experience22 El-Malt M, Ceelen W, Boterberg T, Claeys G, de Hemptinne B, de Neve W, Pattyn P. Does the addition of glutamine to total parenteral nutrition have beneficial effect on the healing of colon anastomosis and bacterial translocation after preoperative radiotherapy? Am J Clin Oncol. 2003;26(3):e54-9. PMID: 12796616.
3 Bedirli A, Kerem M, Karahacioglu E, Ofluoglu E, Yilmaz TU, Pasaoglu H, Tater OP, Sakrak O, Pak Y. Effects of two conventional preoperative radiation schedules on anastomotic healing in the rat colon. Eur Surg Res. 2007;39(3):141-7. PMID: 17337891.-44 Kerem M, Bedirli A, Karahacioglu E, Pasaoglu H, Sahin O, Bayraktar N, Yilmaz TU, Sakrak O, Goksel F, Oguz M. Effects of soluble fiber on matrix metalloproteinase-2 activity and healing of colon anastomosis in rats given radiotherapy. Clin Nutr. 2006;25(4):661-70. PMID: 16677740.. Most of the research available is based on radiation given by teletherapy machines. Those machines are used for the treatment of cancer patients and are usually unavailable (or of difficult access) for research purposes.
In order to try to find other means of studying radiotherapy, we delineated this study, which investigates the feasibility and reproducibility of an animal model of actinic enteritis using a natural source of radiation in a nuclear technology research center (Centro de Desenvolvimento da Tecnologia Nuclear) without the use of teletherapy industrialized machines that are used for treating patients with cancer.
Methods
The study was approved by the Research Ethics Committee of the Universidade Federal de Minas Gerais (CEUA UFMG Project No. 408/2015).
Sixteen female Wistar rats weighing around 220-280g and aged 2-3 months were used in this study, obtained from the Universidade Federal de Minas Gerais (UFMG). The rats were housed in polycarbonate cages (49×34×16cm), with n= 4/cage, under controlled conditions (temperature, humidity, air flux). Throughout the experimental period, all mice had access to food (Purina Lab Chow, Curitiba, Brazil) and filtered water ad libitum.
The rats were randomly distributed into four groups of four animals each: group A (control without irradiation); group B (single dose 10Gy irradiation and euthanized after two weeks); group C (single dose 10Gy irradiation and euthanized after five weeks); group D (single dose 10Gy irradiation and euthanized after eight weeks).
All experimental procedures were performed on anesthetized rats. Anesthesia was maintained with ketamine and xylazine (60mg/Kg and 8mg/Kg i.p.). After properly anesthetized, the rats were set in an acrylic cylindrical compartment (Figure 1). They were transferred into a protected room which has the MDS Nordion Cobalt-60 source in the center. This room has a complex protection mechanism that rises the source from an enclosed cage from under the ground only when it is fully locked and safe. We developed a covering device (15cm-thick plumb bricks with a 32mm round opening in the center to provide a collimator) to protect the rest of the animal's body from the radiation shade (Figure 2). This protective plumb device did not cover the back of the animal, in order to allow radiation to disperse after collimation and prevent it from reflecting and spreading in the surrounding tissues (Figure 3). A TLD dosimetry system was used in the pilot experiment so as to check the proper radiation dose in the irradiated field and it also proved that the dose outside the radiation beam was lower than 1% of the dose inside it. The beam axis was determined using a laser, in order to center and align the collimator to the source (Figure 4). The total radiation single dose was 10Gy, which was defined after another pilot experiment in which we observed rectal wall necrosis after a 25Gy single dose radiation and no enteropathy after a 5Gy single dose. The animals were set in the device which was placed two meters away from the source. The radiation dose rate was 76.38Gy/h and the animals were irradiated for 7minutes and 51 seconds in order to achieve the 10Gy total dose. In the date of the irradiation, the source had an activity of 1.3 x 1015 Bq.
After irradiating groups B, C and D, the animals were maintained in the experimental surgery animal care facility and received tap water and rat chow ad libitum throughout the experiments. At the end of the observation period (according to each group), the animals were submitted to euthanasia by anesthetic overdose (intraperitoneal 200 mg/Kg ketamine + 25 mg/Kg xylazine), the rectum (irradiated colon) and transverse colon (non-irradiated colon) were obtained through laparotomy.
The rectum and transverse colon were fixed in 10% buffered formalin, dehydrated in successive solutions of alcohols and embedded in paraffin. The paraffin blocks were sectioned in a microtome, obtaining 4 μm sections, which later were stained with hematoxylin/eosin (HE) and stained Gomori Trichrome for histopathological analysis. Slides were examined by the same pathologist under a light microscope (Nikon Eclipse E600), twice, in a blinded manner. Each specimen was graded as follows, according to Northway et al.55 Northway MG, Scobey MW, Geisinger KR. Radiation proctitis in the rat. Sequential changes and effects of anti-inflammatory agents. Cancer. 1988;62(9):1962-9. PMID: 3167809. and Kang et al.66 Kang S, Chun M, Jin YM, Cho MS, Oh YT, Ahn BO, Oh TY. A rat model for radiation-induced proctitis. J Korean Med Sci. 2000;15(6):682-9. PMID: 11194195.:
-
0- Normal or minor alterations which cannot be correlated to radiation with certainty;
-
1- Slight radiation damage (mild inflammation and/or slight crypt change);
-
2- Mild damage (more significant inflammation and/or crypt damage);
-
3- Moderate damage (must have prominent loss of epithelium and/or variable degree of inflammation);
-
4- Severe damage (ulcer, necrosis).
Results
There were no deaths after irradiation, neither from anesthesia nor from the radiation effects.
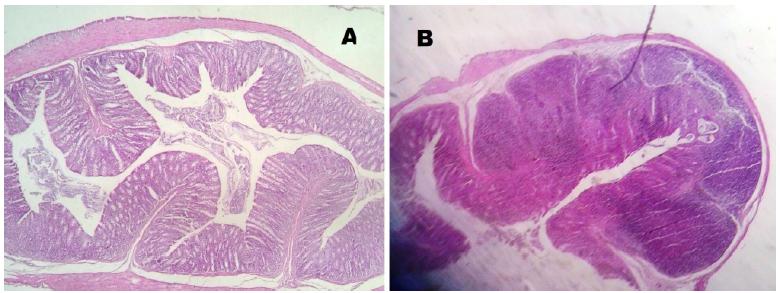
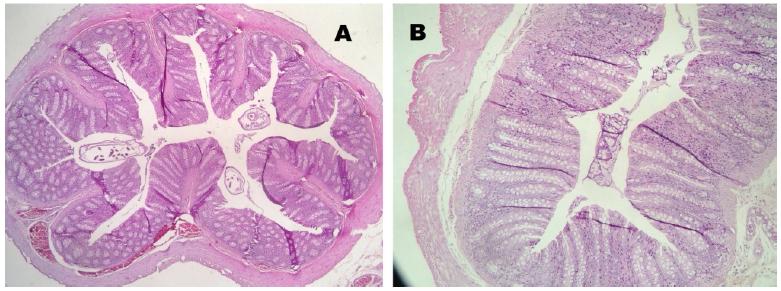
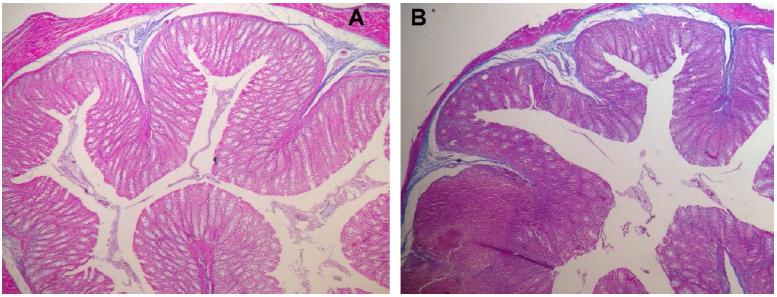
No mucosal damage was noted in the group A (control) - score 0 for all animals. In group B (sacrifice after 2 weeks of irradiation), the median score for the irradiated colon (rectum) was 3.5 and the median score for the non- irradiated colon (transverse colon) was 2.5 (Figure 5). In group C (sacrifice after 5 weeks of irradiation), we noted a median score for the irradiated colon (rectum) of 1.5 and the median score for the non-irradiated colon (transverse colon) was 1. In group D (sacrifice after 8 weeks of irradiation), the median score for the irradiated colon (rectum) was 1 and the median score for the non-irradiated colon (transverse colon) was 0.5. In the irradiated colon of group D, analyzing the Gomori trichrome slides, we observed intense collagen submucosal deposition in all four animals, which was not observed in the other groups (Figures 6 to 8).
Colon histology (Group B) - Hematoxylin/eosin. A- Non irradiated; B- Irradiated (Acute inflammatory phase).
Colon histology (Group D) - Hematoxylin/eosin. A- Non irradiated; B- Irradiated (Chronic fibrotic phase).
Discussion
The pathological changes noted after applying radiation to the intestine can be divided into acute and chronic changes. The first pathological changes include a loss of lymphocytes in the lamina propria and microscopic damage to mucosal epithelial cells and vascular endothelial cells. These changes manifest as marked submucosal edema and villous blunting and a decrease in crypt regenerative cells. These chronic effects later manifest as progressive fibrosis leading to mucosal atrophy, stricture formation, and thrombosis, causing secondary ischemic damage. Radiation colitis in the chronic phase demonstrates a very significant crypt distortion, vascular telangiectasia, and fibrosis of the lamina propria. Progressive endarteritis appears to be the central mechanism by which the chronic effects occur55 Northway MG, Scobey MW, Geisinger KR. Radiation proctitis in the rat. Sequential changes and effects of anti-inflammatory agents. Cancer. 1988;62(9):1962-9. PMID: 3167809.
6 Kang S, Chun M, Jin YM, Cho MS, Oh YT, Ahn BO, Oh TY. A rat model for radiation-induced proctitis. J Korean Med Sci. 2000;15(6):682-9. PMID: 11194195.-77 Indaram AV, Visvalingam V, Locke M, Bank S. Mucosal cytokine production in radiation-induced proctosigmoiditis compared with inflammatory bowel disease. Am J Gastroenterol. 2000;95(5):1221-5. PMID: 10811331..
The results in our study are in accordance to the literature, as the acute changes (inflammatory damage) are observed in the irradiated colon of group B as it reached the highest score. In group D, as expected, we found a lower inflammatory score, once the chronic changes are more related to fibrosis (as noted by the submucosal collagen deposition). Group C demonstrated a transition from acute to chronic phase as it displayed intermediate values. The non-irradiated colon of groups B, C and D presented a lower score compared to the rectum of the same groups, which proves that the shield was effective in preventing surrounding tissues damage.
Many drugs are being tested as having radio-protective effects, such as N-Acetyl-Cysteine, Amifostine, L-carnitine, with encouraging results88 Demir EO, Cakmak GK, Bakkal H, Turkcu UO, Kandemir N, Demir AS, Tascilar O. N-acetyl-cysteine improves anastomotic wound healing after radiotherapy in rats. J Invest Surg. 2011;24(4):151-8. PMID: 21675850.
9 Ozdemir CS, Burgazli KM, Beken-Ozdemir E, Akdere H, Mericliler M, Ozcelik MF. The effect of Amifostine (Ethyol) on intestinal anastomosis in rats with radiation enteritis. Eur Rev Med Pharmacol Sci. 2013;17(10):1351-9. PMID: 23740449.-1010 Caloglu M, Caloglu VY, Yalta T, Yalcin O, Uzal C. The histopathological comparison of L-carnitine with amifostine for protective efficacy on radiation-induced acute small intestinal toxicity. J Cancer Res Ther. 2012;8(2):260-5. PMID: 22842372.. The development of new effective radiation techniques is essential for the expansion of such research, and we described here an effective way of irradiating the pelvis without compromising machines used for cancer patients' treatment1111 Simões Neto J, Reis Neto JA, Matos D. Effects of preoperative irradiation using fractioned electron beam on the healing process of colocolonic anastomosis in rats undergoing early and late surgical intervention. Acta Cir Bras. 2013;28(1):72-7. PMID: 23338117.,1212 Franca A, Ramalho FS, Ramalho LN, da Rocha JJ, Féres O. Effects of preoperative pelvic irradiation on colonic anastomosis healing. An experimental study in rats. Acta Cir Bras. 2008;23(Suppl 1):24-30. PMID: 18516444.. Further studies are needed in order to validate this method and also try to determine the molecular changes that also occur in this irradiated colon1313 Karliczek A, Zeebregts CJ, Benaron DA, Coppes RP, Wiggers T, van Dam GM. Preoperative irradiation with 5 x 5 Gy in a murine isolated colon loop model does not cause anastomotic weakening after colon resection. Int J Colorectal Dis. 2008;23(11):1115-24. PMID: 18629517.,1414 Kerem M, Bedirli A, Karahacioglu E, Pasaoglu H, Sahin O, Bayraktar N, Yilmaz TU, Sakrak O, Goksel F, Oguz M. Effects of soluble fiber on matrix metalloproteinase-2 activity and healing of colon anastomosis in rats given radiotherapy. Clin Nutr. 2006;25(4):661-70. PMID: 16677740..
Conclusion
In order to create new alternatives to investigate the effects of radiation in the organism, we have described a new model of radiation damage in rats without the usage of radiotherapy machines, which proved to be effective and feasible, as the changes found in intestines subjected to radiation exposure in this study were compatible with actinic enteritis.
References
-
1Hogan NM, Kerin MJ, Joyce MR. Gastrointestinal complications of pelvic radiotherapy: medical and surgical management strategies. Curr Probl Surg. 2013;50(9):395-407. PMID: 23930906.
-
2El-Malt M, Ceelen W, Boterberg T, Claeys G, de Hemptinne B, de Neve W, Pattyn P. Does the addition of glutamine to total parenteral nutrition have beneficial effect on the healing of colon anastomosis and bacterial translocation after preoperative radiotherapy? Am J Clin Oncol. 2003;26(3):e54-9. PMID: 12796616.
-
3Bedirli A, Kerem M, Karahacioglu E, Ofluoglu E, Yilmaz TU, Pasaoglu H, Tater OP, Sakrak O, Pak Y. Effects of two conventional preoperative radiation schedules on anastomotic healing in the rat colon. Eur Surg Res. 2007;39(3):141-7. PMID: 17337891.
-
4Kerem M, Bedirli A, Karahacioglu E, Pasaoglu H, Sahin O, Bayraktar N, Yilmaz TU, Sakrak O, Goksel F, Oguz M. Effects of soluble fiber on matrix metalloproteinase-2 activity and healing of colon anastomosis in rats given radiotherapy. Clin Nutr. 2006;25(4):661-70. PMID: 16677740.
-
5Northway MG, Scobey MW, Geisinger KR. Radiation proctitis in the rat. Sequential changes and effects of anti-inflammatory agents. Cancer. 1988;62(9):1962-9. PMID: 3167809.
-
6Kang S, Chun M, Jin YM, Cho MS, Oh YT, Ahn BO, Oh TY. A rat model for radiation-induced proctitis. J Korean Med Sci. 2000;15(6):682-9. PMID: 11194195.
-
7Indaram AV, Visvalingam V, Locke M, Bank S. Mucosal cytokine production in radiation-induced proctosigmoiditis compared with inflammatory bowel disease. Am J Gastroenterol. 2000;95(5):1221-5. PMID: 10811331.
-
8Demir EO, Cakmak GK, Bakkal H, Turkcu UO, Kandemir N, Demir AS, Tascilar O. N-acetyl-cysteine improves anastomotic wound healing after radiotherapy in rats. J Invest Surg. 2011;24(4):151-8. PMID: 21675850.
-
9Ozdemir CS, Burgazli KM, Beken-Ozdemir E, Akdere H, Mericliler M, Ozcelik MF. The effect of Amifostine (Ethyol) on intestinal anastomosis in rats with radiation enteritis. Eur Rev Med Pharmacol Sci. 2013;17(10):1351-9. PMID: 23740449.
-
10Caloglu M, Caloglu VY, Yalta T, Yalcin O, Uzal C. The histopathological comparison of L-carnitine with amifostine for protective efficacy on radiation-induced acute small intestinal toxicity. J Cancer Res Ther. 2012;8(2):260-5. PMID: 22842372.
-
11Simões Neto J, Reis Neto JA, Matos D. Effects of preoperative irradiation using fractioned electron beam on the healing process of colocolonic anastomosis in rats undergoing early and late surgical intervention. Acta Cir Bras. 2013;28(1):72-7. PMID: 23338117.
-
12Franca A, Ramalho FS, Ramalho LN, da Rocha JJ, Féres O. Effects of preoperative pelvic irradiation on colonic anastomosis healing. An experimental study in rats. Acta Cir Bras. 2008;23(Suppl 1):24-30. PMID: 18516444.
-
13Karliczek A, Zeebregts CJ, Benaron DA, Coppes RP, Wiggers T, van Dam GM. Preoperative irradiation with 5 x 5 Gy in a murine isolated colon loop model does not cause anastomotic weakening after colon resection. Int J Colorectal Dis. 2008;23(11):1115-24. PMID: 18629517.
-
14Kerem M, Bedirli A, Karahacioglu E, Pasaoglu H, Sahin O, Bayraktar N, Yilmaz TU, Sakrak O, Goksel F, Oguz M. Effects of soluble fiber on matrix metalloproteinase-2 activity and healing of colon anastomosis in rats given radiotherapy. Clin Nutr. 2006;25(4):661-70. PMID: 16677740.
-
Financial source:
CNPq -
1
Research performed at Experimental Surgery Laboratory, Department of Surgery, Medical School, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte-MG, Brazil. Part of PhD degree thesis, Postgraduate Program in Surgery and Ophthalmology. Tutor: Ivana Duval-Araujo.
Publication Dates
-
Publication in this collection
Apr 2017
History
-
Received
19 Dec 2016 -
Reviewed
16 Feb 2017 -
Accepted
20 Mar 2017