Abstract
Purpose:
To investigate the therapeutic potential of human immature dental pulp stem cells in the treatment of chronic spinal cord injury in dogs.
Methods:
Three dogs of different breeds with chronic SCI were presented as animal clinical cases. Human immature dental pulp stem cells were injected at three points into the spinal cord, and the animals were evaluated by limb function and magnetic resonance imaging (MRI) pre and post-operative.
Results:
There was significant improvement from the limb function evaluated by Olby Scale, though it was not supported by the imaging data provided by MRI and clinical sign and evaluation.
Conclusion:
Human dental pulp stem cell therapy presents promising clinical results in dogs with chronic spinal cord injuries, if used in association with physical therapy.
Key words:
Cell- and Tissue-Based Therapy; Spinal Cord Injuries; Dental Pulp; Dogs
Introduction
Spinal cord injury (SCI), with loss of neurological function, represents a major challenge, not only in terms of adjusting patients, owners, and their environment to daily life with a dog with SCI, but also regarding the therapeutic options that improve the outcome in patients11 Frostell A, Mattsson P, Persson JKE, Hedman B, Nordblom J, Lindenryd A, Trok K, Brundin L, Svensson M. Neurophysiological evaluation of segmental motor neuron function of the thoracic cord in chronic SCI. Spinal Cord. 2012;50(4):315-9. doi: 10.1038/sc.2011.155.
https://doi.org/10.1038/sc.2011.155...
.
Ramón Y Cajal22 Ramón Y Cajal S. Degeneration and regeneration of the nervous system. J Neurol Psychopathol London. 1928;9(36):760. PMID: 1038529. was the first to describe the limited regeneration capacity of the central nervous system. Only after five decades were these information questioned by Richardson et al.33 Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurones regenerate into PNS grafts. Nature. 1980;284(5753):264-5. doi: 10.1038/284264a0.
https://doi.org/10.1038/284264a0...
. These authors performed an assay on rats by removing axons from the spinal cord and inserting them in the peripheral nervous system. They noticed that the regeneration difficulties of the CNS neural cells were more related to their cellular niche than to their regenerative or reposition capability.
Animal models are important tools for the study of the underline physiopathology, and possibilities of new therapies44 Capecchi MR. The new mouse genetics: altering the genome by gene targeting. Trends Genet. 1989;5(3):70-6. PMID: 2660363.. Stem cell therapy may become more effective than other therapeutic alternatives, such as transplantations and drugs, as long as some limiting factors are taken into account, among which the characteristics of the glial scar would be the most important aspect in this context.
A great number of studies have been performed with stem cells from different sources using animal models of spinal cord injury, and even therapeutic attempts on humans, presenting promising results55 Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007;8(3):275-82. PMID: 17679775.
6 Silva AJ, Junior JAV, Fracaro L, Rebelatto CLK, Barchiki F, Moura SAB, Dominguez AC, Abud APR, Locatelli-Dittrich R, Brofman PRS, Quitzan JG. Effect of mesenchymal stem cells on movement and urination of rats with spinal cord injury. Semina Ciênc Agrárias. 2014;35(6):3205-14. doi: 10.5433/1679-0359.2014v35n6p3205.
https://doi.org/10.5433/1679-0359.2014v3...
-77 Li K, Javed E, Scura D, Hala TJ, Seetharam S, Falnikar A, Richard JP, Chorath A, Maragakis NJ, Wright MC, Lepore AC. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Exp Neurol. 2015;271:479-92. doi: .1016/j.expneurol.2015.07.020.. However, there are some difficulties to perform a direct comparison of such results, due to the large variability of characterization grade of the transplanted cells, different animal models, and the time fluctuation between injury event and its treatment88 Barnabé-Heider F, Frisén J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3(1):16-24. doi: 10.1016/j.stem.2008.06.011.
https://doi.org/10.1016/j.stem.2008.06.0...
.
Commonly, dogs present clinical injuries of the thoracic and lumbar spinal medulla mainly due to herniation or protrusion of the intervertebral discs99 Seim HB. Conditions of the thoracolumbar spine. Semin Vet Med Surg (Small Anim). 1996;11(4):235-53. PMID: 9020577.. Animals that exhibit limb paralysis, but with preserved deep pain reflexes are suitable for decompressive spinal surgery. If the dog has lost the deep pain reflex for more than 48 hours, they usually are no longer suitable for surgical treatment because previous studies demonstrated poor prognosis on the return of the locomotive function in these cases1010 Knecht CD. Results of surgical treatment for thoracolumbar disc protrusion. J Small Anim Pract. 1972;13(8):449-53. doi: 10.1111/j.1748-5827.1972.tb06872.x.
https://doi.org/10.1111/j.1748-5827.1972...
.
On the other hand, some studies performed in rats reported coherent evidences of functional recovery in cases of spinal cord injury treated with mesenchymal stem cells (MSC)1111 Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99(4):2199. PMID: 11854516.,1212 Lee KH, Suh-Kim H , Choi JS , Jeun SS , Kim EJ , Kim SS , Yoon DH , Lee BH . Human mesenchymal stem cell transplantation promotes functional recovery following acute spinal cord injury in rats. Acta Neurobiol Exp (Wars). 2007;67(1):13-22. PMID: 17474317.. These “non-neural” cellular types display different mechanisms for recovery in spinal cord injury, in which there is no reposition of neurons or glial cells. It is highly probable that these types of cells provide trophic support, modulate inflammatory response or provide substratum for axon growth, guiding the spinal cord regeneration and therefore promoting functional recovery1313 Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95(7):3908-13. PMID: 9520466.,1414 Orlandin JR, Ambrósio CE, Lara VM.Glial scar-modulation as therapeutic tool in spinal cord injury in animal models. Acta Cir Bras. 2017;32(2):168-74. doi: 10.1590/s0102-865020170209.
https://doi.org/10.1590/s0102-8650201702...
.
The ideal stem cells for transplantations should be immunologically inert, developed from easily accessible sources, possess fast in vitro expansibility, present competence for both long term survival and integration to the host site of interesting, besides being suitable for transfection and expression of exogenous genes1515 Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531-5. doi: 10.1177/154405910208100806.
https://doi.org/10.1177/1544059102081008...
.
Human dental pulp was uncovered as a source of MSC derivative from the neural crest, with promising therapeutic possibilities. Many authors have studied the capacity of self-regeneration, multi-lineage differentiation and clonogenic efficiency of dental pulp stem cells. They reported their capacity of differentiation into neural cells and adipocytes1616 Zhou Z, Chen Y, Zhang H, Min S, Yu B, He B, Jin A. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy. 2013;15(4):434-48. doi: 10.1016/j.jcyt.2012.11.015.
https://doi.org/10.1016/j.jcyt.2012.11.0...
-1717 Kano F, Yamamoto A, Mita T, Ueda M. Mechanisms of functional recovery of spinal cord injury after primates using dental pulp stem cells. J Oral Maxillofac Surg. 2014;72(9):e121. doi: 10.1016/j.joms.2014.06.213.
https://doi.org/10.1016/j.joms.2014.06.2...
.
Kerkis et al.1818 Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI, Cerruti HF. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184(3-4):105-16. doi: 10.1159/000099617.
https://doi.org/10.1159/000099617...
reported the isolation and characterization of human immature dental pulp stem cells (h-IDPSC) using various embryonic stem cell markers. The authors differentiated these cells in both skeletal and smooth muscle, bones, cartilage and neurons in pre-defined culture conditions, suggesting the use of such cells in pre-clinical assays.
The aim of thestudy was to transplant human dental pulp stem cells in chronic spinal cord injury in dogs with intervertebral disc herniation in order to evaluate the condition of the canine patients using magnetic resonance imaging and behavioral clinical tests before and after they underwent the experimental surgical stem cell application associated with a physical therapy protocol.
Methods
The experiment was carried out and approved according to the ethical principles of the Ethics Committee on Animal Use of The Veterinarian Medicine and Animal Science College, Universidade de São Paulo (USP), protocol number 2081/2010.
Three dogs of different breeds selected from local private veterinary practices within the state of São Paulo, Brazil, in the period between December of 2008 and January of 2011. The criteria of inclusion used were: paraplegia, absence of conscious proprioception, presence of sciatic reflexes, exaggerated patellar and cranial tibialr eflexes, absence of deep pain reflexes, and the diagnosis of thoracolumbar intervertebral disc disease on the MRI. Animal number one was a six-year-old male Lhasa Apso dog with chronic spinal cord injury for over two years, that has been treated with the use of ozone therapy and physical therapy for over a year, although unsuccessfully so far. Animal number two was a nine-year-old male Dachshund dog with hindlimb paralysis for at least one and a half year. Subject number two had never undergone surgical treatment, and has been in ozone therapy for a year. Animal number three was a nine-year-old male Yorkshire Terrier with chronic paralysis for two years, under physical therapy for a year. This animal had undergone previous surgical spinal decompression.
Human IDPSC isolation and culture
Human immature dental pulp stem cells (hIDPSC, 2n=46, XY) were isolated and previously characterized and cultured as described by Kerkis et al.2323 Meng XT, Li C, Dong ZY, Liu JM, Li W, Liu Y, Xue H, Chen D. Co-transplantation of bFGF-expressing amniotic epithelial cells and neural stem cells promotes functional recovery in spinal cord-injured rats. Cell Biol Int. 2008;32(12):1546-58. doi: 10.1016/j.cellbi.2008.09.001.
https://doi.org/10.1016/j.cellbi.2008.09...
. These cells were cryopreserved and available at the Stem Cell Laboratory (SCL), USP. In vitro expansion and differentiation followed the protocols already developed by this group: the cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM)/Ham’s F12 (1:1, Invitrogen, Carlsbad, Calif., USA), containing 15% Fetal Bovine Serum (FBS, HyClone, Logan, Utah, USA), 100U/mL Penicillin + 100μg/ml Streptomycin, 2mM L-Glutamine and 2mM Non-Essential Amino Acids.
Surgical procedure
Intervertebral disc disease was diagnosed by magnetic resonance imaging (MRI). The animals were pre-medicated with deep intramuscular application of 0.05 mg/Kg acepromazine maleate in association with 2 mg/Kg meperidine. Anesthesia was induced by propofol (5 mg/Kg) and maintained with isoflurane. The animals were monitored through electrocardiogram and pulse oximetry. The same anesthetical protocol was used in the performance of the surgical procedure.
Spinal decompressive surgery was performed a few weeks after the MRI. Hemilaminectomy procedure was performed on the ipsilateral side to the spinal cord compression, as determined by the MRI results. The herniated disc was removed and 1x1066 Silva AJ, Junior JAV, Fracaro L, Rebelatto CLK, Barchiki F, Moura SAB, Dominguez AC, Abud APR, Locatelli-Dittrich R, Brofman PRS, Quitzan JG. Effect of mesenchymal stem cells on movement and urination of rats with spinal cord injury. Semina Ciênc Agrárias. 2014;35(6):3205-14. doi: 10.5433/1679-0359.2014v35n6p3205.
https://doi.org/10.5433/1679-0359.2014v3...
hiDPSC were injected in three different sites: directly at the site of the spinal cord injury, 2mm cranially and 2mm caudally to the lesion (Figure 1). Post-operative management included the administration of antimicrobials and analgesics, as well as urinary incontinence management, and nursing care to prevent scar pressure ulcers.
Procedure for human immature dental pulp stem cell (h-IDPSC) intramedullary injection in dog with chronic spinal cord injury. A. Hemilaminectomy surgical approach for spinal cord. B. Intramedullary injection of h-IDPSC in site of disc compression.
All animals received a veterinary physical therapy protocol including hydrotherapy, in three times per week sessions during two months post-surgery. The animals were placed on underwater treadmill to eliminate the animal’s weight and facilitating ambulation for strengthening the pelvic muscles. No procedures were used to interfere with the inflammatory process or to avoid interference with stem cell chemotaxis by inflammation. The sessions were recorded at date times 0, 15, 30, 60 (in days), when scores were assigned for each sessions by two veterinarians, according to the Olby Scale1919 Olby NJ, De Risio L, Muñana KR, Wosar MA, Skeen TM, Sharp NJ, Keene BW. Development of a functional scoring system in dogs with acute spinal cord injuries. Am J Vet Res. 2001;62(10):1624-8. PMID: 11592330. (Table 1).
Assessment of pelvic limb function by Olby Scale1919 Olby NJ, De Risio L, Muñana KR, Wosar MA, Skeen TM, Sharp NJ, Keene BW. Development of a functional scoring system in dogs with acute spinal cord injuries. Am J Vet Res. 2001;62(10):1624-8. PMID: 11592330..
The protocol for MRI exams was sagittal and transversal T1 and T2 weighted images, dorsal STIR (short-tau inversion recovery), and transversal 3DHyce (a type of Balanced Steady State Free Precession). Recheck MRI evaluation was performed approximately 60 days after the transplantation of the hIDPSCs, in order to check the spinal cord status.
Results
Case 1
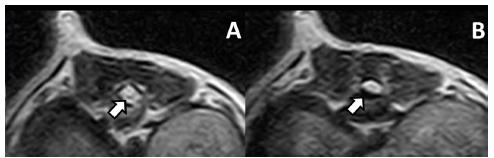
On animal 1, the pre-surgical MRI showed a right ventrolateral extradural myelopathy with adjacent syringohydromyelia on T12-13. Neurofunctional evaluation by the Olby Scale was then performed. The animal showed irregular movements of the hind limbs and did not support its own weight. Based on these data, this animal was scored 3, according to the Olby Scale.
In surgery, the disc material was removed and 1 x 1066 Silva AJ, Junior JAV, Fracaro L, Rebelatto CLK, Barchiki F, Moura SAB, Dominguez AC, Abud APR, Locatelli-Dittrich R, Brofman PRS, Quitzan JG. Effect of mesenchymal stem cells on movement and urination of rats with spinal cord injury. Semina Ciênc Agrárias. 2014;35(6):3205-14. doi: 10.5433/1679-0359.2014v35n6p3205.
https://doi.org/10.5433/1679-0359.2014v3...
cells diluted in 300µl PBS were applied at three points (cranial, caudal and in the center of the spinal cord injury). Post-operative eurofunctional evaluation by the Olby Scale was then performed, and this animal showed weight-bearing protraction of pelvic limb about 25% of the time, which scored a final 7 points for this animal. No improvements were observed in final MRI (Figure 2).
Case 1 pre-operative MRI. A. White arrow points to central channel dilation. B. White arrow points to ventrolateral extradural compression.
Case 2
On the MRI study of the animal 2, was observed presence of moderate to severe ventromedial extradural compressive myelopathy in L4-5 and areas of gliosis or focal hemorrhages at T13 and L1, with probable syringohydromyelia from T11 to L2. Pre-operative analysis of this animal gives him 2 points in Olby Scale, with no pelvic limb movement, but presence of voluntary movement of the tail.
Surgical decompression was performed and human dental pulp stem cells were then applied. This application was made three times with 1 x 106 hIDPSC diluted in 300 µl PBS, from which 100 µl were applied each in the T11-12 and T12-13 intervertebral spaces, and the remaining 100 µl were applied in the L4-5 space. After that, the animal showed an improvement in Olby Scale, getting a 4 in final score with no improvement in final MRI (Figure 3).
Case 2 pre and post-operative MRI. A. Central channel dilation. B. Severe ventromedian extradural compressive myelopathy in L4-5. C. Gliosis or focal hemorrhages at T13-L1. D. White arrow points to permanent central channel. E. Right ventrolateral compression.
Case 3
Dog number 3 also underwent pre-surgical MRI that demonstrated spinal cord atrophy in the thoracic-lumbar region (mainly from T10 to L2) and syringohydromyelia at the level of L1. Previous surgical intervention, probably left hemilaminectomy was noted on T11-12, and associated adjacent muscular paravertebral atrophy was also observed. This animal presents no pelvic limb movement and deep pain sensation, getting score 1 in Olby Scale.
The surgical procedure was performed in this animal, differently from the others. The hemilaminectomy was not necessary, since no disc material on the vertebral canal was present. Three applications of immature human dental pulp stem cells, diluted in 300 µl PBS were made, 100 µl each on the T10-11, T11-12 and T12-13 intervertebral spaces. After surgery, the animal was evaluated by Olby Scale and presents movement of one joint, with minimal non-weight-bearing protaction, getting a 3 in final score. MRI evaluation showed no improvement (Figure 4).
Case 3 pre and post-operative MRI evaluation. A. Medullary hypersinal. B. Previous hemilaminectomy surgery. C. Permanent medullary hypersinal. D. Medullary central channel dilation.
In all cases, using the Olby Scale1919 Olby NJ, De Risio L, Muñana KR, Wosar MA, Skeen TM, Sharp NJ, Keene BW. Development of a functional scoring system in dogs with acute spinal cord injuries. Am J Vet Res. 2001;62(10):1624-8. PMID: 11592330. it was observed clinical improvement of the scores through time, despite it was not supported by the post-surgical MRI data, performed approximately 60 days after the surgical procedure (Figure 5).
Olby Scale assessment of three dogs with chronic spinal cord injury undergoing human immature dental pulp stem cell transplantation.
Discussion
There are few studies that use intramedullary stem cell transplantation, of which the most notorious is the one performed by Mackay-Sim et al.2020 Mackay-Sim A, Féron F, Cochrane J, Bassingthwaighte L, Bayliss C, Davies W, Fronek P, Gray C, Kerr G, Licina P, Nowitzke A, Perry C, Silburn PAS, Urquhart S, Geraghty T. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain. 2008;131(Pt 9):2376-86. doi: 10.1093/brain/awn173.
https://doi.org/10.1093/brain/awn173...
. Intramedullary injections of stem cells were performed in dogs with spinal cord injury in this study, favoring the delivery of cells in a form that is more efficient than the epidural or intravenous access.
Furthermore, it is probable that the cellular niche is the provider of nourishment and subsides for the differentiation of the injected cells in nervous origin cell. Such statements were confirmed in previous studies by Paul et al.2121 Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B. Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine (Phila Pa 1976). 2009;34(4):328-34. doi: 10.1097/BRS.0b013e31819403ce.
https://doi.org/10.1097/BRS.0b013e318194...
in an assay using rats. In this study, a number of 1 x 106 human IDPSC were injected in 3 pre-defined points in the spinal cord injury, in the same manner as in the current study. This same method was proposed by Ryu et al.2222 Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS, Lee YW, Kim WH, Kang KS, Kweon OK. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;10(4):273-84. PMID: 19934591., in experimentations on dogs with induced acute spinal cord injury and treatment with stem cells derived from the adipose tissue. Another study was performed by Lee et al.1212 Lee KH, Suh-Kim H , Choi JS , Jeun SS , Kim EJ , Kim SS , Yoon DH , Lee BH . Human mesenchymal stem cell transplantation promotes functional recovery following acute spinal cord injury in rats. Acta Neurobiol Exp (Wars). 2007;67(1):13-22. PMID: 17474317. with dogs whose spinal cord was injured through compression with an angiography balloon and treated with umbilical cord stem cells. They showed significant improvement in the behavioral scores in the Olby Scale without apparent improvement in MRI, the same result being found like in the present study. The injury caused in both studies, however, was of acute character, that probably developed a spontaneous resolution.
The cases selected for the present study had chronic spinal cord injury with no response to either usual or alternative therapies that are currently used in veterinarian medicine. Despite the behavioral clinical improvement shown by the animals in this experimentation, there is no evidence of spinal cord regeneration after post-surgical MRI. Ryu et al.2222 Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS, Lee YW, Kim WH, Kang KS, Kweon OK. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;10(4):273-84. PMID: 19934591. reported that even though there was improvement in behavior, there were no changes in the histopathologycal evaluation, and no improvement in MRI, despite the affirmed presence of the injected adipose stem cells, that were confirmed by the Green Fluorescent Protein - GFP - gene reporter. There are consistent reports of function recovery in rats with spinal cord injury that were treated with mesenchymal stem cells (MSC)1212 Lee KH, Suh-Kim H , Choi JS , Jeun SS , Kim EJ , Kim SS , Yoon DH , Lee BH . Human mesenchymal stem cell transplantation promotes functional recovery following acute spinal cord injury in rats. Acta Neurobiol Exp (Wars). 2007;67(1):13-22. PMID: 17474317.,2323 Meng XT, Li C, Dong ZY, Liu JM, Li W, Liu Y, Xue H, Chen D. Co-transplantation of bFGF-expressing amniotic epithelial cells and neural stem cells promotes functional recovery in spinal cord-injured rats. Cell Biol Int. 2008;32(12):1546-58. doi: 10.1016/j.cellbi.2008.09.001.
https://doi.org/10.1016/j.cellbi.2008.09...
. This fact was observed in the present study by using human dental pulp stem cells, which are an example of MSC. Nosrat et al.2424 Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238(1):120-32. doi: 10.1006/dbio.2001.0400.
https://doi.org/10.1006/dbio.2001.0400...
evaluated the neurogenic potential of these stem cells in vitro, and in rats that underwent spinal cord hemisection. The injection of such cells in those rats increased the survival rate of motor neurons, showing functional bioactivity.
The results obtained in the present study, in association with the results obtained by the referred authors, lead us to the belief that the transplanted cells promoted a functional response in the animals. The mesenchymal stem cells present different mechanics for spinal cord injury recovery, with no reposition of neurons or glial cells88 Barnabé-Heider F, Frisén J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3(1):16-24. doi: 10.1016/j.stem.2008.06.011.
https://doi.org/10.1016/j.stem.2008.06.0...
.
The h-IDPSC that was used in this experimentation with dogs consists of a source of “non-neural” cells with great capacity for neurogenic regeneration. It is highly probable that this kind of cells provides nourishing support, modulates inflammatory response or provides substratum for axon growth, acts a guide for spinal cord regeneration, promoting functional recovery, as previously reported by Hofstetter et al.1111 Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99(4):2199. PMID: 11854516.. Such mechanism is credited for the clinical improvement shown in the Olby behavioral evaluation. Human dental pulp stem cells have already been differentiated into neuronal tissue in cell culture conditions, influenced by supplemented culture medium1818 Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI, Cerruti HF. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184(3-4):105-16. doi: 10.1159/000099617.
https://doi.org/10.1159/000099617...
.
Authors report that these cells have strong tendency to differentiate into neuronal tissue, even without the addition of specific culture media, and this was the main reason for their choice as a cell source for the therapy of the dogs in the present study. The applied cells probably differentiated into neural origin cells, having as influences the chemical substances generated by the cells of the niche to where they were transplanted. Studies using clinical tests with therapy and stem cells in dogs with spontaneous spinal cord injury are rare in the literature2525 Penha EM, Meira CS, Guimarães ET, Mendonça MV, Gravely FA, Pinheiro CM, Pinheiro TM, Barrouin-Melo SM, Ribeiro-Dos-Santos R, Soares MB. Use of autologous mesenchymal stem cells derived from bone marrow for the treatment of naturally injured spinal cord in dogs. Stem Cells Int. 2014;2014:437521. doi: 10.1155/2014/437521.
https://doi.org/10.1155/2014/437521...
, but with increase of data reports during last years.
In the present study, we chose to use animals with spontaneous spinal cord injury, because this presentation is very common in dogs and also due to the possibility of evaluating the results of stem cell therapy in a real life situation.
Even though significant improvement was achieved in the dogs that underwent treatment, our results confirmed the findings by Sykova et al.2626 Syková E, Homola A, Mazanec R, Lachmann H, Konrádová SL, Kobylka P, Pádr R, Neuwirth J, Komrska V, Vávra V, Stulík J, Bojar M. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15(8-9):675-87. PMID: 17269439., who affirmed that in complete and extended injuries, the isolated application of stem cells is not enough for tissue repairing, and so, a search for other alternatives that can complement the action of the cells and fill the gap caused by tissue death is required. Up-to-date, studies aim to the resolution of the glial scar associated to the stem cell therapy, as a treatment for chronic spinal cord injury1414 Orlandin JR, Ambrósio CE, Lara VM.Glial scar-modulation as therapeutic tool in spinal cord injury in animal models. Acta Cir Bras. 2017;32(2):168-74. doi: 10.1590/s0102-865020170209.
https://doi.org/10.1590/s0102-8650201702...
,2727 Mitsui T, Kakizaki H, Tanaka H, Shibata T, Matsuoka I, Koyanagi T. Immortalized neural stem cells transplanted into the injured spinal cord promote recovery of voiding function in the rat. J Urol. 2003;170(4 Pt 1):1421-25. doi: 10.1097/01.ju.0000075501.05758.33.
https://doi.org/10.1097/01.ju.0000075501...
. Immunization with recombinant vaccines that act on the site of the axonal growth inhibitors (Nogo A, AMG e OMP) in the axonal cells2828 Yu P, Huang L, Zou J, Zhu H, Wang X, Yu Z, Xu XM, Lu PH. DNA vaccine against NgR promotes functional recovery after spinal cord injury in adult rats. Brain Res. 2007;1147:66-76. PMID: 17362886., and even treatment with anti-Nogo A antibodies2929 Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Anti-Nogo-A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates - re-examination and extension of behavioral data. Eur J Neurosci. 2009;29(5):983-96. doi: 10.1111/j.1460-9568.2009.06642.x .
https://doi.org/10.1111/j.1460-9568.2009...
show promising results among the therapies for spinal cord injury. Other alternative therapies are related with benefic effects for the spinal cord injury3030 Goldshmit Y, Kanner S, Zacs M, Frisca F, Pinto AR, Currie PD, Pinkas-Kramarski R. Rapamycin increases neuronal survival, reduces inflammation and astrocyte proliferation after spinal cord injury. Mol Cell Neurosci. 2015;68:82-91. doi: 10.1016/j.mcn.2015.04.006.
https://doi.org/10.1016/j.mcn.2015.04.00...
, and in association with stem cells, might be a reasonable hope in the search for the cure of this disease.
Conclusions
Human immature dental pulp stem cell therapy presents promising clinical results in dogs with chronic spinal cord injuries, if used in association with physical therapy. The imaging data gathered, however, does not support the clinical results presented.
The postoperative evaluation of only 60 days is not enough to evidence the results presented, and it is also worth mentioning that a single application was made, despite the high concentration of cells used.
Acknowledgement
To Francisco Hato, for the MRI studies and the whole structure of his hospital and human material for this work.
References
-
1Frostell A, Mattsson P, Persson JKE, Hedman B, Nordblom J, Lindenryd A, Trok K, Brundin L, Svensson M. Neurophysiological evaluation of segmental motor neuron function of the thoracic cord in chronic SCI. Spinal Cord. 2012;50(4):315-9. doi: 10.1038/sc.2011.155.
» https://doi.org/10.1038/sc.2011.155 -
2Ramón Y Cajal S. Degeneration and regeneration of the nervous system. J Neurol Psychopathol London. 1928;9(36):760. PMID: 1038529.
-
3Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurones regenerate into PNS grafts. Nature. 1980;284(5753):264-5. doi: 10.1038/284264a0.
» https://doi.org/10.1038/284264a0 -
4Capecchi MR. The new mouse genetics: altering the genome by gene targeting. Trends Genet. 1989;5(3):70-6. PMID: 2660363.
-
5Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007;8(3):275-82. PMID: 17679775.
-
6Silva AJ, Junior JAV, Fracaro L, Rebelatto CLK, Barchiki F, Moura SAB, Dominguez AC, Abud APR, Locatelli-Dittrich R, Brofman PRS, Quitzan JG. Effect of mesenchymal stem cells on movement and urination of rats with spinal cord injury. Semina Ciênc Agrárias. 2014;35(6):3205-14. doi: 10.5433/1679-0359.2014v35n6p3205.
» https://doi.org/10.5433/1679-0359.2014v35n6p3205 -
7Li K, Javed E, Scura D, Hala TJ, Seetharam S, Falnikar A, Richard JP, Chorath A, Maragakis NJ, Wright MC, Lepore AC. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Exp Neurol. 2015;271:479-92. doi: .1016/j.expneurol.2015.07.020.
-
8Barnabé-Heider F, Frisén J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3(1):16-24. doi: 10.1016/j.stem.2008.06.011.
» https://doi.org/10.1016/j.stem.2008.06.011 -
9Seim HB. Conditions of the thoracolumbar spine. Semin Vet Med Surg (Small Anim). 1996;11(4):235-53. PMID: 9020577.
-
10Knecht CD. Results of surgical treatment for thoracolumbar disc protrusion. J Small Anim Pract. 1972;13(8):449-53. doi: 10.1111/j.1748-5827.1972.tb06872.x.
» https://doi.org/10.1111/j.1748-5827.1972.tb06872.x -
11Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99(4):2199. PMID: 11854516.
-
12Lee KH, Suh-Kim H , Choi JS , Jeun SS , Kim EJ , Kim SS , Yoon DH , Lee BH . Human mesenchymal stem cell transplantation promotes functional recovery following acute spinal cord injury in rats. Acta Neurobiol Exp (Wars). 2007;67(1):13-22. PMID: 17474317.
-
13Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95(7):3908-13. PMID: 9520466.
-
14Orlandin JR, Ambrósio CE, Lara VM.Glial scar-modulation as therapeutic tool in spinal cord injury in animal models. Acta Cir Bras. 2017;32(2):168-74. doi: 10.1590/s0102-865020170209.
» https://doi.org/10.1590/s0102-865020170209 -
15Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531-5. doi: 10.1177/154405910208100806.
» https://doi.org/10.1177/154405910208100806 -
16Zhou Z, Chen Y, Zhang H, Min S, Yu B, He B, Jin A. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy. 2013;15(4):434-48. doi: 10.1016/j.jcyt.2012.11.015.
» https://doi.org/10.1016/j.jcyt.2012.11.015 -
17Kano F, Yamamoto A, Mita T, Ueda M. Mechanisms of functional recovery of spinal cord injury after primates using dental pulp stem cells. J Oral Maxillofac Surg. 2014;72(9):e121. doi: 10.1016/j.joms.2014.06.213.
» https://doi.org/10.1016/j.joms.2014.06.213 -
18Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI, Cerruti HF. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184(3-4):105-16. doi: 10.1159/000099617.
» https://doi.org/10.1159/000099617 -
19Olby NJ, De Risio L, Muñana KR, Wosar MA, Skeen TM, Sharp NJ, Keene BW. Development of a functional scoring system in dogs with acute spinal cord injuries. Am J Vet Res. 2001;62(10):1624-8. PMID: 11592330.
-
20Mackay-Sim A, Féron F, Cochrane J, Bassingthwaighte L, Bayliss C, Davies W, Fronek P, Gray C, Kerr G, Licina P, Nowitzke A, Perry C, Silburn PAS, Urquhart S, Geraghty T. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain. 2008;131(Pt 9):2376-86. doi: 10.1093/brain/awn173.
» https://doi.org/10.1093/brain/awn173 -
21Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B. Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine (Phila Pa 1976). 2009;34(4):328-34. doi: 10.1097/BRS.0b013e31819403ce.
» https://doi.org/10.1097/BRS.0b013e31819403ce -
22Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS, Lee YW, Kim WH, Kang KS, Kweon OK. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;10(4):273-84. PMID: 19934591.
-
23Meng XT, Li C, Dong ZY, Liu JM, Li W, Liu Y, Xue H, Chen D. Co-transplantation of bFGF-expressing amniotic epithelial cells and neural stem cells promotes functional recovery in spinal cord-injured rats. Cell Biol Int. 2008;32(12):1546-58. doi: 10.1016/j.cellbi.2008.09.001.
» https://doi.org/10.1016/j.cellbi.2008.09.001 -
24Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238(1):120-32. doi: 10.1006/dbio.2001.0400.
» https://doi.org/10.1006/dbio.2001.0400 -
25Penha EM, Meira CS, Guimarães ET, Mendonça MV, Gravely FA, Pinheiro CM, Pinheiro TM, Barrouin-Melo SM, Ribeiro-Dos-Santos R, Soares MB. Use of autologous mesenchymal stem cells derived from bone marrow for the treatment of naturally injured spinal cord in dogs. Stem Cells Int. 2014;2014:437521. doi: 10.1155/2014/437521.
» https://doi.org/10.1155/2014/437521 -
26Syková E, Homola A, Mazanec R, Lachmann H, Konrádová SL, Kobylka P, Pádr R, Neuwirth J, Komrska V, Vávra V, Stulík J, Bojar M. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15(8-9):675-87. PMID: 17269439.
-
27Mitsui T, Kakizaki H, Tanaka H, Shibata T, Matsuoka I, Koyanagi T. Immortalized neural stem cells transplanted into the injured spinal cord promote recovery of voiding function in the rat. J Urol. 2003;170(4 Pt 1):1421-25. doi: 10.1097/01.ju.0000075501.05758.33.
» https://doi.org/10.1097/01.ju.0000075501.05758.33 -
28Yu P, Huang L, Zou J, Zhu H, Wang X, Yu Z, Xu XM, Lu PH. DNA vaccine against NgR promotes functional recovery after spinal cord injury in adult rats. Brain Res. 2007;1147:66-76. PMID: 17362886.
-
29Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Anti-Nogo-A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates - re-examination and extension of behavioral data. Eur J Neurosci. 2009;29(5):983-96. doi: 10.1111/j.1460-9568.2009.06642.x .
» https://doi.org/10.1111/j.1460-9568.2009.06642.x -
30Goldshmit Y, Kanner S, Zacs M, Frisca F, Pinto AR, Currie PD, Pinkas-Kramarski R. Rapamycin increases neuronal survival, reduces inflammation and astrocyte proliferation after spinal cord injury. Mol Cell Neurosci. 2015;68:82-91. doi: 10.1016/j.mcn.2015.04.006.
» https://doi.org/10.1016/j.mcn.2015.04.006
-
Financial source:
FAPESP (Process Number: 09/51605-4)
-
1
Research performed at Department of Surgery, Stem Cell Laboratory, and Laboratory of Stem Cell and Gene Therapy, Veterinary Medicine Department, Faculty of Animal Science and Food Engineering, Universidade de São Paulo (USP), Pirassununga-SP, Brazil. Part of PhD degree thesis, Postgraduate Program in Anatomy in Domestic and Wild Animals. Tutor: Prof. Carlos Eduardo Ambrósio.
Publication Dates
-
Publication in this collection
July 2017
History
-
Received
02 Mar 2017 -
Reviewed
08 May 2017 -
Accepted
05 June 2017