Abstract
Purpose:
To investigate the use Aldefluor® and N, N - Dimethylaminobenzaldehyde (DEAB) to design a protocol to sort keratinocyte stem cells from cultured keratinocytes from burned patients.
Methods:
Activated Aldefluor® aliquots were prepared and maintained at temperature between 2 to 8°C, or stored at -20°C. Next, the cells were collected following the standard protocol of sample preparation.
Results:
Best results were obtained with Aldefluor® 1.5µl and DEAB 15 µl for 1 x 106 cells, incubated at 37°C for 15 minutes. Flow cytometer range for keratinocyte stem cells separation was evaluated. There were 14.8% of stem cells separated in one sample of keratinocyte culture used to pattern the protocol. After being defined the ideal concentration, the same test pattern was performed in other keratinocyte samples. We observed a final mean of 10.8%.
Conclusion:
Aldefluor® has been shown as a favorable marking of epidermal keratinocyte stem cells for subsequent separation on a flow cytometer, with detection of 10.8% of epidermal keratinocyte stem cells, in this protocol.
Key words:
Keratinocytes; Flow Cytometry; Adult Stem Cells.
Introduction
Keratinocyte is the predominant cell type in the epidermis, the outermost layer of the skin. In addition to structural function, keratinocytes play an important role as a fundamental defense mechanism of the body, such as a physical barrier and immune defense11 Gragnani A, Ipolito MZ, Sobral CS, Brunialti MK, Salomao R, Ferreira LM. Flow cytometry of human primary epidermal and follicular keratinocytes. Eplasty. 2008;8:e14. PMID: 2258552..
Keratinocyte stem cells are found in the basal layer of the epidermis, which is the innermost layer of the epithelium, and has a basic proliferative function. These cells divide to give rise to transient amplifying cells which may continue to multiplicate or activate differentiation pathways. According to specific signaling, these cells can activate differentiation pathways, acquiring morphological, generic and protein expression changes in the upper epidermal layers changing their location and reaching specific function in differentiation. On the other hand, these cells might maintain themselves as a mere proliferative cell, in a quiescent phase, keeping a population of undifferentiated cells that may proliferate responding to some stimuli22 Pullar CE, Isseroff RR. Cyclic AMP mediates keratinocyte directional migration in an electric field. J Cell Sci. 2005;118(Pt 9):2023-34. PMID: 15840650..
When keratinocyte culture is performed, the cells that adhere to the flask proliferate to form large colonies that at confluence fuse to form a sheet of keratinocytes33 Corti S, Locatelli F, Papadimitriou D, Donadoni C, Salani S, Del Bo R, Strazzer S, Bresolin N, Comi GP. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24(4):975-85. PMID: 16293577.. Cultured epidermal sheets have been extensively used as autografts to treat burn victims, establishing that stem cells survive in culture33 Corti S, Locatelli F, Papadimitriou D, Donadoni C, Salani S, Del Bo R, Strazzer S, Bresolin N, Comi GP. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24(4):975-85. PMID: 16293577..
Nowadays, stem cells can be isolated from almost all types of tissues33 Corti S, Locatelli F, Papadimitriou D, Donadoni C, Salani S, Del Bo R, Strazzer S, Bresolin N, Comi GP. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24(4):975-85. PMID: 16293577.. There are some techniques used to separate them after their isolation and growth. Although the existence of a set of universal markers for stem cells44 Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3(12):931-40. PMID: 12459723. is in quarrel, the most used in the literature are CD34, CD44, AC133, Keratin15, and gene expression of ABCG2, p63 and BMI1.
There are three main methods of cell separation: fluorescence activated, magnetically selection, and single separation. Fluorescence-activated cell sorting (FACS), which uses flow cytometry, allows objective and quantitative analysis of intra- and extracellular properties, which does not consider cell morphology. Magnetic cell sorting (MACS) is a method of enriching a heterogeneous cell mixture, by means of proteins or cell surface antigens, by MACS that is based on cell passage by magnetic column, or by the separation system (SEP system), in which a tube of labeled cells is placed in a magnetic field, and positive cells are retained in the tube while the negative cells are transfered to the suspension liquid. Simple separation is indicated for heterogeneous mixing based on intra- and extracellular properties, such as IsoRaft array and DEPArray chip technology.
Another option is labeling cells with Aldefluor®, a marker that was first developed to detect hematopoietic cells in human blood and bone marrow, but it has also been used in protocols for separation of non-hematopoietic cells, based on the activity and expression of Aldehyde dehydrogenase enzyme (ALDH) specific for each cell type55 Cai J, Cheng A, Luo Y, Lu C, Mattson MP, Rao MS, Furukawa K. Membrane properties of rat embryonic multipotent neural stem cells. J Neurochem. 2004;88(1):212-26. PMID: 14675165.. There is a lack of protocols, in the current literature, regarding the use of Aldefluor® in epidermal keratinocytes, and therefore, the definition of a protocol for isolated keratinocyte stem cells was pivotal. The importance of flow cytometry in keratinocyte populations is discussed together with the potential for investigation the keratinocyte growth and differentiation66 Clausen OP. Flow cytometry of keratinocytes. J Cutan Pathol. 1983;10(1):33-51. PMID: 6188766., as it maintains viable cells after assortment.
Among the different types of aldehyde dehydrogenase a stem cell can express, in undifferentiated cutaneous keratinocytes of mice, the most active type of hydrophobic aldehyde dehydrogenase is Aldh3a2, an enzyme associated with the Sjögren-Larsson Syndrome in humans77 Naganuma T, Takagi S, Kanetake T, Kitamura T, Hattori S, Miyakawa T, Sassa T, Kihara A. Disruption of the Sjörgen-Larsson Syndrome gene Aldh3a2 in mice increases keratinocyte growth and retards skin barrier recovery. J Biol Chem. 2016 May 27;291(22):11676-88. PMID: 27053112..
In keratinocytes from corneal limbus, there are 3 ALDH enzymes expressed at distinct moment of cell differentiation: ALDH1A1, ALDH1A3 and ALDH3A1. A study evaluating the importance of these enzymes in the differentiation process of keratinocytes from the corneal limbus characterized the expression of the enzyme ALDH1A1 in already differentiated cells, just the opposite to what is found in hematopoietic cells. The enzyme ALDH1A3 is expressed in cells in differentiation stages, and the enzyme ALDH3A1 in cells with potential of regeneration 88 Ahmad S, Kolli S, Li DQ, de Paiva CS, Pryzborski S, Dimmick I, Armstrong L, Figueiredo FC, Lako M. A putative role for RHAMM/HMMR as a negative marker of stem cell-containing population of human limbal epithelial cells. Stem Cells. 2008;26(6):1609-19. PMID: 18356573.,1010 Muzio G, Maggiora M, Paiuzzi E, Oraldi M, Canuto RA. Aldehyde dehydrogenases and cell proliferation. Free Radic Biol Med. 2012;52(4):735-46. PMID: 22206977.. The expression of ALDH3A1 in keratinocytes was also linked to the ability of regeneration in oral mucosa and cutaneous epithelium, reinforcing the significance of this enzyme expression in more primordial cells. Fat-derived stem cells, when exposed to certain stimuli, differentiate into keratinocytes stem cells and also lead off to express ALDH3A11111 Du Y, Roh DS, Funderburgh ML, Mann MM, Marra KG, Rubin JP, Li X, Funderburgh JL. Adipose-derived stem cells differentiate to keratocytes in vitro. Mol Vis. 2010;16:2680-9. PMID: 21179234..
The substance diethylaminobenzaldehyde (DEAB) was formerly used to inhibit an ALDH1A1 enzyme but it is also able to curb other isoenzymes, such as ALDH1A2, ALDH2 and ALDH1A31212 Marcato P, Dean CA, Pan D, Araslanova R, Gillis M, Joshi M, Helyer L, Pan L, Leidal A, Gujar S, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells. 2011;29(1):32-45. PMID: 21280157.,1313 Moreb JS, Ucar D, Han S, Amory JK, Goldstein AS, Ostmark B, Chang LJ. The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance. Chem Biol Interact. 2012;195(1):52-60. PMID: 22079344..
The ALDH enzymes are divided into 5 families. The enzyme ALDH3A1 present in keratinocyte stem cells is not significantly inhibited by DEAB. But DEAB functions as a competitive inhibition to ALDH1A1, ALDH1A3, ALDH1B1 and ALDH5A1; and as a covalent inhibitor to ALDH2 and ALDH1A21414 Morgan CA, Parajuli B, Buchman CD, Dria K, Hurley TD. N,N-diethylaminobenzaldehyde (DEAB) as a substrate and mechanism-based inhibitor for human ALDH isoenzymes. Chem Biol Interact. 2015;234:18-28. PMID: 25612087..
Based on these definitions, it became possible to draft a protocol to sort epidermal stem cells using Aldefluor®. This process preserves the cells of interest feasible therefore may be the basis for future experiments. The objective of the present study was to define a protocol to separate stem cells from primary epidermal keratinocytes cultured employing Aldefluor®.
Methods
The development of present protocol is part of a project developed in the Laboratory of Translational Surgery entitled “Keratinocyte growth factor in the gene expression in wound healing process and immune system of stem cells of burned patients”, with approval of Research Ethics Committee UNIFESP number 197.342 of 08/02/2013.
Epidermal keratinocyte Culture
Skin fragments were obtained from patients who underwent surgical procedures on Burns Treatment Unit, Discipline of Plastic Surgery in Hospital São Paulo and the culture of primary human keratinocytes was performed on Translational Surgery Laboratory following the protocol used routinely11 Gragnani A, Ipolito MZ, Sobral CS, Brunialti MK, Salomao R, Ferreira LM. Flow cytometry of human primary epidermal and follicular keratinocytes. Eplasty. 2008;8:e14. PMID: 2258552..
After keratinocytes isolation and culture, when the cell confluence reached 80%, the keratinocytes were transferred to new flasks. The cells in the third passage were used in these experiments. All patients signed an informed consent.
Aldefluor®
The protocol was performed according to Aldefluor® guidelines and adapted to the cell type employed in this study. First, was necessary the Aldefluor® activation (Stemcell Technologies, WA, USA), a one-time process, before it initially usage. All necessary material kit reagents was separated and left at 15 to 25°C degrees to stabilize before use. After that time, 25 μl of DMSO (Sigma, SP, Brazil) was added to the vial containing Aldefluor® , it was well mixed and allowed to stand for 1 minute at room temperature. Aldefluor® powder has an orange color and when mixed with DMSO it changes to a bright green. Subsequently, 25 μL of 2N hydrochloric acid (HCl) was added and well mixed to Aldefluor® vial, it is important to follow this order, because if the 2N HCl were prior added, it will be inatived. Then the vial was stand for 15 minutes at room temperature and finally 360 μl of buffer was added and homogenized to Aldefluor® vial. At the end, Aldefluor® aliquots were prepared, and those that would be used for the experiment were kept at a temperature between 2 to 8°C and those to be stored were stocked at -20°C.
On the experiment day, the cultured cells were withdrawn from the flask, following the standard protocol, to apportion on analysis samples33 Corti S, Locatelli F, Papadimitriou D, Donadoni C, Salani S, Del Bo R, Strazzer S, Bresolin N, Comi GP. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24(4):975-85. PMID: 16293577..
Each tube was give out with a total of 5x106 cells in 2 ml of buffer. These 5x106/2ml tube were subdivided in another three 15 ml tubes. One named as test, other as control and the third as white. From the tube containing 2 ml of buffer with suspension of homogenized cells, 250 μL was withdrawn and placed in the control tube, 250 μL in the white tube and 1.5 mL in the test tube. The white tube served as the normal cells fluorescence.
In the control tube, 10 μl of DEAB, a specific ALDH inhibitor (Stemcell Technologies, WA, USA), were added and immediately closed as DEAB was diluted in 95% to prevent evaporation. In the control tube, 1.5 μL of Aldefluor® was added, well homogenized and immediately transferred 250 μL of this to test tube. The enzymatic reaction begins immediately after adding the activated Aldefluor® substrate to the cells in suspension. It was imperative that an aliquot containing cells activated with Aldefluor® was added to the test tube with DEAB without delay.
The same procedure was repeated for each sample analyzed. All tubes were left incubating for 15 minutes at 37°C. After the incubation period, the tubes were centrifuged for 5 minutes at 250G and the supernatant from each tube was removed, always being careful not to undo the cell pellet. The white and test tube cell pellets were resuspended in 200μl buffer, and the control tube in 300μl buffer. Upon completion, the tubes were stored on ice or at a temperature between 2 to 8°C, to decrease the cell fluorescent product efflux. When prepared they were submitted to the analysis process in the flow cytometer.
Flow cytometry - BDFACSAria ll (Cell sorter)
Defined as a cell sorter, this flow cytometer, in addition to analyzing also separates the cells into distinct containers, such as the stem cells are collected in a specific repository and the other cell types in another, both containing cell culture medium. This process allows cell culture or RNA extraction from samples. After the complete series of steps of reagents dilution, the procedure in the cytometer is standard, following protocol of the cell sorter19,20.
Each keratinocyte sample was assessed by flow cytometry and the analysis was performed in triplicates.
Results
In order to obtain a protocol, it was necessary to setup the concentrations of Aldefluor® and DEAB to adapt to cell type in quest. For this purpose, groups were sort out according to different cell numbers, incubation time, Aldefluor® and DEAB concentrations (Table 1).
Different cells concentration in different incubation time to a same Aldefluor® and DEAB ratio.
In the first flow cytometry analysis, all cells from the above scheme were presented as ALDHBri at these reagent concentrations. All cells ware labeled by Aldefluor® without DEAB inhibition.
It was defined that the cells with 1x106 in 300 μl of buffer in a 15 minute incubation period at 37°C would be used, as they were the ones that presented the best response among all.
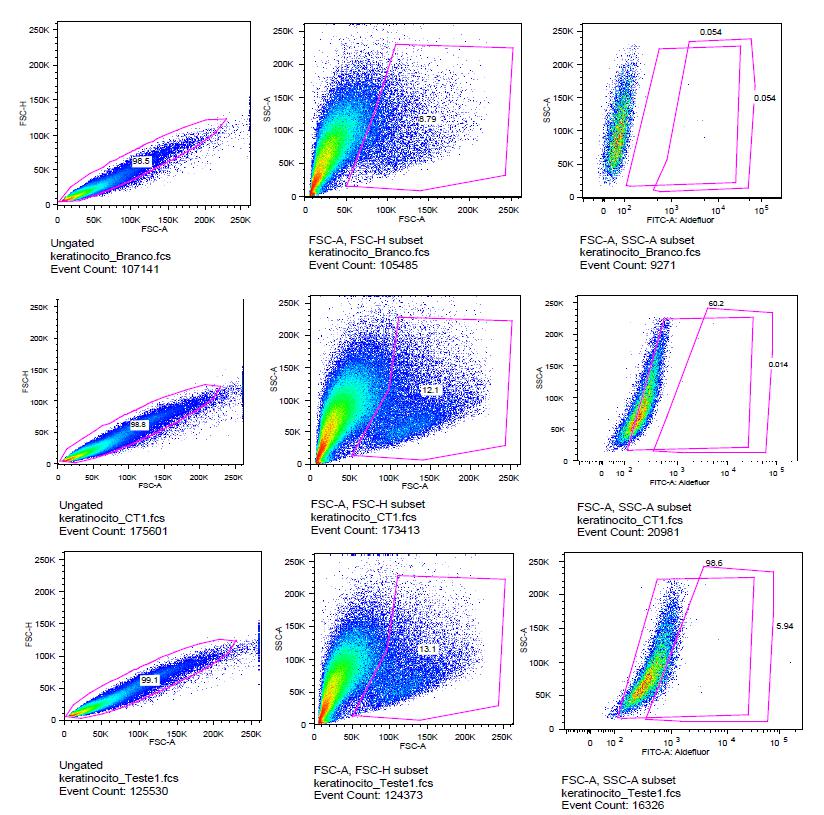
In the second turn, the amount of DEAB was rised to 15 μl and Aldefluor® ebbed to 2.5 μl attempting to counterweight the Aldefluor® marking. The results were still similar to that found in the first analysis, intense stamp by Aldefluor® and low inhibition by DEAB (Figure 1).
Top row - white group results, which contains cells without any marking. In the middle row - control group graphics, stained cells by Aldefluor® without DEAB inhibition. Lower row - test group graphs - cells marked with Aldefluor inhibited by DEAB [The graphics in the first column are Forward-scatter (FSCH) x Forward-scatter (FSCA), in the second column are Side-scatter (SSCA) x Forward-scatter (FSCA), in the third column graphics Side-scatter (SSCA) x Aldefluor® Fluorescent Marker].
Based on these results, two other concentrations were suggested. One with 20 μl of DEAB and 2 μl of Aldefluor®, and other with 10 μl DEAB and 1 μl Aldefluor®. A new sort tube was added to study, this containing 2x106 cells. All the vials underwent the same incubation time variation as done before (Figure 2).
Based on the same principles as in the first figure, this graph corresponds to the first group with 2 x 106 cells with 20 μl of DEAB and 2 μl of Aldefluor®.
Based on analyzed results, a better amount of Aldefluor® marking was 1x106 cells with 1 μl of Aldefluor® and 10 μl of DEAB (Figure 3).
Based on the same principles of the first figure, this graph corresponds to the second group, with 1 x 106 cells with 10 μl of DEAB and 1 μl of Aldefluor®.
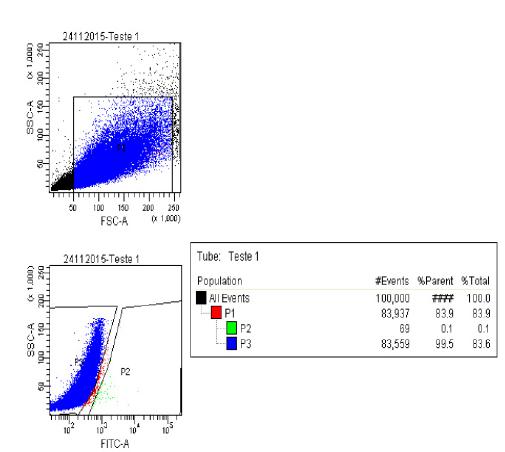
From 8x106 cells three 15 ml tubes were apportioned. The first one named as white was prepared 1x106 cells in 500 μl of buffer. The second named as control placed 6x106 cells with 6 μl of Aldefluor® in 3 ml of buffer. And the third named as test placed 1x106 cells with 10 μl DEAB and 1µl de Aldefluor® in 500 μl of buffer. In the first cell sorter cytometer analysis, 1x106 cells were passed from the test tube to the control tube. Of these, only 0.1% of stem cells were separated. However, the cells were poorly marked and very inhibited. So, the amount of Aldefluor® was increased (Figure 4).
100.000 events were analyzed. In P1, the total population excluded debris, in P3 the ALDHDim cells (which were not labeled with Aldefluor® by the use of DEAB) and finally in P2 (0.1%) ALDHBri cells (which were labeled with Aldefluor® with DEAB).
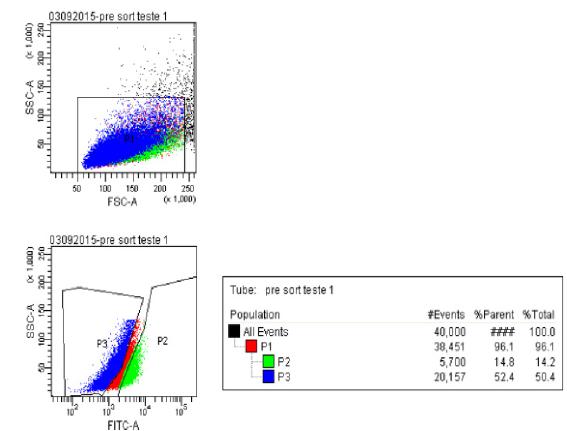
In the second cell sorter cytometry analysis performed to define the standards, 5x106 cells were used. 1x106 cells were placed in 250 μl of buffer in the white tube, 1x106 cells with 15 μM DEAB in 250 μl of buffer and in the test tube and 3x106 cells with 4.5 μl of Aldefluor® in 1500 μl of buffer on control tube, of which 250 μl was transferred to test tube. All tubes were incubated at 37°C for 15 minutes. Of these, 14.8% of stem cells were separated (Figure 5).
40.000 events were analyzed. In P1 the total population is observed, in P3 the ALDHDim cells (which were not labeled with Aldefluor® by the use of DEAB) 52.7% and, finally, in P2 the ALDHBri cells (which were labeled with Aldefluor despite the use of DEAB) corresponding to 14.8% of non-differentiated cells.
The cell number and reagents proportion was used in another 3 samples of keratinocyte cultures. Through the use of a flow cytometer it was possible to separate, on average, 10,8% of stem cells using the separation by the program.
None compensation was needed. As there were no groups to be compared, and only the analysis of a cell line were performed, with variations in cell numbers, time of exposure to the reagents, Aldefluor® and DEAB in different concentrations. It was not necessary to perform statistical tests, being presented here the final mean of experiments performed in triplicate for each keratinocyte culture.
Discussion
Flow cytometry is a biological tool that allow us to separate subpopulations of cultured keratinocytes constituted of differentiated and undifferentiated cells. Having the ability to sort skin stem cells will give us the possibility to better understand the molecular profile of this subpopulation of cells. Skin stem cells culture knowledge is extremely important due to its clinical use in large burned patients and chronic wounds difficult to treat. Keratinocyte culture was proved to be an effective tool for profiling skin cells from large burn since the 1980s, but up to now there is a lack of information about this subpopulation of stem cells1515 Amoh Y, Katsuoka K, Hoffman RM. Peripheral-nerve and spinal-cord regeneration in mice using Hair-Follicle-Associated Pluripotent (HAP) stem cells. Methods Mol Biol. 2016;1453:21-32. PMID: 27431243.. Flow cytometry is a technique that simultaneously measures and analyzes multiple characteristics of particles, usually cells, suspended in a liquid media and submitted to a unicellular flux. The parameters assessed by this assay are particle size, granularity or complexity and fluorescence intensity.
Stem cells are usually isolated for cell culture in a plate for further culture techniques. Recently, phenotypic markers have been described for these cells. Some of the methods used for separation were primary antibodies, separation by magnetically activated cells, fluorescence activated cell sorting, cell cycle analysis, immunostaining, reverse transcriptase polymerase chain reaction analysis, etc1616 Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116(Pt 9):1827-35. PMID: 126165563..
Aldefluor® is a kit of reagents used to identify hematopoietic human cells expressing high levels of aldehyde dehydrogenase (ALDH) enzymes. Aldefluor® active reagent, BODIPY-aminoacetaldehyde (BAAA), is a non-toxic Aldefluor® substrate that freely diffuses into intact and viable cells1414 Morgan CA, Parajuli B, Buchman CD, Dria K, Hurley TD. N,N-diethylaminobenzaldehyde (DEAB) as a substrate and mechanism-based inhibitor for human ALDH isoenzymes. Chem Biol Interact. 2015;234:18-28. PMID: 25612087..
The amount of the fluorescence reaction of the product is proportional to the ALDH activity in the cells. Viable cells stained by Aldefluor® can be isolated with a sorter. An ALDH inhibitor, diethylaminobenzaldehyde (DEAB), is used to control the emitted fluorescence1414 Morgan CA, Parajuli B, Buchman CD, Dria K, Hurley TD. N,N-diethylaminobenzaldehyde (DEAB) as a substrate and mechanism-based inhibitor for human ALDH isoenzymes. Chem Biol Interact. 2015;234:18-28. PMID: 25612087.. DEAB functions as a competitive inhibitor ALDH1A1, ALDH1A3, ALDH1B1 and ALDH5A1, greatly reducing the reaction rate and leaving the cells less fluorescent, and as covalent inhibitor ALDH2 and ALDH1A2, preventing the reaction from occurring1313 Moreb JS, Ucar D, Han S, Amory JK, Goldstein AS, Ostmark B, Chang LJ. The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance. Chem Biol Interact. 2012;195(1):52-60. PMID: 22079344..
Different concentrations and incubation times were analyzed in order to obtain the best parameters for marking populations of keratinocytes stem cells, which are well known for other cell lines but not for keratinocytes. Since no specific protocol for epidermal keratinocytes was found in the literature, the objective of this study was to define a protocol to standardize keratinocyte stem cells marking using ALDH for subsequent separation using a cell sorter flow cytometer. The data demonstrated here in this study may assist other researchers who will run similar protocols, although these results were obtained under specific conditions, which include the primary lineage of large burned patients, a culture technique routinely performed in our laboratory1,3 and also the number of cells and concentrations of Aldefluor® and DEAB employed in the present study.
After determining the optimum concentrations for running this protocol, the same test pattern was performed repeatedly for the accuracy of the results observed. We obtained a final mean of 10.8% of stem cells when using the separation by the program settings, from 5x106 cells, into the white tube with 1x106 cells in 250 μl of buffer, in the test tube 1x106 cells with 15 μl DEAB in 250 μl of buffer and in the control tube 3x106 cells with 1.5 μl of Aldefluor® in 1500 μl of buffer, of which 250 μl were transferred to the test tube. All tubes were incubated at 37° C for 15 minutes. This protocol allowed us to observe and sort a high percentage of keratinocytes stem cell in our cell culture using Aldefluor, which maintains cell viability and is atoxic for a putative clinical use.
Conclusion
The protocol here presented for sorting keratinocyte stem cells with Aldefluor® was found to be useful in labeling primary epidermal keratinocytes stem cells from burned patients for subsequent separation on a flow cytometer sorter, yielding an average of 10.8% of stem cells using the separation program.
Acknoledgements
To Profa. Milena Karina Coló Brunialti and Prof. Dr. Reinaldo Salomão of the Laboratory of Immunology, Division of Infectious Diseases, UNIFESP, and Profa. Daniela Teixeira and Prof. Dr. Alexandre Basso from the Laboratory of Immunology, Division of Microbiology, Immunology and Parasitology, UNIFESP.
References
-
1Gragnani A, Ipolito MZ, Sobral CS, Brunialti MK, Salomao R, Ferreira LM. Flow cytometry of human primary epidermal and follicular keratinocytes. Eplasty. 2008;8:e14. PMID: 2258552.
-
2Pullar CE, Isseroff RR. Cyclic AMP mediates keratinocyte directional migration in an electric field. J Cell Sci. 2005;118(Pt 9):2023-34. PMID: 15840650.
-
3Corti S, Locatelli F, Papadimitriou D, Donadoni C, Salani S, Del Bo R, Strazzer S, Bresolin N, Comi GP. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24(4):975-85. PMID: 16293577.
-
4Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3(12):931-40. PMID: 12459723.
-
5Cai J, Cheng A, Luo Y, Lu C, Mattson MP, Rao MS, Furukawa K. Membrane properties of rat embryonic multipotent neural stem cells. J Neurochem. 2004;88(1):212-26. PMID: 14675165.
-
6Clausen OP. Flow cytometry of keratinocytes. J Cutan Pathol. 1983;10(1):33-51. PMID: 6188766.
-
7Naganuma T, Takagi S, Kanetake T, Kitamura T, Hattori S, Miyakawa T, Sassa T, Kihara A. Disruption of the Sjörgen-Larsson Syndrome gene Aldh3a2 in mice increases keratinocyte growth and retards skin barrier recovery. J Biol Chem. 2016 May 27;291(22):11676-88. PMID: 27053112.
-
8Ahmad S, Kolli S, Li DQ, de Paiva CS, Pryzborski S, Dimmick I, Armstrong L, Figueiredo FC, Lako M. A putative role for RHAMM/HMMR as a negative marker of stem cell-containing population of human limbal epithelial cells. Stem Cells. 2008;26(6):1609-19. PMID: 18356573.
-
9Kato H, Izumi K, Saito T, Ohnuki H, Terada M, Kawano Y, Nozawa-Inoue K, Saito C, Maeda T. Distinct expression patterns and roles of aldehyde dehydrogenases in normal oral mucosa keratinocytes: differential inhibitory effects of a pharmacological inhibitor and RNAi-mediated knockdown on cellular phenotype and epithelial morphology. Histochem Cell Biol. 2013;139(6):847-62. PMID: 23250514.
-
10Muzio G, Maggiora M, Paiuzzi E, Oraldi M, Canuto RA. Aldehyde dehydrogenases and cell proliferation. Free Radic Biol Med. 2012;52(4):735-46. PMID: 22206977.
-
11Du Y, Roh DS, Funderburgh ML, Mann MM, Marra KG, Rubin JP, Li X, Funderburgh JL. Adipose-derived stem cells differentiate to keratocytes in vitro. Mol Vis. 2010;16:2680-9. PMID: 21179234.
-
12Marcato P, Dean CA, Pan D, Araslanova R, Gillis M, Joshi M, Helyer L, Pan L, Leidal A, Gujar S, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells. 2011;29(1):32-45. PMID: 21280157.
-
13Moreb JS, Ucar D, Han S, Amory JK, Goldstein AS, Ostmark B, Chang LJ. The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance. Chem Biol Interact. 2012;195(1):52-60. PMID: 22079344.
-
14Morgan CA, Parajuli B, Buchman CD, Dria K, Hurley TD. N,N-diethylaminobenzaldehyde (DEAB) as a substrate and mechanism-based inhibitor for human ALDH isoenzymes. Chem Biol Interact. 2015;234:18-28. PMID: 25612087.
-
15Amoh Y, Katsuoka K, Hoffman RM. Peripheral-nerve and spinal-cord regeneration in mice using Hair-Follicle-Associated Pluripotent (HAP) stem cells. Methods Mol Biol. 2016;1453:21-32. PMID: 27431243.
-
16Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116(Pt 9):1827-35. PMID: 126165563.
-
Financial source:
FAPESP (process number 2013/10905-0)
-
1
Research performed at Translational Surgery Laboratory, Division of Plastic Surgery, Universidade Federal de São Paulo (UNIFESP), Brazil.
Publication Dates
-
Publication in this collection
Nov 2017
History
-
Received
03 July 2017 -
Reviewed
05 Sept 2017 -
Accepted
08 Oct 2017