Abstract
Purpose:
To evaluate the toxicity of Erbitux as well as its biosimilar APZ001 antibody (APZ001) in pre-clinical animal models including mice, rabbits and cynomolgus monkeys.
Methods:
We performed analysis of normal behavior activity, autonomic and non-autonomic nervous functions, nervous-muscle functions, nervous excitability and sensorimotor functions on CD-1 mice. Subsequently, we studied that effects of APZ001 and Erbitux on respiratory system, cardiovascular system and kidney in Cynomolgus monkey models and performed local tolerance experiments on New Zealand rabbits.
Results:
The comparisons between APZ001 and Erbitux showed no significant differences in mice autonomic nervous system, nervous muscle functions, non-autonomic nervous functions, nervous excitability and sensorimotor functions between treated and untreated group (p>0.05). APZ001 and Erbitux showed negative effect on CD-1 mice in the present of pentobarbital sodium anesthesia (p>0.05). Single administrations of high, medium or low doses of APZ001 did not lead to monkey urine volume alterations (p>0.05). In human tissues, APZ001 and Erbitux showed positive signals in endocardium, lung type II alveolar epithelial cell and surrounding vessels, but showed negative results in kidney and liver tissues. No hemolysis phenomenon and serious side-effects in vessels and muscles were observed in rabbits when administrated with APZ001 and Erbitux respectively.
Conclusion:
The safety comparisons between APZ001 antibody and Erbitux showed that these two antibodies showed highly similarities in mice, rabbits and cynomolgus monkey animal models in consideration of pharmaceutical effects, indicating APZ001 might be a suitable substitute for Erbitux.
Key words:
Cetuximab; Biosimilar Pharmaceuticals; Macaca fascicularis
Introduction
Biosimilar developments have stepped into an explosive era, accompanied with more and more innovator drug patents becoming expired. A vital factor to assess the biosimilar is regarded as the comparability to the original innovator drug. But how similarity is acceptable under current regulatory guidelines? The U.S. Food and Drug Administration defines biosimilarity as ‘‘the biological product that is highly similar to the reference product, notwithstanding minor differences in clinically inactive components’’ with ‘‘no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency of the product’’. In addition to the above definition, although FDA accepts minor modifications or truncations at the NH2- and COOH-terminus, the completed same amino acid sequence with innovator drug is the basic criteria. Generally, pharmacological and toxicological comparability tests in vitro are required in FDA and EMA11 Rossert J. EMEA guidelines on biosimilars and their clinical implications. Kidney Blood Press Res. 2007;30 Suppl 113-7.doi: 10.1159/000107276.
https://doi.org/10.1159/000107276...
. In China, PD and PK evaluation as well as consideration for a repeat dose toxicology study are required22 Cohen AD, Wu JJ, Puig L, Chimenti S, Vender R, Rajagopalan M, Romiti R, de la Cruz C, Skov L, Zachariae C, Young HS, Foley P, van der Walt JM, Naldi L, Blauvelt A. Biosimilars for Psoriasis: worldwide overview of regulatory guidelines, uptake and implications for dermatology clinical practice. Br J Dermatol. 2017 Dec;177(6):1495-502. doi: 10.1111/bjd.15756.
https://doi.org/10.1111/bjd.15756...
.
Erbitux (Cetuximab) is a genetically mouse-human chimeric IgG1 anti-EGFR monoclonal antibody (mAb), which is approved for the treatment of metastatic colorectal cancer and head and neck cancer. However, the expensive cost of Erbitux limited their clinical applications. To develop an economic therapeutic mAb for clinic use, we generated Erbitux biosimilar, an antibody named APZ001. APZ001 is manufactured under GMP standard conditions. According to regulatory criteria for biosimilar equivalent evaluation, we assessed the safety of Erbitux and APZ001 in CD-1 mice, rabbits and Cynomolgus monkeys. The assessments include autonomic activity behavior, nervous activity, acute allergy in tissues and vessels, organs side-effects and organs cross activity. Previously, we have showed the efficacy of APZ001 in xenograft mice models, indicating similar therapeutic effects compared to Erbitux. In the pharmacokinetic parameters assessment in Cynomolgus monkey, APZ001 and Erbitux showed identical results in PK, as well as in the toxicology and immunogenicity. In addition to previous data, we further studied the safety of APZ001 in mice, rabbits and Cynomolgus monkeys, and its comparison to Erbitux.
Methods
Chemicals
Stable DAB Peroxidase Substrates (#54-11-00), Blocking Solution Concentrate for Immunohistochemistry (#71-00-10) and Streptavidin Peroxidase Labeled (#474-3000) were purchased from KPL. Non-specific human IgG was from Thermo Fisher company (#12000C). Haematoxylin (lot: 090721, Kelong Chemical Inc.). Pentobarbital sodium (lot: 090205) was bought from the Sinopharm Chemical Reagent Co., Ltd (Beijing, China).
Animal experiment ethic and animal welfare
CD-1 mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd.. Experiments using CD-1 mouse were licensed by local Ethic Committee (animal use license No. SYXK (Guangdong): 2008-0003).
Mice were raised in PVC cage with 25cm*15cm*15cm in size, with room temperature 20.1~23.7ºC and humidity 63.0~69.8%, 12/12hrs dark and light alternation and 15 times/hr fresh air ventilation. After experiments, all living mice were sacrificed with cervical dislocation and dissected for overall examination.
The usage of Cynomolgus monkey was licensed by local ethic committee [No. SYXK (Guangdong) 2009-0099]. Cynomolgus monkeys and forage were purchased from Guangdong Landau Biotechnology Ltd. Two Cynomolgus monkeys were kept in a 140cm*90cm*90cm stainless steel cage. Monkey feeding condition was setup as: room temperature 16.3~19.7ºC, 58.3~69.2% relative humidity, 8-10 times/hr fresh air change, and 12/12hrs light-dark alteration. For anesthesia and euthanasia, Cynomolgus monkeys were firstly given ketamine hydrochloride 8mg/kg (0.1ml/kg.bw) via muscle injection. Subsequently, monkeys were given 30mg/kg pentobarbital sodium (1.0 ml/kg, bw, i.v.).
The usage of New Zealand rabbits was approved by local ethic committee [license No. SYXK (Guangdong) 2008-0003]. Rabbits were provided by Animal Center of Guangdong Province (SPF grade). Rabbit was kept in 55 cm*37 cm*40 cm stainless steel cages individually. Animal room condition was setup as: room temperature 21.0~26.0ºC, humidity.34.1~66.5%, air refreshment 8-10 times/hr, 12/12hrs dark and light (150~300Lx) alternation. Rabbits could freely access to drinking water and forage, which would be refreshed daily and cleaned.
Cross-reactivity test
Different organ tissue sections of Cynomolgue monkey and human patients were examined via antibody cross-reactivity test by Immunohistochemistry (IHC) staining. Briefly, tissues were fixed using 4% formalin and waxed subsequently. Tissues were sectioned at 3μm per slice, followed by drying at 50ºC overnight. Sections were de-waxed by xylene and gradient ethanol. Sections were blocked by 2% BSA for 1 hr, before biotin-labeled antibody incubation overnight (1:10). Then the slices were washed with 1xPBS for four times. Streptavidin-HRP substrate (1:20) was applied as secondary antibody and DAB substrates were used to detect the signal. To reduce non-specific binding, we generated biotin-labelled primary antibody, and used Streptavidin-HRP substrates to replace secondary antibody. Briefly, commercial anti-EGFR monoclonal antibody was used as positive control, and non-specific human IgG in 1xPBS as negative control. Biotin-labelled APZ001, Erbitux or anti-EGFR antibody was applied (1:10), and Streptavidin-HRP was used as secondary antibody (1:20).
Sections were observed under microscopy and recorded (Olympus, BX41, Japan). There sections included heart, liver, spleen, lung, kidney, ureter, stomach, pituitary body, renicapsule etc. Positive ranking standard was determined according to darkness of color. For example, light yellow was considered as 1+, brown yellow as 2+ and dark brown yellow as 3+. Patients were diagnosed by more than two licensed pathologists.
CD-1 mouse behavior test
CD-1 mice were divided into 5 groups, with 5 male and 5 female in each group, namely, ①negative control group, ② 120mg/kg APZ001, ③ 38mg/kg APZ001, ④ 12mg/kg APZ001 and ⑤ 38mg/kg Erbitux group. We observed and recorded autonomic activities, autonomic nervous functions, non-autonomic nervous functions, nervous excitability, nervous-muscle functions, and sensorimotor functions. We recorded 15min, 0.5hrs, 1hr, 2hrs, 4hrs data after drug administration. We also observed effects on hypnosis induced by subthreshold dose of pentobarbital sodium by the disappearance of righting reflex.
Urinary test
In the urinary function experiment, Cynomolgus monkeys were grouped and administrated with 0.9% NaCl injection for negative control, and three doses of APZ001 or Erbitux. In detail, monkeys were received 120 mg/kg, 38 mg/kg and 12 mg/kg APZ001 administration, which were 1.33, 0.41 and 0.13 folds of human clinical dosage. Erbitux was applied using 38 mg/kg as positive control. Monkey urine was collected at two periods, 0-6hrs and 6-24hrs post-drug treatments. Urine Na+, K+ and Cl- concentrations were analyzed by a Chemistry Analyzer respectively.
Respiratory and cardiovascular alteration test
30 Cynomolgus monkeys were equally divided into five groups, namely ① negative control, ② high dose APZ001, ③ medium dose of APZ001, ④ low dose APZ001 and ⑤ clinical dose Erbitux. Monkeys were received 120 mg/kg, 38 mg/kg and 12 mg/kg APZ001 administration as described before. Erbitux concentration was set at 38 mg/kg as positive control, and 0.9% saline was applied as negative control. Before deep anesthesia maintaining by isoflurane (MIDMARK Matrx VME, USA), monkeys were given 80 mg/ml ketamine for induction. 16 channels electro-physiolograph was used to record the data of blood pressures, cardiogram and breath. We collected data pre-/post-drug administration at different timepoints (5min, 10min, 30min, 45min, 1hr, 2hrs, 3hrs and 5hrs). Heart function parameters included systolic pressure (SP), diastolic pressure (DP), mean pressure (MP), type II electrocardiogram and heart rate. Breath rate and breathing depth were also collected.
Local tolerance and hemolytic experiment
Twenty four New Zealand rabbits were used for vascular stimulation and muscular stimulation tests, 12 for each experiment, equal male and female number.
In vascular stimulation test, left and right sides of rabbit ear was given tested drug and normal saline for comparison. Left body side were administrated Erbitux or APZ001 20 mg/kg (injection volume: 4 ml/kg) with 3 ml/min transfusion. Right side was given equal volume 0.9% normal saline as negative control simultaneously. For muscular stimulation test, left and right sides of rabbit quadriceps femoris was given tested drug and normal saline for comparison, administration volume was 1 ml for each side for 3 times. Three rabbits in each group were analyzed 48hrs post- last time drug administration. Rabbits were sacrificed by bleeding to death after anaesthesia.
We observed and recorded if vasodilatation, swelling, dropsy and blood stasis were occurred in vessel of ear. Rabbit ear tissues were recorded in thickness and necrosis status. Rabbit muscles from injection side were sectioned for pathological analysis. We chose another 3 rabbits for hemolysis test. In the preparation of 2% red cell suspension, rabbit blood was centrifuged at 1620g for 10 min. Red cell pellets were resuspended and washed by 0.9% saline for 5times. When performing hemolysis test, different drugs were added into tubes and mixed with 2ml of red cell suspension buffer. Samples were incubated at 37ºC for 3hrs. We observed every 15 min if red cell lysis occurred.
Statistical analysis
Student’s t-test or Mann-Whitney was performed for two samples comparison after F-test. Paired animal experiment was used t-test after variance equality test (F-test). P<0.05 was regarded as statistically significance in this study (***p<0.05, **p<0.01, ***p<0.001). All statistical analysis was performed using EXCEL software.
Results
Effects of Erbitux and APZ001 on normal behavior, activity and nervous function in CD-1 mice
Mice that received 120 mg/kg, 38 mg/kg and 12 mg/kg APZ001 administration, which are 1.33, 0.41 and 0.13 folds of human clinical dosage according to body surface calculation. We recorded the mice normal behavior, nervous excitability, autonomic and non-autonomic nervous function, nervous-muscle function and coordination function.
In mice behavior observation, various dose of APZ001 and Erbitux showed negative effects on CD-1 mice from timepoint 15min to 4hrs compared to negative group (p>0.05, Table 1, n=20 per group). The comparison between group 38 mg/kg APZ001 and group 38 mg/kg Erbitux showed no significant difference (p>0.05, Table 1). In autonomic nervous system ranking and scoring, different doses of APZ001 and Erbitux did not show significant alterations as well. Comparisons among APZ001 groups and Erbitux group were also remained negative (p>0.05, Table 2). In nervous muscle function evaluation, all drug-treated mice showed the same scoring level 5, indicating negative effects were caused by drug treatments (p>0.05, Table 3). Additionally, the comparison between non-autonomic nervous functions (Table 4), nervous excitability (Table 5), and sensorimotor functions (Table 6) all showed non-significant differences (p>0.05).
Collectively, there were no obvious changes in between pre- and post- drug treated mice. No positive response mice were observed among different concentration groups (p>0.05). Both 120 mg/kg APZ001 and 38 mg/kg Erbitux showed negative effects on CD-1 mice.
Effect of subthreshold doses of pentobarbital sodium anesthesia in CD-1 mice
In this experiment, mice were received high, medium and low doses of APZ001 and medium doses of Erbitux. 0.5hrs later, mice were given equal dosage of pentobarbital sodium (i.p.) for anesthesia. We recorded the anesthesia rates in the first 30min post-pentobarbital administration. Three mice of control group fall asleep, indicating 15% of anesthesia-induced asleep. The number of asleep mice were 3, 4, and 4 in high, medium and low APZ001 dose group, indicating 20% sleep rate (Table 7). 4 mice fell asleep in Erbitux group, showing a 20% sleep rate. All group comparisons were statistically insignificant (p>0.05).
Effects of APZ001 on respiratory system and cardiovascular system in Cynomolgus monkeys
In the evaluation of drugs on Cynomolgus monkeys, we tested and recorded blood pressure, breath parameters and electrocardiogram alteration. Heart function parameters, including systolic and diastolic pressure, average pressure and heart rate, were recorded and compared. There were no significant differences between drug groups and negative control group in heart function. Various doses of APZ001 showed insignificant difference with Erbitux in heart rate (p>0.05; Tables 8 and 9) and pressure in pentobarbital induced aneasthesia (p>0.05; Table 8). In monkeys’ breath parameters analysis, breath rate and depth were recorded. No significant differences were observed between control group and drug-treated groups under aneasthesia (p>0.05, Tables 8 and 9). In electrocardiogram analysis, we compared PR interval, QT interval, duration of QRS, ST average voltage, T wave maximum voltage and adjusted QT interval (QTcF) independently. Results showed that none of the above markers malfunction were observed (p>0.05).
Effects of APZ001 on urinary system in Cynomolgus monkey
Another important biological marker is drug-related urinary volume alteration. We collected urine from timepoint 6hr to 24hr post-drug treatment. Medium APZ001 group showed higher Na+ concentration after drug treatment (*p<0.05), indicating APZ001 could increase urine secretion. High APZ001 group have higher Cl- concentration than control group (*p<0.05). However, other groups showed negative alteration in urine volume when compared with negative groups (p>0.05; Table 10). In monkey’s urine volume analysis, we collected monkey urine every hour. Single administration of high, medium and low dose of APZ001 did not cause monkey urine volume alteration (p>0.05, compared with control group; Table 10). The comparison between high dose APZ001 and medium Erbitux was also showed insignificant difference (p>0.05).
Cross-reactivity analysis between Cynomolgus monkeys and human tissues
IHC staining results showed anti-human-EGFR antibody have cross activity with cynomolgus monkey tissues (Table 11, scoring standard was mentioned in materials and methods part).
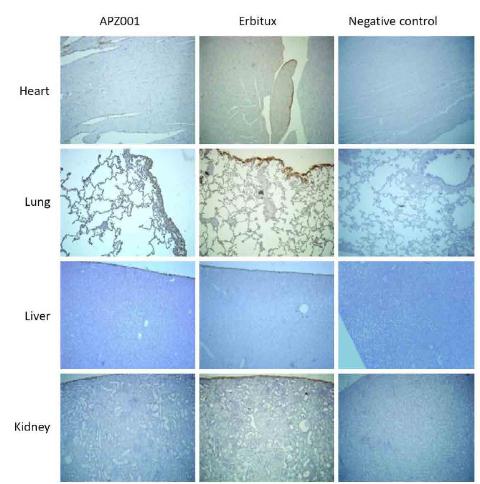
The comparison of positive staining in tissue between APZ001 and Erbitux was not significant (p>0.05). Positive APZ001 and Erbitux groups staining were shown in endocardium, lung type II alveolar epithelial cell and surrounding vessels, liver tunicle and Kidney tunicle (Figure 1). we did not detect positive signal in following tissue sections, including cardiac muscle fibers, lung type II alveolar epithelial cell, liver parenchymal cell, spleen parenchymal cells, kidney parenchymal cells, pituitary body, adrenal essence, hyroid follicular epithelium, parathyroid glands, lymph node parenchyma, bladder mucosa epithelium, cerebellum, cerebral cortex, cornea and nervous, pancreas, skin dermis, muscle fibers, thymicparenchyma, mammary duct, uterus, oarium, oviduct epithelial cell, prostate, testis parenchyma, spinal cord parenchyma and bone marrow (IHC staining data not shown). In human tissues, APZ001 and Erbitux both showed positive signal in endocardium, lung typeⅡ alveolar epithelial cell and surrounding vessels, but showed negative staining results in kidney and liver tissues (Figure 2; Table 12). The following tissues exhibited weak positive signals, such muscle and percreta layer of ureter, gastric submucosa and percreta, adrenal gland tunicle, and thyroid follicular stroma (Table 12).
Cross activity staining by IHC in Cynomolgus monkey organs of APZ001 and Erbitux. In heart, both APZ001 and Erbitux showed heart Endocardium positive staining, but negative in fibers. Lung type I alveolar epithelial cells and surrounding vessel were positive stained, while negative in type II alveolar epithelial cells. APZ001 group showed positive staining in liver tunicle, negative in liver parenchymal cells, as well as that Erbitux group. Kidney tunicle was positive stained by APZ001 and Erbitux, but negative in kidney parenchymal cells (all sections were observed in 100 fold magnification.
Cross reactivity staining by IHC in human organs of APZ001 and Erbitux. Both APZ001 and Erbitux showed positive staining in heart endocardium, but negative in muscle fibers. Lung type I alveolar epithelial cells and surrounding vessel were positive stained, while type II alveolar epithelial cells showed negative staining. Both liver and kidney tissues were negative stained.
Effects of APZ001 on vessel and muscle stimulation in New Zealand rabbits
During the periods of drug administration and recovery, none abnormal reaction was observed. In perusal and microscopy observation of rabbit ear tissues where needles were injected, obvious pathological reaction and damage were not detected in injection spot at 48hrs and 14th day. Results suggested that both Erbitux and APZ001 did not cause rabbit ear vessels acute allergic reaction. In hemolysis in vitro experiment, rabbit blood was incubated with 5 mg/ml Erbitux or APZ001. No hemolysis phenomenon was observed after 3hrs incubation in antibody treated tubes (data not shown). After 48hrs of single injection Erbitux or APZ001, three rabbits showed dark-red plaque reaction in perusal observation. In microscopic observation, the above spots exhibited muscle deconstruction, muscle fibers bumping and breaking. Muscle fiber gaps were enlarged and loosen, infiltrated with inflammatory white cells. In 14th days, the dark-red plaque disappeared in all rabbits in perusal. The microscopy results showed normal muscle fibers structure and integrated, indicating both Erbitux and APZ001 caused slight muscle acute reaction, and such reaction was reversible.
Discussion
In a GLP standard laboratory, we performed APZ001-treated normal behavior activity experiment in a CD-1 mice model. In this in vivo experiment, possible side-effects and un-predicable effects were analyzed, as well as the effects caused by innovator drug named Erbitux. These non-clinical experiments could provide scientific basis for clinical trials. Autonomic activity is one of the major markers which reflect the instinct activity. Compared to pre-drug treatment status, various doses of APZ001 and Erbitux decreased mice autonomic activity significantly (*p<0.05), but the comparison among drug groups showed no differences (p>0.05). In addition, various doses of APZ001 and Erbitux were no difference on mice activity scoring, indicating negative effects of drug on autonomic activity. It was in accordance with peers’ study reports that Erbitux did not show vital side-effects on mice33 Fukuoka S, Kojima T, Koga Y, Yamauchi M, Komatsu M, Komatsuzaki R, Sasaki H, Yasunaga M, Matsumura Y, Doi T, Ohtsu A. Preclinical efficacy of Sym004, novel anti-EGFR antibody mixture, in esophageal squamous cell carcinoma cell lines. Oncotarget. 2017;8(7):11020-9.PMID: 5355242. doi: 10.18632/oncotarget.14209.
https://doi.org/10.18632/oncotarget.1420...
4 Kim S, Prichard CN, Younes MN, Yazici YD, Jasser SA, Bekele BN, Myers JN. Cetuximab and irinotecan interact synergistically to inhibit the growth of orthotopic anaplastic thyroid carcinoma xenografts in nude mice. Clin Cancer Res. 2006;12(2):600-7. PMID: 1403833. doi: 10.1158/1078-0432.CCR-05-1325.
https://doi.org/10.1158/1078-0432.CCR-05...
-55 Hsu YF, Ajona D, Corrales L, Lopez-Picazo JM, Gurpide A, Montuenga LM, Pio R. Complement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivo. Mol Cancer. 2010;9:139. PMID: 2893457. doi: 10.1186/1476-4598-9-139.
https://doi.org/10.1186/1476-4598-9-139...
. In the assessment of autonomic nervous system function experiment, mice defecation, eyelids activity, pupillary light reflex and saliva secretion were recorded. We found that muscle rigidity was occasionally occurred in APZ001 injected-, Erbitux treated-groups, as well as the negative control. We speculated that the muscle abnormal activity might be associated with functional nervous system in the present of overdue stimulation. Overall, after comparison of APZ001 with Erbitux, or drug groups with negative control group, we did not observe the significant difference. In clinical, occasionally muscle rigidity were observed, which could be recovered and released without sever outcome66 Rich A, Toro TZ, Tanksley J, Fiske WH, Lind CD, Ayers GD, Piessevaux H, Washington MK, Coffey RJ. Distinguishing Menetrier's disease from its mimics. Gut. 2010;59(12):1617-24. PMID: 3020399. doi: 10.1136/gut.2010.220061.
https://doi.org/10.1136/gut.2010.220061...
. Collectively, we did not find the significant difference between various doses of APZ001 and negative control group, as well as between Erbitux and negative control. In the bioequivalence assessment, APZ001 showed highly similarity with Erbitux in mice normal behavior activity and nervous system associated functions.
In the evaluation of drug-induced side-effects on cynomolgus monkeys, we recorded and compared monkey cardiovascular functions, urinary system alterations, tissue cross activities and acute allergy reactions. In single injection of Erbitux, the pharmacokinetics was similar to the present study, which was also applied as a biosimilar on monkeys77 Oitate M, Masubuchi N, Ito T, Yabe Y, Karibe T, Aoki T, Murayama N, Kurihara A, Okudaira N, Izumi T. Prediction of human pharmacokinetics of therapeutic monoclonal antibodies from simple allometry of monkey data. Drug Metab Pharmacokinet. 2011;26(4): 423-30. PMID: 21606605.. In the dosages ranging from 7.5 to 75 mg/kg in rhesus monkeys studies, Erbitux injection did not cause severe side-effects77 Oitate M, Masubuchi N, Ito T, Yabe Y, Karibe T, Aoki T, Murayama N, Kurihara A, Okudaira N, Izumi T. Prediction of human pharmacokinetics of therapeutic monoclonal antibodies from simple allometry of monkey data. Drug Metab Pharmacokinet. 2011;26(4): 423-30. PMID: 21606605.,88 Che J, Wang H, Chen Z, Li X, Hou Y, Shan C, Cheng Y. A new approach for pharmacokinetics of single-dose cetuximab in rhesus monkeys by surface plasmon resonance biosensor. J Pharm Biomed Anal. 2009;50(2):183-8. doi: 10.1016/j.jpba.2009.04.009.
https://doi.org/10.1016/j.jpba.2009.04.0...
. As reported by researches on Erbitux or its biosimilars, they reported that Erbitux did not cause vital organs pathological alterations, except rarely reported heart associated heart failure and increased heart beating rate99 Deeken JF, Shimkus B, Liem A, Hill D, Gurtler J, Berghorn E, Townes L, Lu H, Trifan O, Zhang S. Evaluation of the relationship between cetuximab therapy and corrected QT interval changes in patients with advanced malignancies from solid tumors. Cancer Chemother Pharmacol. 2013;71(6):1473-83. doi: 10.1007/s00280-013-2146-5.
https://doi.org/10.1007/s00280-013-2146-...
.
In species cross activity analysis, heart endocardium, lung type II alveolar epithelial cell and surrounding vessels showed moderate positive staining of EGFR expression, but not in cardiac muscle fibers, indicating potential crossing binding APZ001 or anti-EGFR mAb drugs on heart endocardium cells and lung epithelial cells. Heart side-effects caused by Erbitux were reported1010 Gronich N, Lavi I, Barnett-Griness O, Saliba W, Abernethy DR, Rennert G. Tyrosine kinase-targeting drugs-associated heart failure. Br J Cancer. 2017;116(10):1366-73. PMID: 5482733. doi: 10.1038/bjc.2017.88.
https://doi.org/10.1038/bjc.2017.88...
11 Overbeek JA, Zhao Z, van Herk-Sukel MP, Barber BL, Gao S, Herings RM. Costs of hospital events in patients with metastatic colorectal cancer. J Med Econ. 2011;14(5):656-61. doi: 10.3111/13696998.2011.610394.
https://doi.org/10.3111/13696998.2011.61...
-1212 Primrose J, Falk S, Finch-Jones M, Valle J, O'Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M, Iveson T, Hickish T, Butler R, Stanton L, Dixon E, Little L, Bowers M, Pugh S, Garden OJ, Cunningham D, Maughan T, Bridgewater J. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15(6):601-11. doi: 10.1016/S1470-2045(14)70105-6.
https://doi.org/10.1016/S1470-2045(14)70...
recently. They reported that patients might suffer from heart rate increase, sense of suppression in the chest, breathe hard and fatigue susceptibility, and even heart failure1010 Gronich N, Lavi I, Barnett-Griness O, Saliba W, Abernethy DR, Rennert G. Tyrosine kinase-targeting drugs-associated heart failure. Br J Cancer. 2017;116(10):1366-73. PMID: 5482733. doi: 10.1038/bjc.2017.88.
https://doi.org/10.1038/bjc.2017.88...
. Overbeek et al.1111 Overbeek JA, Zhao Z, van Herk-Sukel MP, Barber BL, Gao S, Herings RM. Costs of hospital events in patients with metastatic colorectal cancer. J Med Econ. 2011;14(5):656-61. doi: 10.3111/13696998.2011.610394.
https://doi.org/10.3111/13696998.2011.61...
reported that Erbitux exposure was 1.72 fold OR (1.10-2.69) associated with increased heart failure risk. One patient died of heart failure during the chemotherapy plus Erbitux in 128 patients cohort1212 Primrose J, Falk S, Finch-Jones M, Valle J, O'Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M, Iveson T, Hickish T, Butler R, Stanton L, Dixon E, Little L, Bowers M, Pugh S, Garden OJ, Cunningham D, Maughan T, Bridgewater J. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15(6):601-11. doi: 10.1016/S1470-2045(14)70105-6.
https://doi.org/10.1016/S1470-2045(14)70...
. Collectively, in the assessment of Erbitux and APZ001 in pre-clinical and clinical trials, we concluded that these clinical side-effects were associated with tissue expression of EGFR and its subsequent signal transductions and biological reaction individually. Therefore, we should monitor patients more frequently who have basic diseases of cardiovascular or lung, if the patients want to use Erbitux or its biosimilar.
Hypersensitivity reactions (HSR) and infusion reactions (IRs) could be defined as allergy associated with vessels and surrounding tissues. In clinical application of Erbitux, allergy was reported by many researchers1313 Touma W, Koro SS, Ley J, Wildes TM, Michel L, Tao Y, Adkins D. Risk factors for and pre-medications to prevent cetuximab-induced infusion reactions in patients with squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50(9):895-900. PMID: 4130782. doi: 10.1016/j.oraloncology.2014.06.017.
https://doi.org/10.1016/j.oraloncology.2...
,1414 O'Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, Goldberg RM. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25(24):3644-8. doi: 10.1200/JCO.2007.11.7812.
https://doi.org/10.1200/JCO.2007.11.7812...
. However, vessel high grade sever reaction on skin or surrounding vessels indicated more benefits from Erbitux therapeutics against cancers1515 Michel S, Scherer K, Heijnen IA, Bircher AJ. Skin prick test and basophil reactivity to cetuximab in patients with IgE to alpha-gal and allergy to red meat. Allergy. 2014;69(3):403-5. doi: 10.1111/all.12344.
https://doi.org/10.1111/all.12344...
,1616 Dupont B, Mariotte D, Clarisse B, Galais MP, Bouhier-Leporrier K, Grellard JM, Le Mauff B, Reimund JM, Gervais R. Risk factors associated with hypersensitivity reactions to cetuximab: anti-cetuximab IgE detection as screening test. Future Oncol. 2014;10(14):2133-40. doi: 10.2217/fon.14.153.
https://doi.org/10.2217/fon.14.153...
. Researches suggested that we should particularly observe closely during their first infusion, specially, if patient has allergy history1414 O'Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, Goldberg RM. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25(24):3644-8. doi: 10.1200/JCO.2007.11.7812.
https://doi.org/10.1200/JCO.2007.11.7812...
. In the current experiments on Cynomolgus monkeys, we found such skin allergy reaction on the injection spots, or in muscle tissues. In rabbit vessel allergy reaction, muscle fiber gaps were enlarged and loosen, infiltrated with inflammatory white cells. However, muscle hyperplasia and oedema cases were rarely reported in clinical use of Erbitux, except a report from treatment of Ménétrier’s disease that muscle side-effect was observed66 Rich A, Toro TZ, Tanksley J, Fiske WH, Lind CD, Ayers GD, Piessevaux H, Washington MK, Coffey RJ. Distinguishing Menetrier's disease from its mimics. Gut. 2010;59(12):1617-24. PMID: 3020399. doi: 10.1136/gut.2010.220061.
https://doi.org/10.1136/gut.2010.220061...
. As reported conclusions by researchers on allergy induced by Erbitux medication, we also concluded that doctor should monitor patient’s HSR and IRs, if patients received APZ001 for cancer therapeutics.
Conclusions
Safety assessment and bioequivalence of APZ001 antibody with Erbitux showed that these two antibodies were showed highly similar pharmaceutical effects in mice, rabbits and cynomolgus monkeys. No severe symptoms and side effects were observed in tested animals, suggesting indicating APZ001 might be a suitable substitute for further therapeutic applications.
References
-
1Rossert J. EMEA guidelines on biosimilars and their clinical implications. Kidney Blood Press Res. 2007;30 Suppl 113-7.doi: 10.1159/000107276.
» https://doi.org/10.1159/000107276 -
2Cohen AD, Wu JJ, Puig L, Chimenti S, Vender R, Rajagopalan M, Romiti R, de la Cruz C, Skov L, Zachariae C, Young HS, Foley P, van der Walt JM, Naldi L, Blauvelt A. Biosimilars for Psoriasis: worldwide overview of regulatory guidelines, uptake and implications for dermatology clinical practice. Br J Dermatol. 2017 Dec;177(6):1495-502. doi: 10.1111/bjd.15756.
» https://doi.org/10.1111/bjd.15756 -
3Fukuoka S, Kojima T, Koga Y, Yamauchi M, Komatsu M, Komatsuzaki R, Sasaki H, Yasunaga M, Matsumura Y, Doi T, Ohtsu A. Preclinical efficacy of Sym004, novel anti-EGFR antibody mixture, in esophageal squamous cell carcinoma cell lines. Oncotarget. 2017;8(7):11020-9.PMID: 5355242. doi: 10.18632/oncotarget.14209.
» https://doi.org/10.18632/oncotarget.14209 -
4Kim S, Prichard CN, Younes MN, Yazici YD, Jasser SA, Bekele BN, Myers JN. Cetuximab and irinotecan interact synergistically to inhibit the growth of orthotopic anaplastic thyroid carcinoma xenografts in nude mice. Clin Cancer Res. 2006;12(2):600-7. PMID: 1403833. doi: 10.1158/1078-0432.CCR-05-1325.
» https://doi.org/10.1158/1078-0432.CCR-05-1325 -
5Hsu YF, Ajona D, Corrales L, Lopez-Picazo JM, Gurpide A, Montuenga LM, Pio R. Complement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivo. Mol Cancer. 2010;9:139. PMID: 2893457. doi: 10.1186/1476-4598-9-139.
» https://doi.org/10.1186/1476-4598-9-139 -
6Rich A, Toro TZ, Tanksley J, Fiske WH, Lind CD, Ayers GD, Piessevaux H, Washington MK, Coffey RJ. Distinguishing Menetrier's disease from its mimics. Gut. 2010;59(12):1617-24. PMID: 3020399. doi: 10.1136/gut.2010.220061.
» https://doi.org/10.1136/gut.2010.220061 -
7Oitate M, Masubuchi N, Ito T, Yabe Y, Karibe T, Aoki T, Murayama N, Kurihara A, Okudaira N, Izumi T. Prediction of human pharmacokinetics of therapeutic monoclonal antibodies from simple allometry of monkey data. Drug Metab Pharmacokinet. 2011;26(4): 423-30. PMID: 21606605.
-
8Che J, Wang H, Chen Z, Li X, Hou Y, Shan C, Cheng Y. A new approach for pharmacokinetics of single-dose cetuximab in rhesus monkeys by surface plasmon resonance biosensor. J Pharm Biomed Anal. 2009;50(2):183-8. doi: 10.1016/j.jpba.2009.04.009.
» https://doi.org/10.1016/j.jpba.2009.04.009 -
9Deeken JF, Shimkus B, Liem A, Hill D, Gurtler J, Berghorn E, Townes L, Lu H, Trifan O, Zhang S. Evaluation of the relationship between cetuximab therapy and corrected QT interval changes in patients with advanced malignancies from solid tumors. Cancer Chemother Pharmacol. 2013;71(6):1473-83. doi: 10.1007/s00280-013-2146-5.
» https://doi.org/10.1007/s00280-013-2146-5 -
10Gronich N, Lavi I, Barnett-Griness O, Saliba W, Abernethy DR, Rennert G. Tyrosine kinase-targeting drugs-associated heart failure. Br J Cancer. 2017;116(10):1366-73. PMID: 5482733. doi: 10.1038/bjc.2017.88.
» https://doi.org/10.1038/bjc.2017.88 -
11Overbeek JA, Zhao Z, van Herk-Sukel MP, Barber BL, Gao S, Herings RM. Costs of hospital events in patients with metastatic colorectal cancer. J Med Econ. 2011;14(5):656-61. doi: 10.3111/13696998.2011.610394.
» https://doi.org/10.3111/13696998.2011.610394 -
12Primrose J, Falk S, Finch-Jones M, Valle J, O'Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M, Iveson T, Hickish T, Butler R, Stanton L, Dixon E, Little L, Bowers M, Pugh S, Garden OJ, Cunningham D, Maughan T, Bridgewater J. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15(6):601-11. doi: 10.1016/S1470-2045(14)70105-6.
» https://doi.org/10.1016/S1470-2045(14)70105-6 -
13Touma W, Koro SS, Ley J, Wildes TM, Michel L, Tao Y, Adkins D. Risk factors for and pre-medications to prevent cetuximab-induced infusion reactions in patients with squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50(9):895-900. PMID: 4130782. doi: 10.1016/j.oraloncology.2014.06.017.
» https://doi.org/10.1016/j.oraloncology.2014.06.017 -
14O'Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, Goldberg RM. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25(24):3644-8. doi: 10.1200/JCO.2007.11.7812.
» https://doi.org/10.1200/JCO.2007.11.7812 -
15Michel S, Scherer K, Heijnen IA, Bircher AJ. Skin prick test and basophil reactivity to cetuximab in patients with IgE to alpha-gal and allergy to red meat. Allergy. 2014;69(3):403-5. doi: 10.1111/all.12344.
» https://doi.org/10.1111/all.12344 -
16Dupont B, Mariotte D, Clarisse B, Galais MP, Bouhier-Leporrier K, Grellard JM, Le Mauff B, Reimund JM, Gervais R. Risk factors associated with hypersensitivity reactions to cetuximab: anti-cetuximab IgE detection as screening test. Future Oncol. 2014;10(14):2133-40. doi: 10.2217/fon.14.153.
» https://doi.org/10.2217/fon.14.153
-
Financial source:
The High-level Leading Talent Introduction Program of Guangdong Academic of Sciences, People’s Republic of China (Grant No. 2016GDASRC-0104 and No. 2016GDASRC-0206)
-
1
Research performed at Department of Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Institute of Applied Biological Resources, Guangdong, China.
Publication Dates
-
Publication in this collection
Aug 2018
History
-
Received
10 Apr 2018 -
Reviewed
12 June 2018 -
Accepted
10 July 2018