Abstract
Purpose:
To evaluate the morphological effects of injected sclerosing agents into the liver.
Methods:
This study was performed on twenty dogs, distributed into five groups: Group 1 (n = 5) - control, Group 2 (n = 5) - injection of 50% glucose solution inside hepatic parenchyma and animals followed during seven days, Group 3 (n = 10) - injection of ethanol inside hepatic parenchyma and animals distribution into two subgroups Subgroup 3A (n = 5) - followed during 24 hours and subgroup 3B (n = 5) - followed during seven days (group 3B), Group 4 (n = 5) - ethanol injection inside left portal vein branch and followed during 24 hours. Livers were macroscopically evaluated, submitted to hepatic arteriography and portography, then histology.
Results:
All animals in Group 4 died within 23 hours due to diffuse hepatic necrosis. The animals of groups 2 and 3 had a satisfactory evolution. Fibrosis formed in the segment reached by the sclerosant solution and interruption of the contrast flow injected into the portal system.
Conclusion:
Intrahepatic parenchymal ethanol injection is well tolerated and causes sclerosis restricted to a specific segment; however, intraportal ethanol injection causes massive hepatic necrosis and can lead to death.
Key words:
Liver; Necrosis; Glucose; Hepatectomy; Sclerosis; Dogs
Introduction
Liver surgery has been improved in the last decades, but the most critical aspect of this procedure is still the parenchymal resection due to the high blood loss11 Manas DM, Figueras J, Azoulay D, Garcia Valdecasas JC, French J, Dixon E, O'Rourke N, Grovale N, Mazzaferro V. Expert opinion on advanced techniques for hemostasis in liver surgery. Eur J Surg Oncol. 2016;42:1597-607. doi: 10.1016/j.ejso.2016.05.008.
https://doi.org/10.1016/j.ejso.2016.05.0...
2 Tranchart H, O'Rourke N, Van Dam R, Gaillard M, Lainas P, Sugioka A, Wakabayashi G, Dagher I. Bleeding control during laparoscopic liver resection: a review of literature. J Hepatobiliary Pa:ncreat Sci. 2015;22:371-8. doi: 10.1002/jhbp.217.
https://doi.org/10.1002/jhbp.217...
-33 Huntington JT, Royall NA, Schmidt CR. Minimizing blood loss during hepatectomy: a literature review. J Surg Oncol. 2014;109:81-8. doi: 10.1002/jso.23455.
https://doi.org/10.1002/jso.23455...
. Several methods have been described with the aim of reducing bleeding. Liver and vascular compression, arterial embolization, blood interruption by tapes or clamps, as well as previous intraparenchymal small vessels ligation and greasing the hepatic parenchyma with several different substances are well accepted procedures11 Manas DM, Figueras J, Azoulay D, Garcia Valdecasas JC, French J, Dixon E, O'Rourke N, Grovale N, Mazzaferro V. Expert opinion on advanced techniques for hemostasis in liver surgery. Eur J Surg Oncol. 2016;42:1597-607. doi: 10.1016/j.ejso.2016.05.008.
https://doi.org/10.1016/j.ejso.2016.05.0...
2 Tranchart H, O'Rourke N, Van Dam R, Gaillard M, Lainas P, Sugioka A, Wakabayashi G, Dagher I. Bleeding control during laparoscopic liver resection: a review of literature. J Hepatobiliary Pa:ncreat Sci. 2015;22:371-8. doi: 10.1002/jhbp.217.
https://doi.org/10.1002/jhbp.217...
3 Huntington JT, Royall NA, Schmidt CR. Minimizing blood loss during hepatectomy: a literature review. J Surg Oncol. 2014;109:81-8. doi: 10.1002/jso.23455.
https://doi.org/10.1002/jso.23455...
4 Roullet S, Maistre E, Ickx B, Blais N, Susen S, Faraoni D, Garrigue D, Bonhomme F, Godier A, Lasne D; GIHP. Position of the French Working Group on Perioperative Haemostasis on viscoelastic tests. Anaesth Crit Care Pain Med. 2018; pii: S2352-5568(17)30283-7. doi: 10.1016/j.
5 Petroianu A, Trapiello VN, Esquerdo CRM, Nishimoto RH, Barbosa AJA. Efeito da secção hepática parcial e omentoplastia na regeneração hepática. Rev Col Bras Cir. 1999;26:181-4.
6 Petroianu A, Silva RTF, Parreia LM, Barbosa AJA. Alterações morfológicas do fígado após a secção hepática parcial e omentoplastia. Rev Col Bras Cir. 1998;25:15-7.-77 Petroianu A. Hemostasis of the liver, spleen and bone achieved by electrocautery greased with lidocaine gel. Surg Today. 2011;41:300-2. doi: 10.1007/s00595-009-4211-6.
https://doi.org/10.1007/s00595-009-4211-...
. Another possibility is the intravascular and intraparenchymal injection of sclerosing substances that seal the small vessels, controlling a diffuse hemorrhage88 Gong C, Wang H, Liu P, Guo T. Impact of intraoperative vascular occlusion during liver surgery on long-term outcomes. Int J Surg. 2017;44:110-6. doi: 10.1016/j.ijsu.2017.
https://doi.org/10.1016/j.ijsu.2017...
9 Fry W, Lester C, Etedali NM, Shaw S, DeLaforcade A, Webster CR. Thromboelastography in dogs with chronic hepatopathies. J Vet Intern Med. 2017;31:419-26. doi: 10.1111/jvim.14639.
https://doi.org/10.1111/jvim.14639...
10 Glowka TR, Paschenda P, Czaplik M, Kalff JC, Tolba RH. Assessment of plasma coagulation on liver tissue in a large animal model in vivo. J Vis Exp. 2018;(138). doi: 10.3791/57355. PMID: 30124636.
https://doi.org/10.3791/57355. PMID: 301...
-1111 Petroianu A. Arterial embolization for hemorrhage caused by hepatic arterial injury. Dig Dis Sci. 2007;52:2478-81. doi: 10.1007/s10620-006-9704-1.
https://doi.org/10.1007/s10620-006-9704-...
.
The purpose of this study was to evaluate the effect of intravascular or intraparenchymal injected sclerosing agents on the hepatic tissue.
Methods
This work was approved by the Committee of Ethics in Animal Experimentation (CETEA), Universidade Federal de Minas Gerais (UFMG), in accord with Brazilian law the Council for International Organization of Medical Sciences-CIOMS and under the protocol number 007/2008. 4.
This study was performed on twenty male mongrel dogs, apparently healthy, weighing between 6 and 12 (9.5 ± 1.8) kg. There was no water or food restriction in the preoperative period. The dogs were kept under observation for the apparent diseases during 15 days, in special cages for medium-sized animals, one animal per cage, with daily hygiene and nutrition consisting of dog food and water at will. During the 12 hours prior to the operation, the animals were fasted. There was no water or food restriction in the postoperative period
The animals were divided into five groups:
-
Group 1 (n = 5) - control, without sclerosing procedure in the liver.
-
Group 2 (n = 5) injection of 50% glucose solution in the hepatic parenchyma and followed during seven days,
-
Group 3 (n = 10) - ethanol injection in the hepatic parenchyma and distribution into two subgroups:
-
Subgroup 3A (n = 5) - followed during 24 hours
-
Subgroup 3B (n = 5) - followed during seven days
-
Group 4 (n = 5) - ethanol injection into the left branch of the portal vein and followed during 24 hours.
The animals of groups 2, 3 and 4 were anesthetized with intravenous pentabarbituric injection at an initial dose of 10 mg/kg body weight. Further injections of anesthetic were administered at a concentration of 2 mg/kg, according to the animal’s motor response to the operative stimulus. The dogs were kept in spontaneous breathing, reserving orotracheal tubing and assisted ventilation when respiratory depression was observed. The anesthetized animals underwent median supra-umbilical laparotomy, with liver exposure. A liver biopsy was performed on the diaphragmatic surface of the left medial lobe. The liver was exposed and the procedure was performed according to the group to which the animal belonged, followed by laparorraphy by planes.
After the postoperative follow-up time, including the non-operated Group 1, dogs of all groups were anesthetized by the technique described above, adding 1 ml of intravenous heparin solution (5,000 IU/ml) and undergoing median xyphopubic laparotomy. The caudal vena cava was attached cranially to the implantation of the renal veins. At this stage, the animals were killed with pentabarbituric injection at the dose of 25 mg/kg, which determined respiratory interruption during the anesthesia period. After the dog’s death, the abdominal incision was enlarged to a right transverse thoracophrenotomy exposing the entire caudal cava vein, which was once more attached above the insertion of the hepatic veins. The cava vein was cut proximally to the liver in relation to the vascular ligatures. All perihepatic ligaments, and the liver pediculum were cut and the hepatectomy was complete.
After removal of the liver from the abdominal cavity, the portal vein and the hepatic artery were cannulated with 6 Fr to the portal and 4 Fr to the artery nelaton catheters. A 0.9% saline solution was instilled through the two catheters until intrahepatic blood was removed. A 5 ml of iodinated contrast solution was injected through the catheter inserted into the portal vein, and portography was performed. Subsequently, 5 ml of iodinated contrast medium was injected through the hepatic artery catheter for arteriography. Radiographic studies evaluated the number of vascular branches and contrast flow to the parenchyma, comparing with the Group 1 livers.
After the radiological study, three samples of the hepatic parenchyma were taken, one of the right lobe, one of the left lobe, and the third one from the site of the sclerosing agent injection. The liver samples were included in 10% formaldehyde and routinely treated for histology with hematoxylin and eosin staining. The sections were analyzed for morphological changes and the degree of necrosis induced by the sclerosant solution. Changes in arterial and venous architectures were compared qualitatively with Group 1.
Results
After the intraparenchymal and through the left portal vein branch injections of sclerosant agents, the hepatic segment immediately changed color to purplish and became fibrous. The color and consistency alterations were restricted to the injection site in groups 2 and 3, but in Group 4, the color and parenchymal consistency modifications spread throughout the all left hepatic lobe.
The animals of Group 2 tolerated the injection of the glucose solution in their parenchyma without systemic repercussion and weight loss. Violaceous color and hardening parenchymal consistency were restricted to the site of injection. There was one death in Group 2, during the second postoperative day due to the rupture of an intraparenchymal hematoma caused by the injection needle, which damaged a large vessel. All dogs of this group developed inflammatory reactions in the surgical wound local subcutaneous abscesses, which were successfully treated with drainage and local cleaning. At macroscopy, the livers preserved the lobular architecture, but microcystic dilations in the hepatic parenchyma were observed at histology. No sign of necrosis occurred in this group. The radiographic images of this group did not differ from those found in Group 1.
In Group 3A, three dogs died on the same day of the operation: two dogs presented respiratory depression after the injection of alcohol. Despite the delayed manual ventilation, these animals did not recover. The third death was due to a massive acute necrosis of the left lobe followed by a rapid general decline to death, even though the animal had awakened from anesthesia. The two surviving dogs had macroscopic changes characterized by purplish color and increased hard consistency of the whole left hepatic lobe associated with pericapsular and intrahepatic abscesses in one of these animals. The the lobular architecture was preserved without areas of necrosis except for the abscesses described above. Otherwise, extense necrosis was observed at the intraparenchymatous injection site. In the two surviving animals, arterial and portal circulations reached the periphery of the organ. However the arterio- and portography performed on the livers of the three animals, which died precociously showed contrast restricted to the hepatic hilum without secondary branches.
The five animals of Group 3B, tolerated the intraparenchymal ethanol injection. Two deaths occurred, both on the second postoperative day, one due to left lobe necrosis and the other due to an hemorrhagic shock provoked by the rupture of an hematoma at the injection site. Weight loss between 1.5 kg and 2 kg (15% to 20% of body weight) was verified second and fourth postoperative days in the remaining three animals, but all recovered the weight until the seventh postoperative day. Macroscopic liver alterations were characterized by wine color at the site of injection and cystic lesion with necrotic material. At histology necrosis was found only at the injection site and no sign of fibrosis was found. The arterial architecture did not present alteration when compared with Group 1, however the portal vein was present only near liver pediculum.
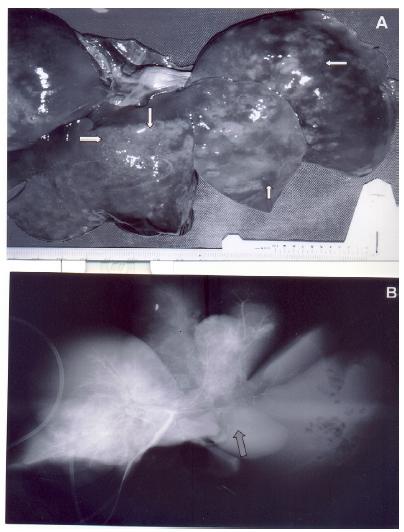
All animals in Group 4 became to be prostrate, right after the surgical recovery, with tachypnea and intense abdominal distension followed by death, in a period ranging from 6 to 72 (23.4 ± 13.0) hours. At necropsy, approximately 300 ml of hemorrhagic fluid and intra-abdominal putrid gas were present, resulting from necrosis by liquefaction of the left lobe. In all hepatic surface small air bubbles friable to the touch were present (Figure 1A). In the liver of these dogs, the radiographic study revealed complete amputation of the left trunk of the hepatic artery and portal vein (Figure 1B). No vessel was histologically evident in the left liver.
Isolated dog liver, 24 hours after absolute alcohol injection in the left branch of the portal vein. A - Liver with areas of necrosis on all surface and small air bubbles friable to the touch (arrows). B - Hepatic arterio- and portography showing amputation of the left vascular tree (arrow).
Discussion
This work is part of an experimental research line related to trauma and hepatic operations55 Petroianu A, Trapiello VN, Esquerdo CRM, Nishimoto RH, Barbosa AJA. Efeito da secção hepática parcial e omentoplastia na regeneração hepática. Rev Col Bras Cir. 1999;26:181-4.
6 Petroianu A, Silva RTF, Parreia LM, Barbosa AJA. Alterações morfológicas do fígado após a secção hepática parcial e omentoplastia. Rev Col Bras Cir. 1998;25:15-7.-77 Petroianu A. Hemostasis of the liver, spleen and bone achieved by electrocautery greased with lidocaine gel. Surg Today. 2011;41:300-2. doi: 10.1007/s00595-009-4211-6.
https://doi.org/10.1007/s00595-009-4211-...
,1111 Petroianu A. Arterial embolization for hemorrhage caused by hepatic arterial injury. Dig Dis Sci. 2007;52:2478-81. doi: 10.1007/s10620-006-9704-1.
https://doi.org/10.1007/s10620-006-9704-...
12 Money SR, Petroianu A, Kimura K, Jaffe BM. Acute hypocalcemic effect of ethanol in dogs. Alcohol Clin Exp Res. 1989;13:453-6. PMID: 2665562.
13 Money SR, Petroianu A, Kimura K, Jaffe BM. The effects of short-term ethanol exposure on the canine jejunal handling of calcium and glucose. Surgery. 1990;107:167-71. PMID: 2300895.
14 Petroianu A. Silva RTF, Parreira LM, Barbosa AJA. Alterações morfológicas do fígado após secção hepática parcial e omentoplastia. Rev Col Bras Cir. 1998;25:15-7.
15 Petroianu A, Trapiello VN, Esquerdo CRM, Nishimoto RH, Barbosa AJA. Efeito da secção hepática parcial e omentoplastia na regeneração hepática de cão. Rev Col Bras Cir. 1999;26:181-4.
16 Petroianu A, Esquerdo CRM, Barbosa AJA, Alberti LR. Regeneração hepática induzida por ressecção segmentar do fígado, em rato. Rev Col Bras Cir. 2004;31:10-4.
17 Sabino KR, Petroianu A, Alberti LR, Machado AN. O efeito do consumo crônico de etanol na absorção duodenal de ferro em camundongos. Arq Gastroenterol. 2010;47:193-6. PMID: 20721467.-1818 Sabino KR, Petroianu A, Alberti LR. Influence of the acute alcoholism on the phagocytic function of the mononuclear phagocytic system. J Med Life. 2011;4:421-3. PMID: 22514578.. Initially, this study was carried out on rats and rabbits, but results achieved on that preliminary step were inconclusive, due to the lack of specificity in hepatic sclerosis and necrosis because the livers were too small. In those pilot studies, necrosis extended to the entire liver, without characterizing specific areas of sclerosis. Based on those results, a special authorization was obtained from CETEA-UFMG to continue this original research without similarity in the literature, in dogs, since a reduced (no more than 20 dogs) amount of animals would be used.
In this study, survival was higher in groups where the sclerosing substance was injected into the hepatic parenchyma than in the groups in which it was injected into the portal vein. The high mortality in the intravenous injection groups was accompanied by endotoxemia without bacteremia, as previously described in the literature1919 Livraghi T. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. Hepatogastroenterology. 2001;48:20-4. PMID: 11268965.
20 Shöenenberg P, Bastid C, Sahiel J. Alcoolisation percutanée guidée par échografie des tumeurs malignes du foie. Schweiz Med Wschr. 1991;121:1686-95. PMID: 1659740.-2121 Johnstone RE, Reier CE. Acute respiratory effects of ethanol in man. Clin Pharmacol Ther. 1973;14:501-3. PMID: 4723257.. According to previous studies, the ethanol used in this research damages the hepatocytes by dehydration, in proportion to its concentration1919 Livraghi T. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. Hepatogastroenterology. 2001;48:20-4. PMID: 11268965.
20 Shöenenberg P, Bastid C, Sahiel J. Alcoolisation percutanée guidée par échografie des tumeurs malignes du foie. Schweiz Med Wschr. 1991;121:1686-95. PMID: 1659740.-2121 Johnstone RE, Reier CE. Acute respiratory effects of ethanol in man. Clin Pharmacol Ther. 1973;14:501-3. PMID: 4723257.. Systemically injected ethanol causes respiratory depression, which can be followed by death when injected at higher concentrations1919 Livraghi T. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. Hepatogastroenterology. 2001;48:20-4. PMID: 11268965.,2121 Johnstone RE, Reier CE. Acute respiratory effects of ethanol in man. Clin Pharmacol Ther. 1973;14:501-3. PMID: 4723257.. This alcohol is also inversely related with hypocalcemia and hypomagnesemia, in proportion to the serum ethanol concentration1212 Money SR, Petroianu A, Kimura K, Jaffe BM. Acute hypocalcemic effect of ethanol in dogs. Alcohol Clin Exp Res. 1989;13:453-6. PMID: 2665562.,1313 Money SR, Petroianu A, Kimura K, Jaffe BM. The effects of short-term ethanol exposure on the canine jejunal handling of calcium and glucose. Surgery. 1990;107:167-71. PMID: 2300895.,2222 Petroianu A, Barquete J, Plentz EA, Bastos CM. Acute effects of alcohol ingestion on the human serum concentration of calcium and magnesium. J Int Med Res. 1991;19:410-3. doi: 10.1177/030006059101900508.
https://doi.org/10.1177/0300060591019005...
,2323 Petroianu A, Barquete J, Plentz EA, Alberti LR. Efeitos da ingestão de álcool nas concentrações séricas humanas de cálcio e magnésio. Rev Bras Med. 2004;61:431-6. PMID: 21152830..
All animals submitted to the injection of ethyl alcohol in the portal vein had respiratory depression immediately after this injection. Next, a tachypnea was observed, which can be considered compensatory for the metabolic acidosis caused by hepatic necrosis. Systemic manifestations indicate that, although the injection was in a specific intrahepatic branch of the portal vein, ethanol reached the systemic circulation and also other regions of the liver.
Changes in hepatic architecture caused by alcohol were observed in portography, with only pre-sinusoidal venous preservation. The damage was mainly to the venous part of the liver. Intraparenchymal injection was accompanied by localized and well-defined fibrosis, with little systemic repercussion. Vascular hypertension or exclusion and systemic response associated to microcirculatory failure and hepatocyte ischemia must be taken into account to explain the adverse effects found mainly in the Group 42424 Biondo-Simões ML, Zamboni CG, Martins E, Lechinewski LD, Ioshii SO, Robes RR. Impact of hypertension in liver regeneration in rats. Acta Cir Bras. 2012;27:460-4. PMID: 22760830.
25 Castro e Silva O, Mente ED, Sankarankutty AK, Souza ME, Gomes MC, Picinato MA, Fina CF, Lopes JR Jr. Biochemical liver function after partial hepatic resection with or without partial hepatic vascular exclusion. Acta Cir Bras. 2011;26 Suppl 2:120-4. PMID: 22030827.
26 Ramalho FS, Fernandez-Monteiro I, Rosello-Catafau J, Peralta C. Hepatic microcirculatory failure. Acta Cir Bras. 2006;21 Suppl 1:48-53. PMID: 17013514.-2727 Galhardo MA, Júnior CQ, Riboli Navarro PG, Morello RJ, Simões Mde J, Montero EF. Liver and lung late alterations following hepatic reperfusion associated to ischemic preconditioning or N-acetylcysteine. Microsurgery. 2007;27:295-9. doi: 10.1002/micr.20359.
https://doi.org/10.1002/micr.20359...
.
Intraparenchymatous injection of hypertonic glucose solution resulted in smaller hepatic lesions than ethanol and did not affect other organs or systems. The glucose solution was not administered by the portal vein because of the velocity and blood volume of the vessel’s flow, leading to a rapid and progressive dilution of the solution, which would not reach adequate concentrations upon contact with the liver tissue. On the other hand, glucose solution has been routinely used in medical practice, such as superficial vascular sclerosants, sometimes associated with some alcohols.
The results of the present study provided informations that support the use of sclerosing substances as ethanol, injected into the parenchyma, to produce ischemia, as in the treatment of neoplastic lesions, however, intravenous injection of alcohol can be lethal. On the other hand, the use of 50% glucose solution was not efficacious in establishing a localized ischemia in the hepatic parenchyma.
Conclusion
Intrahepatic parenchymal ethanol injection is well tolerated and causes sclerosis restricted to a specific segment; however, intraportal ethanol injection causes massive hepatic necrosis and can lead to death.
References
-
1Manas DM, Figueras J, Azoulay D, Garcia Valdecasas JC, French J, Dixon E, O'Rourke N, Grovale N, Mazzaferro V. Expert opinion on advanced techniques for hemostasis in liver surgery. Eur J Surg Oncol. 2016;42:1597-607. doi: 10.1016/j.ejso.2016.05.008.
» https://doi.org/10.1016/j.ejso.2016.05.008 -
2Tranchart H, O'Rourke N, Van Dam R, Gaillard M, Lainas P, Sugioka A, Wakabayashi G, Dagher I. Bleeding control during laparoscopic liver resection: a review of literature. J Hepatobiliary Pa:ncreat Sci. 2015;22:371-8. doi: 10.1002/jhbp.217.
» https://doi.org/10.1002/jhbp.217 -
3Huntington JT, Royall NA, Schmidt CR. Minimizing blood loss during hepatectomy: a literature review. J Surg Oncol. 2014;109:81-8. doi: 10.1002/jso.23455.
» https://doi.org/10.1002/jso.23455 -
4Roullet S, Maistre E, Ickx B, Blais N, Susen S, Faraoni D, Garrigue D, Bonhomme F, Godier A, Lasne D; GIHP. Position of the French Working Group on Perioperative Haemostasis on viscoelastic tests. Anaesth Crit Care Pain Med. 2018; pii: S2352-5568(17)30283-7. doi: 10.1016/j.
-
5Petroianu A, Trapiello VN, Esquerdo CRM, Nishimoto RH, Barbosa AJA. Efeito da secção hepática parcial e omentoplastia na regeneração hepática. Rev Col Bras Cir. 1999;26:181-4.
-
6Petroianu A, Silva RTF, Parreia LM, Barbosa AJA. Alterações morfológicas do fígado após a secção hepática parcial e omentoplastia. Rev Col Bras Cir. 1998;25:15-7.
-
7Petroianu A. Hemostasis of the liver, spleen and bone achieved by electrocautery greased with lidocaine gel. Surg Today. 2011;41:300-2. doi: 10.1007/s00595-009-4211-6.
» https://doi.org/10.1007/s00595-009-4211-6 -
8Gong C, Wang H, Liu P, Guo T. Impact of intraoperative vascular occlusion during liver surgery on long-term outcomes. Int J Surg. 2017;44:110-6. doi: 10.1016/j.ijsu.2017.
» https://doi.org/10.1016/j.ijsu.2017 -
9Fry W, Lester C, Etedali NM, Shaw S, DeLaforcade A, Webster CR. Thromboelastography in dogs with chronic hepatopathies. J Vet Intern Med. 2017;31:419-26. doi: 10.1111/jvim.14639.
» https://doi.org/10.1111/jvim.14639 -
10Glowka TR, Paschenda P, Czaplik M, Kalff JC, Tolba RH. Assessment of plasma coagulation on liver tissue in a large animal model in vivo. J Vis Exp. 2018;(138). doi: 10.3791/57355. PMID: 30124636.
» https://doi.org/10.3791/57355. PMID: 30124636 -
11Petroianu A. Arterial embolization for hemorrhage caused by hepatic arterial injury. Dig Dis Sci. 2007;52:2478-81. doi: 10.1007/s10620-006-9704-1.
» https://doi.org/10.1007/s10620-006-9704-1 -
12Money SR, Petroianu A, Kimura K, Jaffe BM. Acute hypocalcemic effect of ethanol in dogs. Alcohol Clin Exp Res. 1989;13:453-6. PMID: 2665562.
-
13Money SR, Petroianu A, Kimura K, Jaffe BM. The effects of short-term ethanol exposure on the canine jejunal handling of calcium and glucose. Surgery. 1990;107:167-71. PMID: 2300895.
-
14Petroianu A. Silva RTF, Parreira LM, Barbosa AJA. Alterações morfológicas do fígado após secção hepática parcial e omentoplastia. Rev Col Bras Cir. 1998;25:15-7.
-
15Petroianu A, Trapiello VN, Esquerdo CRM, Nishimoto RH, Barbosa AJA. Efeito da secção hepática parcial e omentoplastia na regeneração hepática de cão. Rev Col Bras Cir. 1999;26:181-4.
-
16Petroianu A, Esquerdo CRM, Barbosa AJA, Alberti LR. Regeneração hepática induzida por ressecção segmentar do fígado, em rato. Rev Col Bras Cir. 2004;31:10-4.
-
17Sabino KR, Petroianu A, Alberti LR, Machado AN. O efeito do consumo crônico de etanol na absorção duodenal de ferro em camundongos. Arq Gastroenterol. 2010;47:193-6. PMID: 20721467.
-
18Sabino KR, Petroianu A, Alberti LR. Influence of the acute alcoholism on the phagocytic function of the mononuclear phagocytic system. J Med Life. 2011;4:421-3. PMID: 22514578.
-
19Livraghi T. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. Hepatogastroenterology. 2001;48:20-4. PMID: 11268965.
-
20Shöenenberg P, Bastid C, Sahiel J. Alcoolisation percutanée guidée par échografie des tumeurs malignes du foie. Schweiz Med Wschr. 1991;121:1686-95. PMID: 1659740.
-
21Johnstone RE, Reier CE. Acute respiratory effects of ethanol in man. Clin Pharmacol Ther. 1973;14:501-3. PMID: 4723257.
-
22Petroianu A, Barquete J, Plentz EA, Bastos CM. Acute effects of alcohol ingestion on the human serum concentration of calcium and magnesium. J Int Med Res. 1991;19:410-3. doi: 10.1177/030006059101900508.
» https://doi.org/10.1177/030006059101900508 -
23Petroianu A, Barquete J, Plentz EA, Alberti LR. Efeitos da ingestão de álcool nas concentrações séricas humanas de cálcio e magnésio. Rev Bras Med. 2004;61:431-6. PMID: 21152830.
-
24Biondo-Simões ML, Zamboni CG, Martins E, Lechinewski LD, Ioshii SO, Robes RR. Impact of hypertension in liver regeneration in rats. Acta Cir Bras. 2012;27:460-4. PMID: 22760830.
-
25Castro e Silva O, Mente ED, Sankarankutty AK, Souza ME, Gomes MC, Picinato MA, Fina CF, Lopes JR Jr. Biochemical liver function after partial hepatic resection with or without partial hepatic vascular exclusion. Acta Cir Bras. 2011;26 Suppl 2:120-4. PMID: 22030827.
-
26Ramalho FS, Fernandez-Monteiro I, Rosello-Catafau J, Peralta C. Hepatic microcirculatory failure. Acta Cir Bras. 2006;21 Suppl 1:48-53. PMID: 17013514.
-
27Galhardo MA, Júnior CQ, Riboli Navarro PG, Morello RJ, Simões Mde J, Montero EF. Liver and lung late alterations following hepatic reperfusion associated to ischemic preconditioning or N-acetylcysteine. Microsurgery. 2007;27:295-9. doi: 10.1002/micr.20359.
» https://doi.org/10.1002/micr.20359
-
Financial sources:
FAPEMIG, CNPq, and UFMG
-
1
Research performed at Department of Surgery, School of Medicine, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte-MG, Brazil.
Publication Dates
-
Publication in this collection
Sept 2018
History
-
Received
22 May 2018 -
Reviewed
20 July 2018 -
Accepted
21 Aug 2018