Abstract
Purpose:
To evaluate the effects of hyperbaric oxygenation on prevention of adhesions in the abdominal cavity after laparotomy.
Methods:
Fifty four rats underwent laparotomy; stitches were made in the four quadrant parietal peritoneum and abdominal cavity closure. Animals were divided into three groups: 1 - control; 2 - subjected to high pressures and oxygenation; 3 - subjected to 100% hyperbaric oxygenation. The animals in groups 2 and 3 were daily submitted to oxygenation hyperbaric chamber after surgery. On the seventh day another laparotomy, registration of procedure, assessment of adhesions and biopsies of the peritoneum were held. Professionals analyzed the videos and the biopsies.
Results:
Peritoneal cavity adhesions occurred in animals of three groups with no difference between them. In Group 3, the adhesions presented more fragile and vascular proliferation more pronounced, and there was no difference in comparison with the first and second groups. However, there was no significant difference in the evaluation of these parameters between the animals in groups 1 and 2.
Conclusions:
Postoperative hyperbaric oxygenation in rats submitted to laparotomy did not alter the frequency, but reduced the density of adhesions in the peritoneal cavity and promoted vascular proliferation. The change in atmospheric pressure alone had no influence on the results.
Key words:
Hyperbaric Oxygenation; Tissue Adhesions; Laparotomy; Rats
Introduction
Adhesions represent one of the major problems arising from surgical procedures in the abdominal cavity, since their incidence is high, they cause complications and their prevention is still a challenge. The main consequences are: chronic abdominal pain; secondary infertility in women; obstruction, ischemia and intestinal perforation; more difficult reoperations with longer duration, higher risk of complications and increased mortality. In addition, after surgical treatment of adhesions, complications can arise and require new laparotomies for adhesion removal and treatment of complications11 Alpay Z, Saed GM, Diamond MP. Postoperative adhesions: from formation to prevention. Semin Reprod Med. 2008;26(4):313-21. doi: 10.1055/s-0028-1082389.
https://doi.org/10.1055/s-0028-1082389...
,22 Becker JM, Stucchi AF. Intra-abdominal adhesion prevention: are we getting any closer? Ann Surg. 2004;240(2):202-4. PMID: 15273541..
Surveys were carried out with the aim of finding ways to prevent adhesions formation in the peritoneal cavity33 Schnüriger B, Barmparas G, Branco BC, Lustenberger T, Inaba K, Demetriades D. Prevention of postoperative peritoneal adhesions: a review of the literature. Am J Surg. 2011;201(1):111-21. PMID: 20817145.
4 Kamel RM. Prevention of postoperative peritoneal adhesion. Eur J Obstet Gynecol Reprod Biol. 2010;150:111-8. PMID: 20382467.
5 Lauder CI, Garcea G, Strickland A, Maddern GJ. Abdominal adhesion prevention: still a sticky subject? Dig Surg. 2010;27(5):347-58. PMID: 20847564.
6 Cheung JP, Tsang HH, Cheung JJ, Yu HH, Leung GK, Law WL. Adjuvant therapy for the reduction of postoperative intra-abdominal adhesion formation. Asian J Surg. 2009;32(3):180-6. PMID: 19656760.
7 Viana AET, Daud F, Bonizzia A, Barros PH, Gouvêa E. Comparative study between parietal peritoneum suture and nonsuture in midline laparotomies in rats. Acta Cir Bras. 2008;23(4):348-51. PMID: 18641805.
8 Souza Filho ZA, Greca F, Noronha L, Maranhão A, Calil A, Hubie D, Barbosa F. Abdominal wall healing in reoperated rats. Acta Cir Bras. 2007;22(2):147-51. doi: 10.1590/s0102-86502007000200013.
https://doi.org/10.1590/s0102-8650200700...
9 Claudio RH, Diogo Filho A, Mamede Filho DO. Peritoneostomy with latex coated polypropylene: experimental study in rats. Acta Cir Bras. 2006;21(6):402-8. PMID: 17160253.
10 Berkkanoglu M, Zhang L, Ulukus M, Cakmak H, Kayisli UA, Kursun S, Arici A. Inhibition of chemokines prevents intraperitoneal adhesions in mice. Hum Reprod. 2005;20(11):3047-52. PMID: 16006464.
11 Ellis H. Intraabdominal and postoperative peritoneal adhesions. J Am Coll Surg. 2005;200(5):641-4. PMID: 15848353.
12 Johns A. Evidence-based prevention of post-operative adhesions. Hum Reprod Update. 2001;7(6):577-9. PMID: 11727866.
13 Smaniotto B, Simões M, Artigas G, Silva A, Collaço L, Ramasco G. Effect of streptokinase in the prevention of intra-abdominal adhesions in the rat. Acta Cir Bras. 1997;12(4):240-5. doi: 10.1590/s0102-86501997000400005.
https://doi.org/10.1590/s0102-8650199700...
14 Verreet PR, Fakir C, Ohmann C, Röher HD. Preventing recurrent postoperative adhesions: an experimental study in rats. Eur Surg Res. 1989;21(5):267-73. PMID: 2627981.-1515 Holtz G. Prevention and management of peritoneal adhesions. Fertil Steril. 1984;41(4):497-507. PMID: 6200365.. It is known that the supply of oxygen in tissues is vital in the process of repair after peritoneal trauma and determinant in healing, which reflects in the prevention of adhesions. Studies have shown that hyperbaric oxygenation contributes to the treatment of wounds, either by the shorter healing time or by the decrease of the infection. This therapy has been used in various conditions with good results1616 Al-waili NS, Butler GJ. Effects of hyperbaric oxygen on inflammatory response to wound and trauma: possible mechanism of action. Scientific World J. 2006;6:425-4. PMID: 16604253.,1717 Binda MM, Molinas CR, Koninckx PR. Reactive oxygen species and adhesion formation: clinical implications in adhesion prevention. Hum Reprod. 2003;18(12):2503-7. PMID: 14645163., however, the studies on the formation of adhesions in the abdominal cavity after laparotomy are still incipient.
The present study aimed to evaluate the effects of hyperbaric oxygenation on prevention of adhesions and contribute to the reduction of these frequent complications in patients undergoing laparotomy.
Methods
This research was approved by the Departmental Chamber, Surgery Department of the Medical School, UFMG and by the Animal Research Ethics Committee (No. 169/10).
The sample consisted of 54 rats of the species Rattus novergicus and Wistar strain, with an average of two months of age, whose weight varied from 250 to 280g. The animals were provided by the Institute of Biological Sciences of UFMG and remained in quarantine for 48 hours without signs or symptoms of diseases that would justify their exclusion from research. Surgical procedures were performed in the Nucleus of Animal Experimentation of the UFMG Medical School. During the experiment, the animals were housed in cages, evaluated daily, received industrial feed twice a day (Presence rats and mice®), filtered water at will and daily sanitization of cages and the environment. In the immediate postoperative period they were housed in individual cages to prevent wounds and aggression caused by the surgical wound.
All the animals of the three groups were subjected to the same surgical procedure on the same day and distributed randomly in three groups of 18 animals, according to established standardization for each group, i.e.:
-
Group 1-laparotomy (control)
-
Group 2-laparotomy, oxygenation to 21% and 2.5 atmospheres
-
Group 3-laparotomy, oxygenation to 100% and 2.5 atmospheres
In order to minimize stress, animals were transferred to the experiment room in individual cages moments before the surgical procedure. General anesthesia was performed through the association of Ketamine at a dose of 70 mg/kg and Xylazine at a dose of 10 mg/kg intramuscular injection, according to protocol and guidelines of the Committee of Ethics in Animal Experimentation (CETEA). The animals were immobilized in dorsal decubitus on Styrofoam board capped with plastic by applying strips of tape in the upper and lower limbs. The antisepsis of the ventral region was done with a solution of chlorhexidine.
The surgical procedure consisted of: median incision of 4cm long, starting 1cm of xiphoid process and extending to the pubis; four stitches with 2-0 silk, followed by two stitches in peritoneum parietal 1cm lateral to the midline incision, in all four quadrants, top and bottom, right and left, to stimulate local reaction and formation of adhesions (Figure 1); suture in single plan with 3-0 nylon and cleaning surgical wound with chlorhexidine to remove blood samples and reduce the risk of self-harm. It was used ketoprofen at a dose of 3mg/kg subcutaneously every 12 hours in the first few days after surgery for analgesia.
Digital photograph of the abdominal cavity, showing the 2-0 silk suture, 1.0 cm incision in the upper and lower right quadrants.
The animals from Group 1 (control) were subject only to the surgical procedure.
Animals of groups 2 and 3 were also submitted to the oxygenation in the hyperbaric chamber with 2,5atm pressure, in daily session, lasting 90 minutes during six days after the surgical procedure. The total time of oxygenation was 120 minutes, the first 15 minutes were used to gradual compression and the last 15 for gradual decompression
The animals from Group 2 were submitted to 21% oxygenation and group 3 to 100%. Hyperbaric oxygenation in both groups was held simultaneously in separate chambers.
On the seventh day after surgery (Group 1) and the next day at the end of the hyperbaric oxygenation (groups 2 and 3), which corresponds to the seventh day of the postoperative period, the animals underwent euthanasia in CO2 chamber. In all animals laparotomy were performed in “U” shape to enable the opening of the cavity and evaluate the adhesions without damaging them. Initially it was made a transverse suprapubic incision and two incisions parallel to the first laparotomy scar and laterally to the points with silk stitches applied in the parietal peritoneum.
After opening the abdominal cavity, the abdominal wall elevation and the tension necessary to cause the adhesion rupture were recorded by means of photo and filming. In cases where the adhesion did not rupture by simple traction, anatomical tweezers were used to evaluate the resistance of each one of them. This documentation was recorded in spreadsheet without specifying which group each animal belonged to (Figure 2).
Digital photograph of abdominal cavity and adhesions of the types resistant (red arrow) and fragile (white arrow).
Samples of peritoneum were collected in four quadrants (left and right, top and bottom) and in the surgical wound, totaling five samples for each animal, that were identified by numbers. The material was fixed with 10% formaldehyde and sent to anatomopathological examination with the numbering of each animal, without identifying the group. The slides were stained with hematoxylin and eosin (HE). The peritoneal biopsies histology were evaluated regarding the following aspects: inflammation, fibrosis and vascular proliferation, according to the modified classification by Irkorucu1818 Irkorucu O, Ferahköse Z, Memis L, Ekinci O, Akin M. Reduction of postsurgical adhesions in a rat model: a comparative study. Clinics (Sao Paulo). 2009;64(2):143-8. doi: 10.1590/s1807-593220090000200012.
https://doi.org/10.1590/s1807-5932200900...
. Those biopsies were histological classified among fibrosis, inflammation and vascular proliferation and each of these histologic alterations were graduated between 1 and 2.
-
Fibrosis were considered:
light (1) when there were loose connective stroma;
intense (2) when there were dense connective stroma.
-
Inflammation were considered:
light (1) when had minimal to moderate number of giant cells, occasional lymphocytes, plasma cells eosinophils and neutrophils;
intense (2) when had many inflammatory cells and microabsces.
-
Vascular proliferations were classified as:
light (1) when there were low to moderate vascularization;
intense (2) when it was very vascularized.
The pathologist recorded the individual rating of each slide and, later, the general classification for each rat according to the assessment of the four quadrants.
The animals were divided into two groups, graduate light and intense, with the purpose of statistical analysis of the sample. The histopathological exam has dichotomization between animals classified as light and intense aspect (Figures 3 to 5).
Photomicrographs showing slight fibrosis, loose connective stroma and newly formed blood vessels (A) and (B) with intense dense connective stroma with fibrosis and deposition of fiber collagens thick (HE, x100).
Photomicrographs showing light inflammation (A) and intense inflammatory infiltrate mixed with high number of inflammatory cells mono and polymorphonuclear (B) (HE, x40).
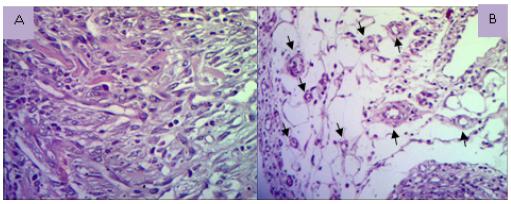
Photomicrographs showing vascular proliferation with denser and loose connective stroma vascular proliferation (A) (HE, x400); loose connective tissue with newly formed vessels (arrows) (B) (HE x100).
Three surgeons evaluated the videos individually, without knowing which group the animal belonged to. Each observer issued the graduation result of adhesions of each animal based on macroscopic classification of Mazuji et al.1919 Mazuji MK, Kalambaheti K, Pawar B. Prevention of adhesions with polyvinylpyrrolidone. Preliminary Report. Arch Surg. 1964;89(6):1011-5. doi: 10.1001/archsurg.1964.01320060079015.
https://doi.org/10.1001/archsurg.1964.01...
, prevailing the largest degree:
-
0 - absent of adhesions;
-
1 - small and irregular;
-
2 - easily separable of medium intensity;
-
3 - tough and difficult separation;
-
4 - very tough difficult separation and homogeneus.
This range made it possible to outline the population under study in order to identify variations of characteristics of adhesions. It was subsequently held the distribution of animals in two groups: fragile adhesions (grade between zero and two) and tough adhesions (animals graded between three and four). This dichotomization has been developed to enable the statistical comparison and evaluation.
For statistical analysis between the groups the animals were classified according to the adhesions characteristics. The fragile are easy to break and possibility of reabsorption (fibrinolysis), as well as potentially failing to present effective risk by generating mechanical obstruction. The toughs are considered difficult to break and probably permanent. The same criteria were applied in the microscopic evaluation of fibrosis, inflammation and vascular proliferation as mild and intense.
The comparison between the three groups of animals, regarding the characteristics of the adhesions, was performed using the Fisher test in the Epi InfoTM7 software, as follows:
-
Groups 1 and 3 were compared in order to verify the changes between two variables - the 100% oxygen and 2.5 atmospheres pressure - and if the association of both would cause changes in the results;
-
Groups 2 and 3 were compared to assess only the variable oxygenation, as the two groups were subjected to the same procedures, displacements and hyperbaric sessions;
-
Groups 1 and 2 were compared to verify if oxygenation pressure change alone would determine changes in the formation of adhesions.
Results
Group 1 (control) and group 2 (21% oxygenation) presented adhesions. The animals from Group 3 (100% hyperbaric oxygenation) showed a significantly higher number of adhesions of the fragile type than the animals in the control group (p = 0.002) and the 21% oxygen group (p = 0.01), and in one of them there was no adhesion.
In the comparison between control and 100% oxygenation groups, it was found the presence of fibrosis, characterized as light and intense, showed no significant difference (p = 0.66), as well as the presence of Inflammation (p = 0.40). On the other hand, vascular proliferation characterized as intense involved most animals undergoing 100%, oxygenation (p = 0.02).
In the comparison between groups of animals with oxygenation at 100% and 21%, that the presence of fibrosis, characterized as light and intense, showed no significant difference (p = 0.66), as well as the presence of Inflammation (p = 0.40). On the other hand, vascular proliferation characterized as intense occurred with more expressiveness in animals subjected to the 100% oxygen (p = 0.02). Regarding the adhesions of the peritoneal cavity characterized as fragile and tough, they were found in both groups, with no significant difference (p = 0.73).
The presence of fibrosis, inflammation and vascular proliferation in the peritoneum was observed in animals of the control group and the 21% oxygen, with no significant difference (p = 1.00) (Table 1).
Comparison between the three groups of animals related to the intensity of adhesions in the peritoneal cavity and microscopic changes during diferent oxigenation levels.
Discussion
This research made it possible to evaluate the pathophysiological changes of the surgical procedure and the prophylactic efficacy of hyperbaric oxygenation. The formation of adhesions relates, for the most part, with injuries that occur during surgery causing mainly imbalance in the interaction of coagulation, fibrinolytic systems and inflammatory2020 Corona R, Verguts J, Schonman R, Binda M, Mailova K, Koninckx P. Postoperative inflammation in the abdominal cavity increases adhesion formation in a laparoscopic mouse model. Fertil Steril. 2011;95(4):1224-8. PMID: 21295297.,2121 Hellebrekers BW, Kooistra T. Pathogenesis of postoperative adhesion formation. Br J Surg. 2011;98(11):1503-16. PMID: 21877324.. Based on this principle, and considering that ischemia is the major inducer of adhesion formation2222 Kece C, Yildiz F, Simsek A, Ozer MT, Deveci S, Ozgurtas T, Sadir S, Korkmaz A, Korkmaz O, Ersoz N, Tufan T. Synergistic effects of hyperbaric oxygen and granulocytecolony stimulating factor on postoperative adhesion formation in a rat cecal abrasion model. Bratisl Lek Listy. 2010;111(3):115-20. PMID: 20437818.,2323 Matsuzaki S, Canis M, Bazin JE, Darcha C, Pouly JL, Mage G. Effects of supplemental perioperative oxygen on post-operative abdominal wound adhesions in a mouse laparotomy model with controlled respiratory support. Hum Reprod. 2007;22(10):2702-6. PMID: 17720702., the proposal was elaborated on the preventive potential of hyperbaric oxygenation. Encouraged by this possibility, the pilot project was carried out, which led to the delineation of the methodology.
The survey was conducted in rats because they have characteristics that facilitate the experiment, as lower morbidity and mortality, good resistance to infections and surgical procedures, easy processing and handling and reproducibility of the experiment.
In a comparative study of adhesion-forming models, in the search for a consistent model, the suture in the parietal peritoneum was proposed by Whang et al.2424 Whang SH, Astudillo JA, Sporn E, Bachman SL, Miedema BW, Davis W, Thaler K. In search of the best peritoneal adhesion model: comparison of different techniques in a rat model. J Surg Res. 2011;167(2):245-50. PMID: 20304431., due to the easy reproducibility that allows standardization of the measurement. We opted for modifications of the model, based on the works of2525 Corrales F, Corrales M, Schirmer C. Preventing intraperitoneal adhesions with vitamin E and sodium hyaluronate/carboxymethylcellulose: a comparative study in rats. Acta Cir Bras. 2008;23(1):36-4. doi: 10.1590/s0102-86502008000100007.
https://doi.org/10.1590/s0102-8650200800...
,2626 Ezberci F, Bulbuloglu E, Ciragil P, Gul M, Kurutas EB, Bozkurt S, Kale IT. Intraperitoneal tenoxicam to prevent abdominal adhesion formation in a rat peritonitis model. Surg Today. 2006;36(4):361-6. PMID: 16554994., namely, interventions in four quadrants, avoiding the concentration of adhesions in one region and the suture wire used was 2-0 silk.
Regarding hyperbaric oxygenation, there is a committee that defines its applicability in tissue ischemia, ischemic ulcers, diabetic and pressure, fascia inflammation, osteomyelitis, carbon monoxide poisoning and gas embolism. However, the indications are increasing over the years2020 Corona R, Verguts J, Schonman R, Binda M, Mailova K, Koninckx P. Postoperative inflammation in the abdominal cavity increases adhesion formation in a laparoscopic mouse model. Fertil Steril. 2011;95(4):1224-8. PMID: 21295297.,2121 Hellebrekers BW, Kooistra T. Pathogenesis of postoperative adhesion formation. Br J Surg. 2011;98(11):1503-16. PMID: 21877324.,2727 Fernandes T D. Hyperbaric medicine. Acta Med Port. 2009;22(4):323-34. doi: 10.20344/amp.1709.
https://doi.org/10.20344/amp.1709...
28 Costa-Val R, Nunes T, Silva R, Souza T. Hyperbaric oxygen therapy in rats submitted to hepatic veins ligation: mortality valuation and histological study of liver and spleen. Acta Cir Bras. 2006;21(1):52-7. doi: 10.1590/s0102-86502006000100012.
https://doi.org/10.1590/s0102-8650200600...
-2929 Gottrup F. Oxygen in wound healing and infection. World J Surg. 2004;28(3):312-5. PMID: 14961190.. Systemic oxygenation is changed by oxygen provided at 100% when applied at high pressure as it increases the partial pressure of oxygen and its solubility in blood plasma. The greater oxygenation in traumatized regions and in ischemic tissue promotes the downfall of pro-inflammatory stimulation by inhibiting the regulation of cytokines and growth factors. Oxygen is directly related to increased resistance to infection, synthesis of collagen and neovascularization. In addition, the alternation of hyperoxia and normal oxygenation improves microvascular perfusion, by stimulating neovascularization in the region because it causes the interpretation of low oxygenation after hyperoxia as a state of hypoxia. With the increased oxygen intake, mediators like prostaglandins, cytokines and nitric oxide, responsible for the inhibition of fibrinolysis and collagen deposition, decrease considerably and contribute to the adhesions to be undone and not organized into resistant and definitive.
Aware of these factors, in the present study, hyperbaric oxygenation sessions were initiated the day after the surgical procedure to avoid stress other than surgical stress and increased morbidity and mortality of the animals by moving them to the hyperbaric treatment center. We opted for a daily session based on studies that have found that intermittent exposure to oxygen can be enough to meet the needs of the ischemic tissue reported that the effectiveness of increasing the concentration of oxygen in the tissues occurs within the period of 90 minutes1818 Irkorucu O, Ferahköse Z, Memis L, Ekinci O, Akin M. Reduction of postsurgical adhesions in a rat model: a comparative study. Clinics (Sao Paulo). 2009;64(2):143-8. doi: 10.1590/s1807-593220090000200012.
https://doi.org/10.1590/s1807-5932200900...
,2323 Matsuzaki S, Canis M, Bazin JE, Darcha C, Pouly JL, Mage G. Effects of supplemental perioperative oxygen on post-operative abdominal wound adhesions in a mouse laparotomy model with controlled respiratory support. Hum Reprod. 2007;22(10):2702-6. PMID: 17720702.,3030 Yagci G, Ozturk E, Ozgurtas T, Gorgulu S, Kutlu OC, Topal T, Cetiner S, Tufan T. Preoperative and postoperative administration of hyperbaric oxygen improves biochemical and mechanical parameters on ischemic and normal colonic anastomoses. J Invest Surg. 2006;19(4):237-44. PMID: 16835138.. In lower periods, the procedure was ineffective and, in longer periods, may increase the risk of complications.
The animal reoperations were programmed for the seventh day of the postoperative, as proposed by Cheong et al.3131 Cheong YC, Laird SM, Li TC, Shelton JB, Ledger WL, Cooke ID. Peritoneal healing and adhesion formation/reformation. Hum Reprod Update. 2001;7(6):556-66. PMID: 11727864.and Molinas et al.3232 Molinas CR, Mynbaev O, Pauwels A, Novak P, Koninckx PR. Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model. Fertil Steril. 2001;76(3):560-7. PMID: 11532482., because these authors reported that in the period of five to eight days, the healing process occurred almost entirely.
In the present study, there was no significant difference between the groups in the frequency of abdominal adhesions. In rats subjected to 100% oxygenation and 2.5atm pressure (group 3), fragile adhesions were observed, with a significant difference in comparison with the other groups. In this group there were also significant increase in vascular proliferation, in comparison with the other groups, which may have contributed to the decline of local ischemia and consequent change of the intensity of intra-abdominal adhesions. This result is in accordance with the statement by Munireddy and colleagues3333 Munireddy S, Kavalukas SL, Barbul A. Intra-abdominal healing: gastrointestinal tract and adhesions. Surg Clin North Am. 2010;90(6):1227-36. PMID: 21074038.that the hyperbaric oxygenation is able to diminish the deleterious effects caused by the surgical trauma, by acting in the process of ischemia and reperfusion. This effect may be due to the reduction of free radicals and nitric oxide, since these substances present in greater quantity in the ischemic tissue, can promote the growth of collagen, causing organization and persistence of adhesions2121 Hellebrekers BW, Kooistra T. Pathogenesis of postoperative adhesion formation. Br J Surg. 2011;98(11):1503-16. PMID: 21877324..
The existence of high frequency of fragile adhesions on the seventh postoperative day of rats submitted to 100% hyperbaric oxygenation (Group 3), suggests that this procedure may be useful in prevention of tough adhesions after laparotomy. In addition, it should be considered that it is a non-invasive intervention, with no increase in morbidity or mortality or the risk of complications inherent in the surgical process, and with a view to reducing the occurrence of suture dehiscences and formation of infectious processes, as well as the acceleration of wound healing. Yagci et al.3030 Yagci G, Ozturk E, Ozgurtas T, Gorgulu S, Kutlu OC, Topal T, Cetiner S, Tufan T. Preoperative and postoperative administration of hyperbaric oxygen improves biochemical and mechanical parameters on ischemic and normal colonic anastomoses. J Invest Surg. 2006;19(4):237-44. PMID: 16835138.concluded that hyperbaric oxygenation on pre and postoperative gastrointestinal anastomosis in patients with deficiency of microcirculation and diabetes decreased the risk of complications.
It is important to note that to take effect, in addition to the 2,5atm pressure, oxygenation is necessary at 100%, since, in the present study, there was no difference in the kind of adhesions when it compared the group of animals subjected to the 21% oxygen and 2.5 atm pressure (Group 2) with the group of animals without hyperbaric oxygenation (control group).
The effectiveness of hyperbaric oxygenation must be refined with beginning the sessions on the day of operation to further improve early tissue oxygenation and thus reduce the initial inflammatory response, the production of cytokines, nitric oxide, prostaglandins and favor the fibrinolytic system since the beginning of the process. In order to effectively assess the effects of hyperbaric oxygenation studies of mesothelial cells or scar tissue are required. The combination of techniques of histology, immunology, biochemistry and immunohistochemistry could identify components involved and changed throughout the healing process.
Conclusions
Hyperbaric oxygenation postoperatively in rats undergoing laparotomy did not alter the frequency of adhesions, but reduced the intensity of the same besides promoting peritoneal vascular proliferation in the affected area. Changing only atmospheric pressure did not influence the formation of adhesions.
References
-
1Alpay Z, Saed GM, Diamond MP. Postoperative adhesions: from formation to prevention. Semin Reprod Med. 2008;26(4):313-21. doi: 10.1055/s-0028-1082389.
» https://doi.org/10.1055/s-0028-1082389 -
2Becker JM, Stucchi AF. Intra-abdominal adhesion prevention: are we getting any closer? Ann Surg. 2004;240(2):202-4. PMID: 15273541.
-
3Schnüriger B, Barmparas G, Branco BC, Lustenberger T, Inaba K, Demetriades D. Prevention of postoperative peritoneal adhesions: a review of the literature. Am J Surg. 2011;201(1):111-21. PMID: 20817145.
-
4Kamel RM. Prevention of postoperative peritoneal adhesion. Eur J Obstet Gynecol Reprod Biol. 2010;150:111-8. PMID: 20382467.
-
5Lauder CI, Garcea G, Strickland A, Maddern GJ. Abdominal adhesion prevention: still a sticky subject? Dig Surg. 2010;27(5):347-58. PMID: 20847564.
-
6Cheung JP, Tsang HH, Cheung JJ, Yu HH, Leung GK, Law WL. Adjuvant therapy for the reduction of postoperative intra-abdominal adhesion formation. Asian J Surg. 2009;32(3):180-6. PMID: 19656760.
-
7Viana AET, Daud F, Bonizzia A, Barros PH, Gouvêa E. Comparative study between parietal peritoneum suture and nonsuture in midline laparotomies in rats. Acta Cir Bras. 2008;23(4):348-51. PMID: 18641805.
-
8Souza Filho ZA, Greca F, Noronha L, Maranhão A, Calil A, Hubie D, Barbosa F. Abdominal wall healing in reoperated rats. Acta Cir Bras. 2007;22(2):147-51. doi: 10.1590/s0102-86502007000200013.
» https://doi.org/10.1590/s0102-86502007000200013 -
9Claudio RH, Diogo Filho A, Mamede Filho DO. Peritoneostomy with latex coated polypropylene: experimental study in rats. Acta Cir Bras. 2006;21(6):402-8. PMID: 17160253.
-
10Berkkanoglu M, Zhang L, Ulukus M, Cakmak H, Kayisli UA, Kursun S, Arici A. Inhibition of chemokines prevents intraperitoneal adhesions in mice. Hum Reprod. 2005;20(11):3047-52. PMID: 16006464.
-
11Ellis H. Intraabdominal and postoperative peritoneal adhesions. J Am Coll Surg. 2005;200(5):641-4. PMID: 15848353.
-
12Johns A. Evidence-based prevention of post-operative adhesions. Hum Reprod Update. 2001;7(6):577-9. PMID: 11727866.
-
13Smaniotto B, Simões M, Artigas G, Silva A, Collaço L, Ramasco G. Effect of streptokinase in the prevention of intra-abdominal adhesions in the rat. Acta Cir Bras. 1997;12(4):240-5. doi: 10.1590/s0102-86501997000400005.
» https://doi.org/10.1590/s0102-86501997000400005 -
14Verreet PR, Fakir C, Ohmann C, Röher HD. Preventing recurrent postoperative adhesions: an experimental study in rats. Eur Surg Res. 1989;21(5):267-73. PMID: 2627981.
-
15Holtz G. Prevention and management of peritoneal adhesions. Fertil Steril. 1984;41(4):497-507. PMID: 6200365.
-
16Al-waili NS, Butler GJ. Effects of hyperbaric oxygen on inflammatory response to wound and trauma: possible mechanism of action. Scientific World J. 2006;6:425-4. PMID: 16604253.
-
17Binda MM, Molinas CR, Koninckx PR. Reactive oxygen species and adhesion formation: clinical implications in adhesion prevention. Hum Reprod. 2003;18(12):2503-7. PMID: 14645163.
-
18Irkorucu O, Ferahköse Z, Memis L, Ekinci O, Akin M. Reduction of postsurgical adhesions in a rat model: a comparative study. Clinics (Sao Paulo). 2009;64(2):143-8. doi: 10.1590/s1807-593220090000200012.
» https://doi.org/10.1590/s1807-593220090000200012 -
19Mazuji MK, Kalambaheti K, Pawar B. Prevention of adhesions with polyvinylpyrrolidone. Preliminary Report. Arch Surg. 1964;89(6):1011-5. doi: 10.1001/archsurg.1964.01320060079015.
» https://doi.org/10.1001/archsurg.1964.01320060079015 -
20Corona R, Verguts J, Schonman R, Binda M, Mailova K, Koninckx P. Postoperative inflammation in the abdominal cavity increases adhesion formation in a laparoscopic mouse model. Fertil Steril. 2011;95(4):1224-8. PMID: 21295297.
-
21Hellebrekers BW, Kooistra T. Pathogenesis of postoperative adhesion formation. Br J Surg. 2011;98(11):1503-16. PMID: 21877324.
-
22Kece C, Yildiz F, Simsek A, Ozer MT, Deveci S, Ozgurtas T, Sadir S, Korkmaz A, Korkmaz O, Ersoz N, Tufan T. Synergistic effects of hyperbaric oxygen and granulocytecolony stimulating factor on postoperative adhesion formation in a rat cecal abrasion model. Bratisl Lek Listy. 2010;111(3):115-20. PMID: 20437818.
-
23Matsuzaki S, Canis M, Bazin JE, Darcha C, Pouly JL, Mage G. Effects of supplemental perioperative oxygen on post-operative abdominal wound adhesions in a mouse laparotomy model with controlled respiratory support. Hum Reprod. 2007;22(10):2702-6. PMID: 17720702.
-
24Whang SH, Astudillo JA, Sporn E, Bachman SL, Miedema BW, Davis W, Thaler K. In search of the best peritoneal adhesion model: comparison of different techniques in a rat model. J Surg Res. 2011;167(2):245-50. PMID: 20304431.
-
25Corrales F, Corrales M, Schirmer C. Preventing intraperitoneal adhesions with vitamin E and sodium hyaluronate/carboxymethylcellulose: a comparative study in rats. Acta Cir Bras. 2008;23(1):36-4. doi: 10.1590/s0102-86502008000100007.
» https://doi.org/10.1590/s0102-86502008000100007 -
26Ezberci F, Bulbuloglu E, Ciragil P, Gul M, Kurutas EB, Bozkurt S, Kale IT. Intraperitoneal tenoxicam to prevent abdominal adhesion formation in a rat peritonitis model. Surg Today. 2006;36(4):361-6. PMID: 16554994.
-
27Fernandes T D. Hyperbaric medicine. Acta Med Port. 2009;22(4):323-34. doi: 10.20344/amp.1709.
» https://doi.org/10.20344/amp.1709 -
28Costa-Val R, Nunes T, Silva R, Souza T. Hyperbaric oxygen therapy in rats submitted to hepatic veins ligation: mortality valuation and histological study of liver and spleen. Acta Cir Bras. 2006;21(1):52-7. doi: 10.1590/s0102-86502006000100012.
» https://doi.org/10.1590/s0102-86502006000100012 -
29Gottrup F. Oxygen in wound healing and infection. World J Surg. 2004;28(3):312-5. PMID: 14961190.
-
30Yagci G, Ozturk E, Ozgurtas T, Gorgulu S, Kutlu OC, Topal T, Cetiner S, Tufan T. Preoperative and postoperative administration of hyperbaric oxygen improves biochemical and mechanical parameters on ischemic and normal colonic anastomoses. J Invest Surg. 2006;19(4):237-44. PMID: 16835138.
-
31Cheong YC, Laird SM, Li TC, Shelton JB, Ledger WL, Cooke ID. Peritoneal healing and adhesion formation/reformation. Hum Reprod Update. 2001;7(6):556-66. PMID: 11727864.
-
32Molinas CR, Mynbaev O, Pauwels A, Novak P, Koninckx PR. Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model. Fertil Steril. 2001;76(3):560-7. PMID: 11532482.
-
33Munireddy S, Kavalukas SL, Barbul A. Intra-abdominal healing: gastrointestinal tract and adhesions. Surg Clin North Am. 2010;90(6):1227-36. PMID: 21074038.
-
Financial source:
none
-
1
Research performed at Surgery Department, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte-MG, Brazil.
Publication Dates
-
Publication in this collection
Sept 2018
History
-
Received
29 May 2018 -
Reviewed
27 July 2018 -
Accepted
26 Aug 2018