Abstracts
This paper has focused on the factors that may affect the permeability of adhesive resins into the demineralized dentin matrix during the development of the bonding process. The effects of surface moisture are discussed respectively to the adhesive systems, and the problems related to incomplete hybrid layer formation presented.
Dentin; Dentin-bonding agents; Hybridization; Permeability
Este trabalho focaliza os aspectos que podem afetar a permeabilidade de resinas adesivas na matriz de dentina desmineralizada durante o desenvolvimento do processo adesivo. Os efeitos da umidade de superfície, respectivamente aos sistemas adesivos e aos problemas relacionados com a formação incompleta da camada híbrida são apresentados.
Dentina; Adesivos dentinários; Hibridização; Permeabilidade
Dentística
Resin diffusion through demineralized dentin matrix
Difusão de resina através da matriz de dentina desmineralizada
Ricardo M. CARVALHO* * Assistant Professor, Dept. of Operative Dentistry, Bauru School of Dentistry, University of São Paulo, Brazil. ** Associate Professor, Dept. of Endodontics, School of Dentistry, University of Geneve, Switzerland. *** Professor, Dept. of Operative Dentistry, School of Dentistry, University of Hokkaido, Japan. **** Assistant Professor, Dept. of Restorative Dentistry, Tokushima School of Dentistry, University of Tokushima, Japan. ***** Regents Professor, Dept. of Oral Biology-Physiology, School of Dentistry, Medical College of Georgia, USA. I Fukushima, personal communication, 1995.
Bernard CIUCCHI** * Assistant Professor, Dept. of Operative Dentistry, Bauru School of Dentistry, University of São Paulo, Brazil. ** Associate Professor, Dept. of Endodontics, School of Dentistry, University of Geneve, Switzerland. *** Professor, Dept. of Operative Dentistry, School of Dentistry, University of Hokkaido, Japan. **** Assistant Professor, Dept. of Restorative Dentistry, Tokushima School of Dentistry, University of Tokushima, Japan. ***** Regents Professor, Dept. of Oral Biology-Physiology, School of Dentistry, Medical College of Georgia, USA. I Fukushima, personal communication, 1995.
Hidehiko SANO*** * Assistant Professor, Dept. of Operative Dentistry, Bauru School of Dentistry, University of São Paulo, Brazil. ** Associate Professor, Dept. of Endodontics, School of Dentistry, University of Geneve, Switzerland. *** Professor, Dept. of Operative Dentistry, School of Dentistry, University of Hokkaido, Japan. **** Assistant Professor, Dept. of Restorative Dentistry, Tokushima School of Dentistry, University of Tokushima, Japan. ***** Regents Professor, Dept. of Oral Biology-Physiology, School of Dentistry, Medical College of Georgia, USA. I Fukushima, personal communication, 1995.
Masahiro YOSHIYAMA**** * Assistant Professor, Dept. of Operative Dentistry, Bauru School of Dentistry, University of São Paulo, Brazil. ** Associate Professor, Dept. of Endodontics, School of Dentistry, University of Geneve, Switzerland. *** Professor, Dept. of Operative Dentistry, School of Dentistry, University of Hokkaido, Japan. **** Assistant Professor, Dept. of Restorative Dentistry, Tokushima School of Dentistry, University of Tokushima, Japan. ***** Regents Professor, Dept. of Oral Biology-Physiology, School of Dentistry, Medical College of Georgia, USA. I Fukushima, personal communication, 1995.
David H. PASHLEY***** * Assistant Professor, Dept. of Operative Dentistry, Bauru School of Dentistry, University of São Paulo, Brazil. ** Associate Professor, Dept. of Endodontics, School of Dentistry, University of Geneve, Switzerland. *** Professor, Dept. of Operative Dentistry, School of Dentistry, University of Hokkaido, Japan. **** Assistant Professor, Dept. of Restorative Dentistry, Tokushima School of Dentistry, University of Tokushima, Japan. ***** Regents Professor, Dept. of Oral Biology-Physiology, School of Dentistry, Medical College of Georgia, USA. I Fukushima, personal communication, 1995.
CARVALHO, R. M.; CIUCCHI, B.; SANO, H.; YOSHIYAMA, M.; PASHLEY, D. H. Resin diffusion through demineralized dentin matrix. Rev Odontol Univ São Paulo, v.13, n.4, p.417-424, out./dez. 1999.
This paper has focused on the factors that may affect the permeability of adhesive resins into the demineralized dentin matrix during the development of the bonding process. The effects of surface moisture are discussed respectively to the adhesive systems, and the problems related to incomplete hybrid layer formation presented.
UNITERMS: Dentin; Dentin-bonding agents; Hybridization; Permeability.
INTRODUCTION
It is generally accepted that the bonding mechanism of current generation dentin bonding systems relies on the permeation of adhesive resin monomers into acid-etched dentin surfaces9,13. Proper infiltration of adhesive resins into the patent open dentinal tubules and the porosities of the intertubular dentin is desirable to create resin tags and hybrid layer. This is expected to optimize bond strengths and sealing of dentin surfaces. Although adhesive systems are expected to uniformly treat dentin surfaces resulting in a uniform bonded layer, this ideal is seldom achieved. Since the ratio of dentinal tubules to intertubular dentin varies a great deal with dentin depth, the contribution of individual bonding mechanisms (resin tags and hybrid layer formation) to the total bond strength also varies accordingly14. At superficial dentin, dentinal tubules may represent as little as 1% of the total surface area11, therefore, hybrid layer formation to intertubular dentin represents the main bonding mechanism. Conversely, at deep dentin, the contribution of hybrid layer to the total bond strength is reduced while the contribution of resin tags increase14. Ideal hybrid layer formation requires the infiltration of adhesive resin monomers to the entire depth of the demineralized zone and proper polymerization of the resin monomers, thereby protecting and reinforcing the exposed collagen network, resulting in a stable, non-permeable adhesive layer. Earlier observations of cut and polished bonded interfaces have shown an apparent compact, fully-infiltrated, acid-resistant hybrid layer formation9,10. However, recent observations of cut, polished and argon ion beam etched and fractured interfaces have questioned the ability of resin monomers to fully infiltrate the demineralized zone1,5,23. This manuscript has focused on the factors that may affect proper resin infiltration into the demineralized zone and its consequences to the adhesion of dentin bonding agents to dentin.
Effects of smear layer and surface moisture
When examining a mineralized fractured dentin surface, it is clear that the only porosities that may permit resin infiltration are the dentinal tubules. Mechanical retention can be achieved by resin penetration into the tubules if they are properly wetted by an hydrophilic primer. Conversely, dentin surfaces that were cut with rotatory instruments present an apparent non-porous surface due to formation of the smear layer. The smear layer and the smear plugs cover the entire cut surface, precluding infiltration of resin monomers into intertubular dentin and dentinal tubules. Acid-etching of dentin is therefore recommended to remove the smear layer and the smear plugs and demineralize the top dentin surface, thus creating opportunity for resin monomers to penetrate the dentinal tubules and the collagen network at the intertubular dentin.
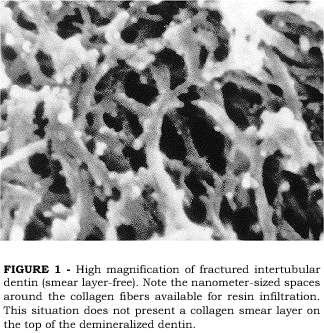
PASHLEY et al.13 investigated the effects of the presence of a smear layer on the permeation of adhesive resins into acid-etched dentin that was either kept wet or air-dried. Examining the intertubular dentin of smear layer-free surfaces that were acid-etched and kept wet, a very porous surface is seen (Figure 1). The loss of the mineral phase in between the collagen fibers left nanometer-sized spaces around the collagen fibers which communicate with the surface, thus offering ideal porosity for resin infiltration. When the same procedure is applied on a smear layer-covered dentin surface, the loss of mineral and interfiber porosities are also seen. However, at the very top of the demineralized zone, a thin, amorphous layer may be seen deposited on the collagen fibers (Figure 2). This crust layer has been described as being the collagen smear layer12. Since the smear layer is composed of the same elements of the sound dentin from which it is formed, cut, denatured collagen debris are present in the smear layer that are not dissolved by the acid-etching. During acid-etching, these collagen debris fall onto the demineralized dentin matrix underneath, forming a thin crust that may interfere with the permeation of adhesive resins through the porous interfiber spaces12. The formation of the collagen smear layer may also be a result of a slight collapse of the top end of the collagen fibers which may occur during etching or during specimen preparation for SEM, even when care is taken to avoid air-drying of the specimen. The same collagen smear layer was not observed in another study which attempted to examine the dentin surface after acid-etching20. The authors claimed that when the surface is kept wet after etching, no crusts that may interfere with resin infiltration are formed. However, the specimens prepared by PASHLEY et al.13, were also kept wet. One possible explanation for that is the way the dentin surface was prepared before etching in both studies. In Pashleys study, the dentin surface was cut with diamond burs while in Tays study the surface was prepared by polishing the dentin with a 600 grit SiC paper. Although both preparations were done under water irrigation, the heat generated by cutting with burs may be somewhat higher, thus causing surface denaturation of some collagen fibers
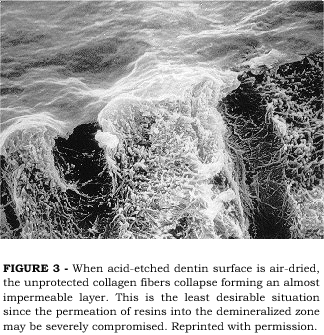
A worse situation may be caused when the demineralized dentin surface is allowed to air-dry. Air-drying demineralized dentin surfaces causes a dramatic collapse of the collagen network onto the sound dentin underneath the demineralized zone2,19. We have demonstrated that demineralized dentin matrix may shrink up to 65% in volume when allowed to air-dry2. The collapse of the collagen network is more evident at the top of the demineralized zone, forming a layer with largely reduced porosity which will create difficulty for resin permeation into the spaces around the collagen fibers. Figure 3 illustrates a non-desirable situation where demineralized dentin was air-dried before preparation for SEM. Even though the subsurface porosity may be preserved, the reduced porosity of the top surface drastically reduces the ability of resin monomers to infiltrate the intertubular dentin within the time period of adhesive resin application in a clinical situation. When resin monomers are applied to this less permeable intertubular dentin surface, full infiltration of the demineralized zone is compromised. The resin monomers may reach the subsurface through lateral diffusion from the demineralized top end of the dentinal tubules and lateral canals, however, the reduced porosity at the top of the demineralized intertubular dentin will cause a reduction in resin concentration at that area. The concentration of resin at the top of the demineralized zone may be as low as 10% and that can be regarded as a weak zone in the bonded interface12.
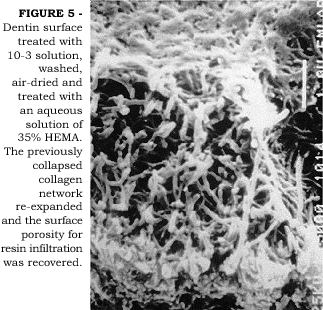
Demineralized, air-dried, shrunken dentin can recover its original volume when immersed in water2. In a clinical situation, the maintenance of a certain degree of moisture on the dentin surface after acid-etching is crucial to maintain the collagen fibers in an expanded state, thereby facilitating the infiltration of resin monomers to a maximum depth. If dentin is allowed to air-dry or the professional blows air to confirm the efficacy of enamel etching, the ideal condition of expanded collagen fibers can be recovered by re-wetting the surface6. Better bond strength data have been reported to dentin when it is in a moist condition than when in dry condition6,7. Better bond strengths are probably a result of improved permeation of adhesive monomers across wet versus dry demineralized dentin matrix27. SUGISAKI19 has pointed out that primer solutions could re-expand air-dried shrunken dentin. In Figure 4 we observe an SEM image of dentin surface that was acid-etched with 10-3 solution, washed and air-dried. The surface is collapsed and the porosity reduced. When a solution of 35% HEMA in water was then applied, the collagen fibers expanded thus increasing the surface porosity (Figure 5). We have tested the hypothesis that a solution of 100% HEMA could re-expand previously air-dried, shrunken dentin2. When air-dried, shrunken demineralized dentin specimens were immersed in 100% HEMA for 24 hours, no re-expansion was observed. However, when the same specimens were immersed in a 50% HEMA/50% water solution, re-expansion was observed. The role that water plays during hybrid layer formation has been extensively discussed in a recent review paper12. Surface moisture is of particular importance when dealing with water-free adhesives because aqueous-based systems may contain enough water in the primer solution to permit partial re-expansion of inadvertently air-dried surface24. It is clear that a critical amount of water is necessary to accomplish optimal permeability of demineralized intertubular dentin to adhesive resins. TAY et al.20,21 have shown that water in excess is also detrimental to good adhesion. Since proper resin infiltration is represented by a complete replacement of the water by the adhesive monomers, the infiltration process is a battle between the molar ratio of the applied adhesive and the molar ratio of water within the fibers. An overwet surface will cause phase separation of the monomers forming blister-like structures on the surface that prevents resin infiltration into the demineralized collagen matrix21. The critical amount of water, or surface moisture, necessary to permit near-perfect resin infiltration is, however, unknown to the present moment and may vary according to the adhesive system used. Complicating these factors, there is the difficulty to achieve ideal moisture over an entire cavity preparation in a clinical situation. The consequence of this is the undesirable yet expected non-uniformity of bonding.
The presence of porosities within the hybrid layer
By etching hybrid layers with argon ion beam, VAN MEERBEEK et al.23 demonstrated that they appeared to be softer than the adhesive resin layer and resin tags. They suggested that the hybrid layer could not be as fully infiltrated with resin as expected. Examination of hybrid layers on cut and highly polished interfaces may not represent the reality since one can inadvertently force polishing debris into porosities that were previously present. If bonded interfaces are fractured and not polished, porosities that represent lack of resin infiltration are readily seen (Figure 6). One could argue that the porosities might be created because of the stresses generated during fracturing of the specimens. We believe that is not the case. SANO et al.16 have used silver nitrate tracing solution to demonstrate leakage at bonded interfaces of gap-free margins. Silver nitrate accumulation could be seen within the nanometer-sized spaces between collagen fibers in the hybrid layer of several adhesive systems. This was named nanoleakage16,18. It is interesting to note that the distribution of the silver grains in the hybrid layer, as examined by TEM (Figure 7), seems to follow the distribution of collagen fibers in the demineralized zone. We have tested the degree of nanoleakage of several dentin bonding systems to the present (unpublished data). Although very different results were found among the systems, all of them showed some degree of nanoleakage.
Newly developed self-etching/self-priming adhesive systems have achieved bond to dentin by simultaneously etching and infiltrating the adhesive monomers into dentin surface4,26. These systems have the advantage that, theoretically, no discrepancies would occur between the etching depth and extension of resin infiltration. Such systems are known to form a thinner hybrid layer which incorporates the remnants of the smear layer on the dentin surface. The quality of the hybrid layer produced by self-etching adhesives is probably improved regarding the presence of porosities. This would explain that although thinner hybrid layers are formed, similar high bond strengths are obtained with these systems when compared to conventional acid-etching systems17,25. Our data suggest that improved sealing is also achieved by self-etching systems when nanoleakage is considered. Since they are applied directly on the smear layer-covered dentin regardless of the surface moisture, one can expect less sensitivity of these systems to the water content of the surface. PEREIRA et al.15 have shown that one self-etching adhesive system was practically insensitive not only to surface moisture but also to dentin depth. Such characteristics are highly desirable when attempting to bond to a dynamic substrate such as dentin.
Consequences of less than optimal resin infiltration into the demineralized zone
As discussed above, the presence of the collagen smear layer associated with a slight collapsing of collagen fibers at the top of the demineralized zone may result in a reduced concentration of resin at the top of the hybrid layer. That area is considered weak and may constitute an area of stress concentration when the bond is stressed to failure. One of the first concerns regarding the efficacy of the hybrid layer as a bonding mechanism of adhesive resins to dentin was the possibility that resin monomers would not reach the bottom of the demineralized zone, thus leaving collagen fibers exposed and unprotected10. Uninfiltrated, exposed collagen fibers were considered a weak link of the hybrid layer to the sound dentin underneath. This bottom layer of exposed collagen fibers would be subjected to hydrolytic degradation whenever oral or dentinal fluids could reach it10. The presence of nanoleakage within the hybrid layer confirms that the porosities communicate with the outer surface and may allow for oral fluids permeation. Degradation of either the exposed collagen fibers or incompletely polymerized resin monomers may compromise the bond. In this regard, it is more likely that the resin monomers will degrade first. If a hybrid layer is not fully formed, the unfilled spaces probably contain residual water that was not replaced by resin. In this case, the presence of water around the resin monomers will result in incomplete polymerization thereby causing easier degradation over time. Demineralized dentin collagen that was stored for 18 months in saline did not show any decrease in its tensile strength and modulus of elasticity3, indicating that hydrolytic effects might have not occurred during such a period. The degradation effects resulting from the nanoleakage may explain the significant decrease in bond strength with time as generally observed in the literature.
Incompletely filled hybrid layers have, however, a possible advantage when we consider the stresses generated at the interface by resin polymerization contraction and occlusal loads. Since it has a low modulus of elasticity, it may function as a stress absorbing layer which compensates the debonding forces, hence, preserving the bond22. Although this may be of importance for bond strength, it lacks benefits for marginal sealing. In fact, we could speculate that the stretching/compressing action of the elastic hybrid layer could cause a squeezing out/sucking in effect, thereby constantly renewing the fluids within the hybrid layer. This could bring in bacterial products and enzymes from colonies harbored at the margins of the restoration.
The stress distribution at bonded interfaces of adhesive resins and dentin is a very complex matter. Abrupt changes in the modulus of elasticity from one layer to another may be responsible for stress concentration and failure at that particular link. However, if we believe that weaker points within the bonded interface would fail first, that could explain the general observation that most bonds fail either at the top or at the bottom of the hybrid layer or in a combination of both. By monitoring the dynamics of failure of a bonded interface during stressing in the SEM chamber, we can observe an example of failure that was initiated at the top of the hybrid layer (Figures 8 e 9). The debonding area is coincident with the area where the collagen smear layer is located.
Understanding the complex mechanisms that are involved during a clinical bonding attempt is crucial to achieve adequate in vivo adhesion to dentin. Once the clinician is aware of the phenomenon described here, he/she will be able to master the bonding technique and obtain the best performance of the adhesive systems.
Concluding remarks
The formation of collagen smear layer on dentin surfaces after acid-etching and air-drying may be a limiting factor to infiltration of resins into dentin.
This may lead to non-uniform hybrid layer formation and non-uniform bonding to dentin surfaces.
Due to incomplete and non-uniform infiltration of resins into the demineralized zone, nanoleakage may occur within the hybrid layer in gap-free margins.
The clinical relevance of this to the durability of the bonding and sealing is yet to be clarified.
Presented at the 5th Generation Adhesive Symposium promoted by BISCO Inc. Rancho Bernardo, San Diego, CA, USA. Abstract previously published in the Proceedings of the same event, 1997.
ACKNOWLEDGEMENTS
This paper was supported, partially, by grants 06427 from the NIDR, USA, 93/2020-3, 95/3895-9 from FAPESP, and 300481/95-0 from CNPq, Brazil.
CARVALHO, R. M.; CIUCCHI, B.; SANO, H.; YOSHIYAMA, M.; PASHLEY, D. H. Difusão de resina através da matriz de dentina desmineralizada. Rev Odontol Univ São Paulo, v.13, n.4, p.417-424, out./dez. 1999.
Este trabalho focaliza os aspectos que podem afetar a permeabilidade de resinas adesivas na matriz de dentina desmineralizada durante o desenvolvimento do processo adesivo. Os efeitos da umidade de superfície, respectivamente aos sistemas adesivos e aos problemas relacionados com a formação incompleta da camada híbrida são apresentados.
UNITERMOS: Dentina; Adesivos dentinários; Hibridização; Permeabilidade.
BIBLIOGRAPHIC REFERENCES
1. CARVALHO, R. M.; YOSHIYAMA, M.; HORNER J. A.; PASHLEY, D. H. Bonding mechanism of Variglass to dentin. Am J Dent, v.8, n.5, p.253-258, Oct. 1995.
2. CARVALHO R. M., YOSHIYAMA M., PASHLEY E. L., PASHLEY D. H. In vitro study of the dimensional changes in dentin after demineralization. Arch Oral Biol, v.41, n.1, p.369-377, Jan. 1996.
3. CARVALHO, R. M.; YOSHIYAMA, M.; SANO, H.; PASHLEY, D. H. Long-term tensile properties of demineralized dentin matrix. J Dent Res, 77, Sp Issue, 168, Abst. nº501, Mar. 1998. [Abstract 501].
4. CHIGIRA, H; YUKITANI, W; HASEGAWA, T; MANABE, A.; ITOH, K.; HAYAKAWA T.; DEBARI, K.; WAKUMOTO, S.; HISAMITSU. Self-etching dentin primers containing Phenyl-P. J Dent Res, v.73, n.5, p.1088-1095, May 1994.
5. ERICKSON, R. L.; GLASSPOOLE, E. A. Bonding to tooth structure: a comparison of glass-ionomer and composite-resin systems. J Esthet Dent, v.6, n.5, p.227-244, Sept. 1994.
6. GWINNETT, A. J. Dentin bonding strength after air drying and rewetting. Am J Dent, v.7, n.4, p.144-148, July 1994.
7. GWINNETT, A. J. Moist versus dry dentin: Its effect on shear bond strength. Am J Dent, v.5, n.3, p.127-129, May 1992.
8. KANCA, J. Resin bonding to wet substrate. I. Bonding to dentin. Quintessence Int, v.23, n.1, p.39-41, Jan. 1992.
9. NAKABAYASHI, N.; KOJIMA, K.; MASUHARA, E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res, v.16, n.2, p.265-273, Feb. 1982.
10. NAKABAYASHI, N.; NAKAMURA, M.; YASUDA, N. Hybrid layer as a dentin bonding mechanism. J Esthet Dent, v.3, n.4, p.133-138, May 1991.
11. PASHLEY, D. H. Dentin bonding: overview of the substrate with respect to adhesive material. J Esthet Dent, v.3, n.2, p.46-50, May 1991.
12. PASHLEY, D. H.; CARVALHO, R. M. Dentine permeability and dentine adhesion. J Dent, v.25, n.5, p.355-372, May 1997.
13. PASHLEY, D. H.; CIUCCHI, B.; SANO, H.; HORNER, J. A. Permeability of dentin to adhesive agents. Quintessence Int, v.24, n.9, p.618-631, Sept. 1993.
14. PASHLEY, D. H.; SANO, H.; CIUCCHI, B.; CARVALHO, R. M.; RUSSELL, C. M. Bond strength vs. dentine structure. A modeling approach. Archs oral Biol, v.40, n.12, p.1109-1118, Dec. 1995.
15. PEREIRA, P. N. R.; OKUDA, M; YOSHIKAWA, T.; SANO, H.; BOSCHIAN, M.; BURROW, M.; INOKOSHI, S.; YAMADA, T.; TAGAMI, J. Effect of water and regional difference on dentin bond strength. J Dent Res, v.76, p.20, Sp Iss., Mar. 1997. [Abstracts 56].
16. SANO, H.; TAKATSU, T; CIUCCHI, B; HORNER, J. A.; MATTHEWS W. G., PASHLEY, D. H. Nanoleakage: Leakage within the hybrid layer. Oper Dent, v.20, n.1, p.18-25, Jan. 1995.
17. SANO, H.; SHONO, T.; SONODA, H.; TAKATSU, T.; CIUCCHI, B.; CARVALHO, R. M.; PASHLEY, D. H. Relationship between surface area for adhesion and tensile bond strength. Evaluation of a microtensile bond test. Dent Mater, v.10, n.4, p.236-240, July 1994.
18. SANO, H.; YOSHIYAMA, M.; EBISU, S.; BURROW, M. F.; TAKATSU, T.; CIUCCHI, B; CARVALHO, R. M.; PASHLEY, D. H. Comparative SEM and TEM observations of nanoleakage within the hybrid layer. Oper Dent, v.20, n.4, p.160-167, Oct. 1995.
19. SUGISAKI, J. The effect of the various primers on the dentin adhesion of resin composites. Jpn J Cons Dent, v.34, n.3, p.228-265, Mar. 1991.
20. TAY, F. R.; GWINNETT, A. J.; PANG, K. U.; WEI, S. H. Y. The overwet phenomenon, an optical, micromorphological study of surface moisture in the acid etched, resin-dentin interface. Am J Dent, v.9, n.1, p.43-48, Jan. 1995.
21. TAY, F. R.; GWINNETT, A. J.; PANG, K. M.; WEI, S. H. Y. Variability in microleakage observed in a total-etch wet-bonding technique under different handling conditions. J Dent Res, v.74, n.5, p.1168-1178, May 1995.
22. UNO, S.; FINGER, W. J. Function of the hybrid zone as a stress-absorbing layer in resin-dentin bonding. Quintessence Int, v.26, n.10, p.733-738, Oct. 1995.
23. VAN MEERBEEK, B.; INOKOSHI, S.; BRAEM, M.; LAMBRECHTS, P.; VANHERLE, G. Morphological aspects of the resin-dentin interdiffusion zone with different adhesive systems. J Dent Res, v.71, n.9, p.1530-1540, Sept. 1992.
24. VAN MEERBEEK, B.; CONN, L. J.; DUKE, E. S.; EICK, J. D.; ROBINSON, S. J.; GUERRERO, D. Correlative transmission electron microscopy examination of nondemineralized and demineralized resin-dentin interfaces formed by two adhesive systems. J Dent Res, v.75, n.3, p.879-888, Mar. 1996.
25. YOSHIYAMA, M.; SANO, H.; EBISU, S.; TAGAMI, J.; CIUCCHI, B.; CARVALHO, R. M.; JOHNOSON, M. H.; PASHLEY, D. H. Regional strengths of bonding agents to cervical sclerotic root dentin. J Dent Res, v.75, n.6, p.1404-1413, June 1996.
26. WATANABE I.; NAKABAYASHI, N.; PASHLEY, D. H. Bonding to ground dentin using a self-etching Phenyl-P primer. J Dent Res, v.73, n.6, p.1212-1220, June 1994.
27. ZHANG, Y.; CARVALHO, R. M.; PASHLEY, D. H. HEMA diffusion through wet versus dry demineralized dentin. J Dent Res, v.76, p.20, Sp Iss., [Abstracts 53], Mar, 1997.
Recebido para publicação em 05/02/99
Enviado para reformulação em 28/06/99
Aceito para publicação em 15/09/99
-
1CARVALHO, R. M.; YOSHIYAMA, M.; HORNER J. A.; PASHLEY, D. H. Bonding mechanism of Variglass to dentin. Am J Dent, v.8, n.5, p.253-258, Oct. 1995.
-
2CARVALHO R. M., YOSHIYAMA M., PASHLEY E. L., PASHLEY D. H. In vitro study of the dimensional changes in dentin after demineralization. Arch Oral Biol, v.41, n.1, p.369-377, Jan. 1996.
-
3CARVALHO, R. M.; YOSHIYAMA, M.; SANO, H.; PASHLEY, D. H. Long-term tensile properties of demineralized dentin matrix. J Dent Res, 77, Sp Issue, 168, Abst. nº501, Mar. 1998.
-
4CHIGIRA, H; YUKITANI, W; HASEGAWA, T; MANABE, A.; ITOH, K.; HAYAKAWA T.; DEBARI, K.; WAKUMOTO, S.; HISAMITSU. Self-etching dentin primers containing Phenyl-P. J Dent Res, v.73, n.5, p.1088-1095, May 1994.
-
6GWINNETT, A. J. Dentin bonding strength after air drying and rewetting. Am J Dent, v.7, n.4, p.144-148, July 1994.
-
8KANCA, J. Resin bonding to wet substrate. I. Bonding to dentin. Quintessence Int, v.23, n.1, p.39-41, Jan. 1992.
-
9NAKABAYASHI, N.; KOJIMA, K.; MASUHARA, E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res, v.16, n.2, p.265-273, Feb. 1982.
-
10NAKABAYASHI, N.; NAKAMURA, M.; YASUDA, N. Hybrid layer as a dentin bonding mechanism. J Esthet Dent, v.3, n.4, p.133-138, May 1991.
-
12PASHLEY, D. H.; CARVALHO, R. M. Dentine permeability and dentine adhesion. J Dent, v.25, n.5, p.355-372, May 1997.
-
13PASHLEY, D. H.; CIUCCHI, B.; SANO, H.; HORNER, J. A. Permeability of dentin to adhesive agents. Quintessence Int, v.24, n.9, p.618-631, Sept. 1993.
-
14PASHLEY, D. H.; SANO, H.; CIUCCHI, B.; CARVALHO, R. M.; RUSSELL, C. M. Bond strength vs. dentine structure. A modeling approach. Archs oral Biol, v.40, n.12, p.1109-1118, Dec. 1995.
-
15PEREIRA, P. N. R.; OKUDA, M; YOSHIKAWA, T.; SANO, H.; BOSCHIAN, M.; BURROW, M.; INOKOSHI, S.; YAMADA, T.; TAGAMI, J. Effect of water and regional difference on dentin bond strength. J Dent Res, v.76, p.20, Sp Iss., Mar. 1997. [Abstracts 56].
-
17SANO, H.; SHONO, T.; SONODA, H.; TAKATSU, T.; CIUCCHI, B.; CARVALHO, R. M.; PASHLEY, D. H. Relationship between surface area for adhesion and tensile bond strength. Evaluation of a microtensile bond test. Dent Mater, v.10, n.4, p.236-240, July 1994.
-
18SANO, H.; YOSHIYAMA, M.; EBISU, S.; BURROW, M. F.; TAKATSU, T.; CIUCCHI, B; CARVALHO, R. M.; PASHLEY, D. H. Comparative SEM and TEM observations of nanoleakage within the hybrid layer. Oper Dent, v.20, n.4, p.160-167, Oct. 1995.
-
19SUGISAKI, J. The effect of the various primers on the dentin adhesion of resin composites. Jpn J Cons Dent, v.34, n.3, p.228-265, Mar. 1991.
-
20TAY, F. R.; GWINNETT, A. J.; PANG, K. U.; WEI, S. H. Y. The overwet phenomenon, an optical, micromorphological study of surface moisture in the acid etched, resin-dentin interface. Am J Dent, v.9, n.1, p.43-48, Jan. 1995.
-
21TAY, F. R.; GWINNETT, A. J.; PANG, K. M.; WEI, S. H. Y. Variability in microleakage observed in a total-etch wet-bonding technique under different handling conditions. J Dent Res, v.74, n.5, p.1168-1178, May 1995.
-
22UNO, S.; FINGER, W. J. Function of the hybrid zone as a stress-absorbing layer in resin-dentin bonding. Quintessence Int, v.26, n.10, p.733-738, Oct. 1995.
-
23VAN MEERBEEK, B.; INOKOSHI, S.; BRAEM, M.; LAMBRECHTS, P.; VANHERLE, G. Morphological aspects of the resin-dentin interdiffusion zone with different adhesive systems. J Dent Res, v.71, n.9, p.1530-1540, Sept. 1992.
-
24VAN MEERBEEK, B.; CONN, L. J.; DUKE, E. S.; EICK, J. D.; ROBINSON, S. J.; GUERRERO, D. Correlative transmission electron microscopy examination of nondemineralized and demineralized resin-dentin interfaces formed by two adhesive systems. J Dent Res, v.75, n.3, p.879-888, Mar. 1996.
-
25YOSHIYAMA, M.; SANO, H.; EBISU, S.; TAGAMI, J.; CIUCCHI, B.; CARVALHO, R. M.; JOHNOSON, M. H.; PASHLEY, D. H. Regional strengths of bonding agents to cervical sclerotic root dentin. J Dent Res, v.75, n.6, p.1404-1413, June 1996.
-
26WATANABE I.; NAKABAYASHI, N.; PASHLEY, D. H. Bonding to ground dentin using a self-etching Phenyl-P primer. J Dent Res, v.73, n.6, p.1212-1220, June 1994.
-
27ZHANG, Y.; CARVALHO, R. M.; PASHLEY, D. H. HEMA diffusion through wet versus dry demineralized dentin. J Dent Res, v.76, p.20, Sp Iss., [Abstracts 53], Mar, 1997.
****
*****
Publication Dates
-
Publication in this collection
01 Mar 2000 -
Date of issue
Dec 1999
History
-
Reviewed
28 June 1999 -
Received
05 Feb 1999 -
Accepted
15 Sept 1999