Abstracts
Bauhinia pentandra (Bong) Vog. ex. Steua seeds were investigated with respect to phenologic aspects (size, mass, hilum and length) and with respect to their chemical composition. The total nitrogen content of the seed flour was determined, and the flour was extracted in different pH values. A lectin was isolated from the seeds by Sepharose-4B affinity chromatography. The homogeneity of the lectin was demonstrated by SDS-PAGE in the presence of beta-mercaptoethanol. Only one protein band with an apparent molecular mass of 30 kDa was found. The B. pentandra lectin showed a carbohydrate specificity for D-galactose, a requirement for divalent metal cations (Ca2+ and Mn2+) for full activity and amino acid composition with a high content of aspartic acid, glutamic acid and alanine and low levels of methionine, cysteine and tryptophan. The lectin agglutinated rabbit and human A group erythrocytes and was relatively stable to heat treatment, retaining half of its original activity after 60 min at 70 ºC.
Bauhinia; Leguminosae; phytohemagglutinin; seed protein; D-galactose
Sementes de Bauhinia pentandra (Bong) Vog. ex. Steua. foram estudadas quanto aos aspectos fenológicos (tamanho, massa e comprimento do hilo) e quanto a sua composição química. A farinha de sementes teve o teor de nitrogênio total determinado e foi submetida à extração de proteínas em diferentes valores de pH. Uma lectina foi isolada de sementes por cromatografia de afinidade em coluna de Sepharose-4B. A homogeneidade da lectina foi demonstrada por eletroforese em gel de poliacrilamida na presença de SDS e beta-mercaptoetanol. A lectina mostra apenas uma banda protéica de massa molecular aparente de 30 kDa. A lectina de B. pentandra mostra especificidade por D-galactose, requer a presença de cátions divalentes (Ca2+ e Mn2+) para exercer sua atividade e tem uma composição de aminoácido caracterizada pelo alto teor de ácido aspártico, ácido glutamico, alanina e pelo baixo teor de metionina, cisteina e triptofano. A lectina aglutina eritrócitos de coelho e humano do grupo A e foi relativamente resistente ao tratamento térmico, retendo parte de sua atividade original mesmo após 60 min a 70 ºC.
Bauhinia; Leguminosae; fitohemaglutinina; proteína de semente; D-galactose
ISOLATION AND PARTIAL CHARACTERIZATION OF A LECTIN FROM BAUHINIA PENTANDRA (BONG) VOG. EX. STEUA.
ANDRÉ LUIS COELHO DA SILVA1 1 Received: 9/7/2001 - Accepted: 17/9/2001 . Eng. Agrônomo, M.Sc., Grupo de Biofísica Molecular e Espectroscopia / USP, IFSC, São Carlos, SP, 13560-970, e-mail: alcoelho@if.sc.usp.br. Corresponding author. 2 . Professor Adjunto, Dr., Depto. de Bioquímica e Biologia Molecular/UFC. 3 . Professor Titular, Dr., Depto. de Bioquímica e Biologia Molecular/UFC. , ANA CECÍLIA GÓES HORTA2 1 Received: 9/7/2001 - Accepted: 17/9/2001 . Eng. Agrônomo, M.Sc., Grupo de Biofísica Molecular e Espectroscopia / USP, IFSC, São Carlos, SP, 13560-970, e-mail: alcoelho@if.sc.usp.br. Corresponding author. 2 . Professor Adjunto, Dr., Depto. de Bioquímica e Biologia Molecular/UFC. 3 . Professor Titular, Dr., Depto. de Bioquímica e Biologia Molecular/UFC. AND RENATO DE AZEVEDO MOREIRA3 1 Received: 9/7/2001 - Accepted: 17/9/2001 . Eng. Agrônomo, M.Sc., Grupo de Biofísica Molecular e Espectroscopia / USP, IFSC, São Carlos, SP, 13560-970, e-mail: alcoelho@if.sc.usp.br. Corresponding author. 2 . Professor Adjunto, Dr., Depto. de Bioquímica e Biologia Molecular/UFC. 3 . Professor Titular, Dr., Depto. de Bioquímica e Biologia Molecular/UFC.
Laboratório de Lectinas e Glicoconjugados, Departamento de Bioquímica e Biologia Molecular, Universidade Federal do Ceará. Caixa Postal 6020, 60451-970, Fortaleza (CE), Brazil.
ABSTRACT-Bauhinia pentandra (Bong) Vog. ex. Steua seeds were investigated with respect to phenologic aspects (size, mass, hilum and length) and with respect to their chemical composition. The total nitrogen content of the seed flour was determined, and the flour was extracted in different pH values. A lectin was isolated from the seeds by Sepharose-4B affinity chromatography. The homogeneity of the lectin was demonstrated by SDS-PAGE in the presence of b-mercaptoethanol. Only one protein band with an apparent molecular mass of 30 kDa was found. The B. pentandra lectin showed a carbohydrate specificity for D-galactose, a requirement for divalent metal cations (Ca2+ and Mn2+) for full activity and amino acid composition with a high content of aspartic acid, glutamic acid and alanine and low levels of methionine, cysteine and tryptophan. The lectin agglutinated rabbit and human A group erythrocytes and was relatively stable to heat treatment, retaining half of its original activity after 60 min at 70 oC.
ADDITIONAL INDEX TERMS: Bauhinia, Leguminosae, phytohemagglutinin, seed protein, D-galactose.
ISOLAMENTO E CARACTERIZAÇÃO PARCIAL DE UMA LECTINA DE BAUHINIA PENTANDRA (BONG) VOG. EX. STEUA.
RESUMO Sementes de Bauhinia pentandra (Bong) Vog. ex. Steua. foram estudadas quanto aos aspectos fenológicos (tamanho, massa e comprimento do hilo) e quanto a sua composição química. A farinha de sementes teve o teor de nitrogênio total determinado e foi submetida à extração de proteínas em diferentes valores de pH. Uma lectina foi isolada de sementes por cromatografia de afinidade em coluna de Sepharose-4B. A homogeneidade da lectina foi demonstrada por eletroforese em gel de poliacrilamida na presença de SDS e b-mercaptoetanol. A lectina mostra apenas uma banda protéica de massa molecular aparente de 30 kDa. A lectina de B. pentandra mostra especificidade por D-galactose, requer a presença de cátions divalentes (Ca2+ e Mn2+) para exercer sua atividade e tem uma composição de aminoácido caracterizada pelo alto teor de ácido aspártico, ácido glutamico, alanina e pelo baixo teor de metionina, cisteina e triptofano. A lectina aglutina eritrócitos de coelho e humano do grupo A e foi relativamente resistente ao tratamento térmico, retendo parte de sua atividade original mesmo após 60 min a 70 oC.
TERMOS ADICIONAIS PARA INDEXAÇÃO: Bauhinia, Leguminosae, fitohemaglutinina, proteína de semente, D-galactose
INTRODUCTION
Lectins are proteins or glycoproteins of non-immune origin derived from plants, animals or microorganisms that have specificity for terminal or subterminal carbohydrate residues. The main characteristic of this class of proteins is their ability to interact with carbohydrates and thus combine with glycocomponents of the cell surface, as well as with cytoplasmatic and nuclear structures and the extracellular matrix of cells and tissues from throughout the animal and plant kingdoms, down to bacteria and viruses (Leathem and Brooks, 1998).
The lectins represent a large group of plant proteins. Lectins have been found in less than 500 species, which indicates that only a limited number of higher plants contain detectable levels of lectins (Van Damme et al., 1998b). The concentration of lectins in seeds varies considerably (from 1 to 10% of the total seed protein). In some species, values to 50% have been reported, whereas in others lectins are barely detectable with techniques currently used (Peumans and Van Damme, 1998).
The use of metabolically active proteins in plant taxonomy is well established. The presence of seed lectins in tribes, genera and species of the same family has been reported and high homology has been found between lectins from taxonomically diverse sources, which makes these proteins a suitable molecular tool for chemotaxonomy studies (Moreira et al., 1993).
Plants of the genus Bauhinia are widely distributed in the tropics and are important for animal nutrition because of their high protein content. Some species also have been used in folk medicine for the treatment of diabetes and as a diuretic.
Previous studies have reported the isolation and characterization of a lectin from Bauhinia purpurea (BPA). B. purpurea seeds contain a typical legume lectin that is purified by affinity chromatography on immobilized N-acetyl-D-galactosamine (Young et al., 1985). BPA is useful for detecting galactose-containing glycoconjugates and mucin-type sugar chains, and can also be used as a marker for Reed-Sternberg cells in Hodgkin's disease (Sarker et al., 1992). Lectins have also been reported from leaves of Bauhinia monandra (Coelho and Silva, 2000).
The isolation and characterization of novel lectins reveal properties which are of practical importance for different areas of biological research. Therefore, the objective of this work was to isolate and characterize a lectin from Bauhinia pentandra seeds.
MATERIAL AND METHODS
Plant material. B. pentandra (Bong) Vog. ex. Steua seeds were collected from plants growing in the State of Ceará (Brazil). Taxonomic identification was carried out at the Department of Biology, Federal University of Ceará (UFC).
Erythrocytes. Human erythrocytes of the ABO system were collected from healthy donors at the Hemotherapy Center of Ceará (HEMOCE). Red blood cells from rabbit, chicken and pigeon were obtained from animals reared at the School of Agronomy, UFC.
Reagents. Sugars, acrylamide and methylene bisacrylamide were from Sigma. Sepharose-4B was from Pharmacia. All other reagents used were of analytical grade.
Hemagglutinating activity. The agglutination of red blood cells by fractions obtained during purification was estimated as described before (Moreira and Perrone, 1977). Serial two-fold dilutions of lectin were carried out in small glass tubes using 0.15 M NaCl containing 5 mM CaCl2 and 5 mM MnCl2 (0.25 ml), and 0.25 ml of erythrocyte suspension was then added. The degree of agglutination was monitored visually after incubation at 37 oC for 30 min and a further 30 min at room temperature. One hemagglutination unit (H.U.) was defined as the reciprocal of the highest dilution still causing a visible agglutination. The specific activity was expressed as hemagglutination units (H.U.). mg-1 and the minimum dose as the minimum amount of protein still promoting a visible agglutination.
Proximate analysis. Moisture, ash and lipid contents were determined as described by Triebold (1949), total nitrogen was determined as previously described (Baethgen and Alley, 1969) and total carbohydrate by difference. The protein content was determined from the total nitrogen, using a conversion factor of 6.25.
Protein determination. The protein concentration of the different fractions was measured according to Bradford (1976), using bovine serum albumine (BSA) as standard. The absorbance at 280 nm was used to estimate the protein concentration in column eluates.
Protein extraction. Dehulled B. pentandra seeds were ground and stirred with 0.1 M glycine-HCl pH 2.6, 0.1 M NaOAc pH 4.0, 0.1 M NaOAc pH 6.0, 0.1 M Tris-HCl pH 7.6 and 0.1M Na-borate buffer pH 10.0, all containing 0.15 M NaCl. The suspensions were left at room temperature for 3 h then spun at 12.000 g for 20 min at 7 ºC. The clear supernatants were used for determining the protein content and hemagglutinating activity.
Ammonium sulfate fractionation.B. pentandra lectin was extracted by treating the seed meal (50 g) with 0.1 M Tris-HCl pH 7.6 containing 0.15 M NaCl at room temperature for 3 h before centrifuging at 12.000 g for 20 min at 7 ºC. The clear supernatant was then precipitated by addition of ammonium sulfate in the saturation levels: 0/25; 25/50; 50/70; 70/100, at 5 ºC overnight. The precipitate was collected by centrifuging at 12.000 g for 20 min, at 7 ºC and then dissolved in 0.15 M NaCl. After exhaustive dialysis against H2O, the fraction was freeze-dried.
Affinity chromatography. A Sepharose-4B column was equilibrated with 0.15 M NaCl containing 5 mM CaCl2 and 5 mM MnCl2 before loading the sample. After sample loading, the column was washed with the same solution until the absorbance at 280 nm was zero. After removing the unbound material, the lectin was desorbed from the column with 0.1 M galactose in the equilibrium solution or with glycine-HCl (pH 2.6) buffer with 0.15 M NaCl containing 5 mM CaCl2 and 5 mM MnCl2.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). The polyacrylamide gel electrophoresis was performed in 2 mm thick vertical slab gels, according to the method of Laemmli (1970), using 3.95% and 12.5% stacking and running gels, respectively. Samples were dissolved in 0.0625 M Tris-HCl pH 6.8, containing 2% SDS buffer and 1% b-mercaptoethanol then incubated at 100 ºC for 10 min. A few crystals of sucrose were dissolved in the samples which were then applied to the gel. Electrophoresis was carried out at a constant current of 20 mA for 3 h. The protein bands were visualized by staining the gel with Coomassie Brilliant Blue-R 250. Estimations of the Mr of the lectin and fragments were based on comparisons using Abrus pulchellus lectin (Ramos et al., 1998) of known Mr as a standard.
Sugar specificity. The carbohydrate-binding specificity of the lectin was estimated by the ability of a series of single sugars to inhibit the hemagglutination of rabbit erythrocytes. Two-fold dilutions of sugars in 0.15 M NaCl containing 5 mM CaCl2 and 5 mM MnCl2 were mixed with 0.25 ml of lectin solution at a concentration such that a two-fold dilution would give the end point agglutination in the absence of inhibitor. The lowest concentration of the sugar giving full inhibition was determined.
Effect of EDTA and Ca2+ and Mn2+. The purified lectin (1 mg) was dissolved and dialyzed dialysed exhaustively (48 h) against 0.2 M EDTA, followed by dialysis against 0.15 M NaCl. Hemagglutinating activity was recovered by adding CaCl2 and MnCl2.
Heat stability. The heat stability of the hemagglutinating activity was determined by incubating the lectin at different temperatures during different times and the residual activity determined.
Amino acid composition. Amino acid analysis was done after hydrolysis of lectin samples (in sealed glass tubes under N2) at 110 ºC for 20 h in 6 M HCl. After hydrolysis, HCl was removed by evaporation and the residue was analyzed, in a BIOCHROM 20 (Pharmacia) amino acid analyzer. Tryptophan was determined spectrophotometrically (Horn and Jones, 1945).
RESULTS AND DISCUSSION
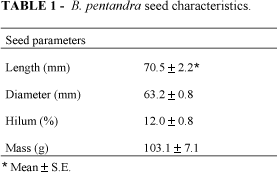
Bauhinia pentandra is a widespread plant, common in tropical and sub-tropical regions, occurring in forests in northeastern Brazil. B. pentandra belongs to the family Leguminosae, sub-family Caesalpinioideae, tribe Cercidae, sub-tribe Cercidinae and genus Bauhinia. In Brazil, this species is commonly called "mororó" or "capa-bode". The seed characteristics (mass, size and hilum length) were determined and are presented in Table 1. The seeds had a high protein content, a characteristic of legumes. The dehulled seed flour contains 37.1% protein (5.9% N2) on a dry weight basis and a high lipid content (Table 2).
The influence of pH on the solubility of the proteins and hemagglutinating activity of B. pentandra was examined (Fig. 1). A maximum protein content was found at pH 2.6 and a minimum content at pH 10.0. The maximum hemagglutinating activity occurred at pH 7.6. The crude extract of B. pentandra seed flour showed hemagglutinating activity only 12 h after the addition of erythrocytes.
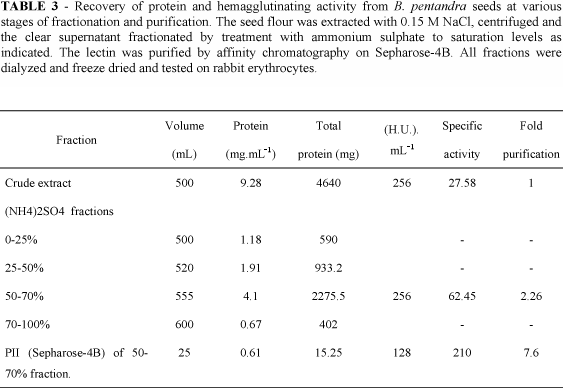
The purification of B. pentandra lectin is summarized in Table 3. The crude saline extract was fractionated by addition of different amounts of solid ammonium sulphate in the cold (5 ºC), overnight. After centrifugation at 12000 g for 20 min at 7 ºC, the precipitates were redissolved and dialysed exhaustively against water and recovered by freeze-drying. This material was redissolved in 0.15 M NaCl containing 5 mM Ca2+ and 5 mM Mn2+ and assayed for protein and hemagglutinating activity (Table 3). Most of the activity was precipitated between 50 and 70% sulfate of ammonium saturation. This fraction (F50/70) was redissolved in 0.15 M NaCl containing 5 mM Ca2+ and 5 mM Mn2+ and applied to a Sepharose-4B column equilibrated with the same solvent. The column was first eluted with the equilibration solution followed by 0.1 M galactose dissolved in he equilibration solution (Fig. 2). All the hemagglutinating activity emerged with the galactose.
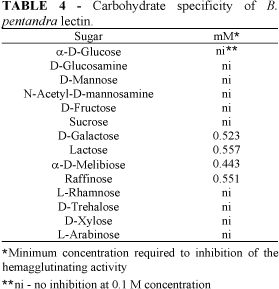
The purified B. pentandra lectin agglutinated rabbit erythrocytes. The minimum lectin concentration required to clump a 2% suspension of rabbit blood cells was about 3 mg.L-1. Human blood group A erythrocytes were poorly agglutinated while human blood group B and O as well as chicken and pigeon erythrocytes were not agglutinated. The hemagglutinating activity of the B. pentandra lectin was inhibited by galactose and galactose-containing sugars and derivatives: galactosamine, a-D-melibiose, lactose and raffinose (Table 4). This carbohydrate-binding specificity is also characteristic of the lectin isolated from Bauhinia purpurea (Young et al., 1985).
The hemagglutinating activity of the lectin against rabbit erythrocytes decreased appreciably after prolonged dialysis of the native protein with 0.2 M EDTA, followed by dialysis against 0.15 M NaCl. However, the addition of Ca2+ and Mn2+ (1, 3, 5, 7 and 10 mM) to the dialysed lectin restored the activity. The optimum concentration for maximum hemagglutinating activity was 3-5 mM for both cations. Similar results were observed for Dioclea altissima lectin (Moreira et al., 1996). The requirement for metal is a general physico-chemical property of most legume lectins (Goldstein and Poretz, 1986; Sharon and Lis, 1990). The B. pentandra lectin was thermostable: hemagglutinating activity was retained even after incubation at 70 ºC for 1 h. However, the activity was completely lost at higher temperatures (80-100 ºC) even after incubation for just 10 min (data not shown).
Polyacrylamide gel electrophoresis of the lectin, treated with SDS and b-mercaptoethanol gave only one protein band with an apparent molecular mass of 30 kDa (Fig. 3). In the absence of b-mercaptoethanol, the B. pentandra lectin also showed only one band (data not shown). The lectin, when dissolved in 0.15 M NaCl, had an absorption of 9.8 at 280 nm in a 1 cm cell (A1 cm, 1%). The amino acid composition was characterized by a very low content of methionine, cysteine and tryptophan, and a high content of aspartic acid, glutamic acid and alanine (Table 5).
ACKNOWLEDGMENTS
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação Cearense de Amparo à Pesquisa (FUNCAP).
- BAETHGEN, W.E. AND ALLEY, M. M. A manual colorimetric procedure for measuring ammonium nitrogen in soil and plant kjeldahl digests. Communication Soil Science Plant, 20: 961-969, 1989.
- BRADFORD, M.M. A rapid sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72: 248-254, 1976.
- COELHO, L.C.B.B AND SILVA, M.B.R. Simple method to purify milligram quantities of the galactose-specific lectin from the leaves of Bauhinia monandra Phytochemistry Analysis, 11: 295-300, 2000.
- GOLDSTEIN, I.J. AND PORETZ, R.D. Isoaltion, physico-chemical characteristics, and carbohydrate-binding specificity of lectins. In: LIENER, I.E; SHARON, N. and GOLDSTEIN, I.J. Ed. The Lectins: properties, functions and applications in biology and medicine Orlando, Florida, Academic Press, 1986. p. 33-247.
- HORN, M.J. AND JONES, D. B. A rapid colorimetric method for the determination of tryptophane in proteins and foods. Journal of Biological Chemistry, 157 :153-160, 1945
- LEAMMLI, U.K. Cleavage of structural proteins during the assembly of bacteriophage T4. Nature, 227: 680-685, 1970.
- LEATHEM, J.A. AND BROOKS, S.A. Lectins methods and protocols. In: Rhodes, J. M. and Milton, J.D. (Eds.) Methods in Molecular Medicine Humana Press Inc., Totowa, NJ, 1998.
- MOREIRA, R.A. AND PERRONE, J.C. Purification and partial characterization of a lectin from Phaseolus vulgaris. Plant Physiology, 59: 783-787, 1977.
- MOREIRA, R.A; CORDEIRO, E.F.; CAVADA, B.S; NUNES, E.P.; FERNANDES, A.G AND OLIVEIRA, J.T.A. Plant seed lectins. A possible marker for chemotaxonomy of the genus Canavalia Revista Brasileira de Fisiologia Vegetal, 5: 127-132, 1993.
- MOREIRA R.A.; MONTEIRO, A.C.O.; HORTA, A.C.G.; OLIVEIRA, J.T.A. AND CAVADA, B. S. Isolation and characterization of Dioclea altissima var. megacarpa seed lectin. Ph ytochemistry, 46: 139-144, 1996.
- PEUMANS, W.J. AND VAN DAMME, E.J.M. Plants lectins: Specific tools for the identification, isolation and characterization of O-linked glycans. Critical Reviews in Biochemistry and Molecular Biology, 33: 209-258, 1998.
- RAMOS, M.V.; MOTA, D.M.; TEIXEIRA, C.R.; CAVADA, B.S. AND MOREIRA, R.A. Isolation and partial characterization of highly toxic lectins from Abrus pulchellus seeds. Toxicon, 36: 477-484, 1998.
- SARKER, A.B.; AKAGI, T.; JEON, H.J.; MIYAKE, K.; MURAKAMI, I.; YOSHINO, T.; TAKAHASHI, K. AND NOSE, S. Bauhinia purpurea: A new marker for Reed-Sternberg cells of Hodgkins disease. A comparison with Leu-M1 (CD15), LN2 (CD74), peanut agglutinin, and BER-H2 (CD30). American Journal of Pathology 141: 19-23, 1992.
- TRIEBOLD, H.O. Quantitative analysis with applications to agricultural and food products D. Van Nostrand Co., New York, 1946.
- VAN DAMME, E.J.M.; PEUMANS, W.J.; PUSZTAI, A. AND BARDOCZ, S. eds. Handbook of plant lectins: Properties and biochemical applications John Wiley AND Sons, Chichester, UK. 1998.
- YOUNG, M.N; WATSON, D.C. AND WILLIAMS, R.E. Lectins and legume taxonomy. Characterization of the N-acetyl-D-galactosamine specific lectin from Bauhinia purpurea FEBS Letters 182: 404-406, 1985.
Publication Dates
-
Publication in this collection
16 July 2002 -
Date of issue
2001
History
-
Received
09 July 2001 -
Accepted
17 Sept 2001