Abstract:
As part of the innovation process, clinical research generates valuable data to assess technological solutions in healthcare, linking scientific research and knowledge transfer to provide beneficial innovations for society. However, the generated clinical data do not appear to be adequately available to the scientific community and society. The present study seeks to analyze the effectiveness of databases of clinical records as a source of relevant information for scientific research. We conducted a comparative analysis of 27 correlations of clinical trials between three different technologies and their scientific articles by consulting two information sources. Making connections between the data from these sources proved to be challenging. In addition, a considerable time lag (40 months on average) was observed between the end of the study and the publication of the results. Among the completed studies, 56% had not published their results in any of the channels studied. In addition to the paucity of reported results, the poor publication record of clinical trials is further evident in the lack of information on these studies in scientific publications. Thus, databases of clinical records are a potential source of information and may come to represent a central tool in the search for new technological solutions in healthcare.
Keywords:
Comparative Research; Technological Innovation; Metadata; Scientific Information
Resumo:
A pesquisa clínica, como parte de um processo de inovação, gera dados úteis para avaliação de diversas soluções tecnológicas em saúde, perfazendo um elo entre a pesquisa científica e a translação do conhecimento em inovações benéficas à sociedade. Entretanto, os dados clínicos gerados parecem não estar disponíveis adequadamente à comunidade científica e à sociedade. O presente estudo buscou analisar o potencial das bases de registros clínicos como fonte de informação relevante às pesquisas científicas e, para isso, foi realizada uma análise comparativa de 27 correlações de estudos clínicos de três diferentes tecnologias e seus artigos científicos em duas fontes de informação distintas. Nesse universo amostral, observou-se importante e limitante dificuldade em associar com exatidão os dados dessas fontes. Foi possível, ainda, observar um grande lapso temporal (40 meses em média) entre o encerramento de um estudo e a publicização dos resultados. Considerando os estudos finalizados, 56% não tiveram seus resultados publicados em nenhum dos canais estudados. A publicação dos estudos clínicos pode se mostrar ainda mais negligenciada quando se percebe que, além da ausência de resultados, não há qualquer informação desses estudos em publicações científicas. Assim, as bases de registros clínicos se apresentam como uma fonte de informação em potencial - e, por vezes, única - para suprir conhecimento na busca por novas soluções tecnológicas em saúde.
Palavras-chave:
Pesquisa comparativa; Inovação tecnológica; Metadados; Informação científica
Introduction
The development of novel medicines, diagnostic methods, and treatments requires connecting various stages on the path from scientific research to commercialization (Ellwood; Williams; Egan, 2020Ellwood, P.; Williams, C.; Egan, J. Crossing the valley of death: Five underlying innovation processes. Technovation, v. 109, n. 102162, p. 1-11, 2020. Doi: https://doi.org/10.1016/j.technovation.2020.102162.
https://doi.org/10.1016/j.technovation.2...
). Specifically, connections must be made between basic research, technological development, Clinical Trials (CTs), and the commercialization of products.
Ideally, the large volume of scientific information generated at each stage should be communicated in scientific journals, patent documents, and CT registration platforms. However, as a social and political process, the transfer of information is not always homogeneous or equitable (Targino, 2001Targino, M. G. Comunicação científica na sociedade tecnológica: periódicos electrônicos em discussão. Comunicação e Sociedade, v. 3, p. 93-112, 2001. Doi: https://doi.org/10.17231/comsoc.3(2001).1317.
https://doi.org/10.17231/comsoc.3(2001)....
). Academic journals have been widely discussed as the vehicle of choice for communicating scientific discoveries. However, a variety of other channels are being used to transmit scientific information in spite of their lower profile within the scientific community (Pimenta, 2017Pimenta, F. P. A patente como fonte de informação (des)necessária para a Biotecnologia em Saúde. Transinformação, v. 29, n. 3, p. 323-332, 2017. Doi: https://doi.org/10.1590/2318-08892017000300009.
https://doi.org/10.1590/2318-08892017000...
).
As part of the innovation process, clinical research generates valuable data for assessing new treatments, diagnoses, drug effects, vaccines, diets, medical devices, and the detection of side effects. This research therefore bridges gaps between basic research and knowledge transfer, resulting in beneficial innovations for society (Ellwood; Williams; Egan, 2020Ellwood, P.; Williams, C.; Egan, J. Crossing the valley of death: Five underlying innovation processes. Technovation, v. 109, n. 102162, p. 1-11, 2020. Doi: https://doi.org/10.1016/j.technovation.2020.102162.
https://doi.org/10.1016/j.technovation.2...
).
According to the resolution RDC N° 39/2008 Anvisa-MS from the Drug Surveillance Agency of the Brazilian Ministry of Health, a clinical trial is:
any research on humans involving therapeutic and diagnostic intervention that uses registered or registrable products to discover or verify their pharmacodynamic, pharmacokinetic, pharmacological, clinical and/or adverse side effects, in addition to determining their safety and/or efficacy (Brasil, 2008Brasil. Resolução RDC n° 39, de 5 de junho de 2008. Regulamento para a realização de pesquisa clínica. [S.l.]: Anvisa, 2008. Disponivel em: Disponivel em: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2008/res0039_05_06_2008.html . Acesso: 20 maio, 2021.
https://bvsms.saude.gov.br/bvs/saudelegi... , online).
The main goals of clinical research include verifying the safety and efficacy of products and technologies (phase I), assessing their effectiveness and investigating their side effects (phase II), confirming their efficacy, and monitoring adverse reactions (phase III). The final phase (phase IV) involves commercialization and pharmacovigilance of the investigated product (Brasil, 2008Brasil. Resolução RDC n° 39, de 5 de junho de 2008. Regulamento para a realização de pesquisa clínica. [S.l.]: Anvisa, 2008. Disponivel em: Disponivel em: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2008/res0039_05_06_2008.html . Acesso: 20 maio, 2021.
https://bvsms.saude.gov.br/bvs/saudelegi...
). Hence, the benefits of clinical research go beyond innovations that are available to the public, since such research is the result of a complex and multidisciplinary process involving several agents like researchers, physicians, regulatory bodies, ethics committees, volunteers, and sponsors (Quental; Salles Filho, 2006Quental, C.; Salles Filho, S. Ensaios clínicos: capacitação nacional para avaliação de medicamentos e vacinas. Revista Brasileira de Epidemiologia, v. 9, n. 4, p. 408-424, 2006. Doi: https://doi.org/10.1590/S1415-790X2006000400002.
https://doi.org/10.1590/S1415-790X200600...
).
In Brazil, all CTs must be evaluated by an institutional review board called the Research Ethics Committee (Comitê de Ética em Pesquisa; CEP) and, if necessary, by a second board, the National Commission for Research Ethics (Comissão Nacional de Ética em Pesquisa; CONEP) (Gouy; Porto; Penido, 2018Gouy, C. M. L.; Porto, T. F.; Penido, C. Avaliação de ensaios clínicos no Brasil: histórico e atualidades. Revista Bioética, v. 26, n. 3, p. 350-359, 2018. Doi: https://doi.org/10.1590/1983-80422018263254.
https://doi.org/10.1590/1983-80422018263...
). Clinical studies must also be registered in a database to avoid the duplication of trials and selective publishing of results, in addition to providing patients and the public with transparency and access to information (Tse; Fain; Zarin, 2018Tse, T.; Fain, K. M.; Zarin, D. A. How to avoid common problems when using ClinicalTrials.gov in research: 10 issues to consider. BMJ, v. 361, n. k1452, p. 1-8, 2018. Doi: https://doi.org/10.1136/bmj.k1452.
https://doi.org/10.1136/bmj.k1452...
). Whether in phase I, II, III, or IV, CTs must be registered in the Brazilian Registry of Clinical Trials (Registro Brasileiro de Ensaios Clínicos; ReBEC) database in accordance with ANVISA resolution RDC 36 of June 27, 2012 (Brasil, 2012Brasil. Resolução RDC nº 36, de 27 junho de 2012. Altera a RDC nº 39, de 05 de junho de 2008. [S.l.]: Anvisa, 2012. Disponível em: Disponível em: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2012/rdc0036_27_06_2012.html . Acesso: 20 maio, 2021.
https://bvsms.saude.gov.br/bvs/saudelegi...
, online).
The registration of CTs begins with the submission of the experimental design, including the intervention plan in general terms. At the end of the study, the publication of results is considered extremely important for the scientific community and society. Traditionally, scientific journals have been the primary vehicles for communicating results, with a parallel role fulfilled by clinical trial databases. However, many CTs are never published (Shrivastava; Shrivastava; Ramasamy, 2018Shrivastava, S.R.; Shrivastava, P. S.; Ramasamy, J. Emphasizing the necessity to register and report the results of all clinical trials: World Health Organization. Medical Journal of Dr. D.Y. Patil Vidyapeeth, v. 11, n. 1, p. 81-82, 2018. Doi: https://doi.org/ 10.4103/MJDRDYPU.MJDRDYPU_105_17.
https://doi.org/10.4103/MJDRDYPU.MJDRDYP...
).
In this context, we seek to demonstrate the potential benefits of databases for CTs as a source of relevant information for developing technological solutions in healthcare.
Methodological Procedures
Selection and identification of technologies
Three technologies were selected for their relevance as potential solutions for public health issues and institutional patent landscape analyses (not yet published). Our study is based on published metadata on these three technologies. We used a scientometrics approach to understand the process of organizing scientific communication (Van Raan, 1997Van Raan, A. F. J. Scientometrics: state-of-the- art. Scientometrics, v. 38, n. 1, p. 205-218, 1997. ; Vanti, 2002Vanti, A. P. Da bibliometria à webometria: uma exploração conceitual dos mecanismos utilizados para medir o registro da informação e a difusão do conhecimento. Ciência da Informação, v. 31, n. 2, p. 369-379, 2002. Doi: https://doi.org/10.1590/S0100-19652002000200016.
https://doi.org/10.1590/S0100-1965200200...
).
The first technology is Eryaspase, a donor-derived red blood cell (RBC) encapsulation technology for the enzyme L-asparaginase. This technology aims to reduce the toxicity observed in treating Acute Lymphoblastic Leukemia (ALL), as well as increase the ability of other oncological treatments to reduce toxicity. Eryaspase is in phase III of CTs conducted by Erytech Pharma SA.
The second technology describes the active compound GSK3186899, similar to current anti-Leishmania drugs. This compound is in phase I of CTs, which were initiated in April 2019, conducted by GlaxoSmithKline plc.
The third technology is the compound DNDI 0690, which is being studied through a partnership between the TB Alliance and Drugs for Neglected Diseases (DNDi). This compound showed excellent in vitro activity against visceral and cutaneous leishmaniasis, proceeding to phase I CTs in March 2020.
Search strategy in clinical studies databases
Metadata from CTs were identified from the International Clinical Trials Registry Platform (ICTRP), an initiative of the World Health Organization (World Health Organization, 2018World Health Organization. International standards for clinical trial registries: the registration of all interventional trials is a scientific, ethical and moral responsibility, version 3.0, 2018. Disponível em: https://apps.who.int/iris/handle/10665/274994. Acesso em: 5 set. 2021.
https://doi.org/10665/274994...
). The ICTRP platform was created to establish “a network of international clinical trials registries,” ensuring a single point of access and unambiguous identification of CTs (https://www.who.int/clinical-trials-registry-platform/about).
Searches were carried out in May 2021 without time limitation, using the simple interface and entering keywords identified as relevant for each of the three technologies in the ‘Search’ field (Table 1).
After performing the searches, the metadata were collected from the fields “Main ID,” “Secondary ID,” “Public Title,” “Scientific Title,” “Sponsor,” “Phase,” “Contacts,” “Affiliation,” “Intervention,” and “Results,” which was then organized in an Excel table with duplicates manually removed. Additionally, when absent, some metadata content was supplemented with the results of searches in the primary databases of CT records from links of the ICTRP platform.
Identification of scientific articles related to clinical trials
As described in section 2, the data collected for each CT were used to search for related scientific articles (SAs) in Medline databases (via Pubmed), Capes Journal Portal, and Google Scholar. The SA metadata were organized into the categories “Title,” “Authors,” “Affiliation,” “Disclosures,” “Conflict of Interest,” “Abstract,” “Introduction,” “Material and Methods,” “Results,” “Discussion,” and “Conclusion” and grouped according to the respective CT. Numerical codes were used for CTs, while SAs were assigned an alphabetic code.
Comparative analysis between metadata from CTs and scientific articles
A comparative analysis was carried out of the CT and SA metadata from the three technological solutions using the criteria listed below. The CT metadata in the categories of “Main ID,” “Secondary ID,” “Phase,” “Sponsor,” “Intervention,” “Contacts,” and “Result” was considered to be present in the SA if they were contained in any part of the related article. The “Public Title” and “Scientific Title” from the CT metadata were considered to be present in the SA when two or more identical or technically similar keywords were present in the article’s title. The “Affiliation” field in the CT metadata were considered to be present in the SA if the article’s “Affiliation,” “Disclosures,” or “Conflict of interest” sections contained matching information.
Analysis of the state of CTs and publication of results
Initially, the metadata on the completion date of the CTs in the primary databases was used to determine studies that had been completed and those that were still in progress.
We used the following fields from CT databases to identify the publication of results: “Results,” “Posted Results,” “Trial Results,” and “Study Information.” In the SA databases, we searched through the following fields to assess whether results had been published: “Publication Date,” “Publication Type,” “Abstract,” “Materials and Methods,” and “Results.”
Finally, these data were used to verify the time elapsed between CT completion and the publication of results. A simple average was used when CT results were published more than once.
Results
Identification of CTs and SAs
After removing duplicates, the searches resulted in 17 records of Eryaspase CTs in the ICTRP database (Table 1). After the first search (I), the term “erytech” was observed in the “Primary Sponsor” field. Based on this information, a new search (II) was designed to increase search recall (I). The new search resulted in 12 CT records, nine of which were already present in the previous search.
An examination of the “Intervention” field during our analysis of search (II) uncovered three different names used for Eryaspase: “l-asparaginase encapsulated in RBC,” “graspa,” and “eryaspase.” Thus, search (III) was designed, from which five more studies were added to the sample, for a total of 17 CT records for Eryaspase.
The searches for CTs for the compounds GSK3186899 and DNDI 0690 used a strategy based on combinations of keywords with concepts related to the disease and the compounds (searches Ia and Ib, respectively) or only the compounds (searches IIa and IIb) (Table 1). Both searches produced the same outcome. A single CT was identified for GSK3186899, while two were found for DNDI-0690.
Table 2 shows the results of the searches for SAs related to the identified CTs. CTs are represented by sequential numbers and SAs by sequential letters, with a corresponding alphanumeric code denoting each SA-CT combination.
A total of 27 SAs were found for Eryaspase, while no SAs were found describing the results of CTs on GSK3186899 and DNDI-0690.
Analysis of relationships between the metadata of CTs and SAs
After the collection, organization, and analysis of the metadata, we observed that all CTs had data for the “Main ID,” “Secondary ID,” “Public Title,” “Scientific Title,” “Sponsor,” “Phase,” and “Intervention” fields. However, CTs 1, 3, and 15 were missing information in the “Contacts,” “Affiliation,” and “Results” fields, respectively, even after complementary verification in the primary databases.
Table 3 shows an overview of data presence or absence for each metadata field in the records of CTs and their respective SAs. For the most part, data are present for all metadata fields in the clinical records and the corresponding SAs. Specifically, CT data in the “Main ID” and “Secondary ID” fields occur in the SA fields “Title,” “Affiliation,” “Abstract,” “Materials and Methods,” and “Results.” Likewise, CT metadata from the “Affiliation” and “Sponsor” fields was found in the “Affiliation,” “Conflict of Interest,” “Abstract,” “Introduction,” “Materials and Methods,” “Results,” and “Discussion” fields of SAs. However, mismatches in some metadata content in the SA and CT records make the searching process more laborious.
Despite being the primary identifier of CT records, the “Main ID” data is not present in all scientific articles. When present, it is primarily located in the “Title” and “Abstract” sections. The lack of this data in SAs is a significant obstacle, making it difficult to trace data from CTs.
The “Secondary ID” proved to be an important data point for identifying CTs, as it was cited in eight SAs that did not cite the “Main ID.” Thus, efforts to trace CTs in the scientific literature should consider both the “Main ID” and the “Secondary ID,” since the latter is frequently cited in the “Title,” “Abstract,” and, particularly, in the “Results” section of SAs.
The public and scientific titles of CTs tended to be close to the titles of the corresponding SAs. This indicates that these metadata can be more effective at obtaining high recall rates despite their low precision.
Finally, although CT results were only rarely available from the ICTRP and primary databases, they could sometimes be found in multiple SA metadata fields, especially “Abstract,” “Results,” “Discussion,” and “Conclusion.”
Another critical aspect to highlight is the absence of content for the “Results” metadata in 15 CT records, indicating a significant gap in scientific knowledge. Hence, only five records among the 20 CT analyzed presented data on results, and only four were reported in eight SAs. Thus, the 166.6% correlation for data on results in Table 3 was mainly due to the data repetition in the metadata of the same scientific article.
Information was analyzed in the primary databases of CTs in order to complement the search for the “Results” metadata; however, no new information was available.
Assessment publication of results from CTs
After observing the low rate of publication of results in the CT databases (n=5), we sought to identify whether the trials were already completed or in progress. We therefore searched for metadata on the completion date of CTs in our sample to identify ones that had already been completed. Of the 20 CTs recorded, 16 were listed as completed (Figure 1 A). For the 16 completed CTs, we observed that nine (56%; trials 4, 5, 7, 13, 14, 15, 16, 17, and 19) had no published results of any kind. Hence, only seven studies (44%) were published, with only four (25%; trials 2, 3, 8, and 11) in both SA and CT databases. Meanwhile, results for two CTs (13%; trials 1 and 10) could only be found in the SA databases, while one (6%; study 6) could only be found in the CT databases (Figure 1 B). Of the CTs with no published results (56%) in any database (Figure 1 B), three CTs (trials 4, 16, and 17) included information on the experimental design in summarized publications. For the other six records, data were only available in CT databases (Figure 1 C). These data indicate that the non-publication of CT results in the scientific literature or clinical databases makes such results unavailable to society, drastically restricting access to information.
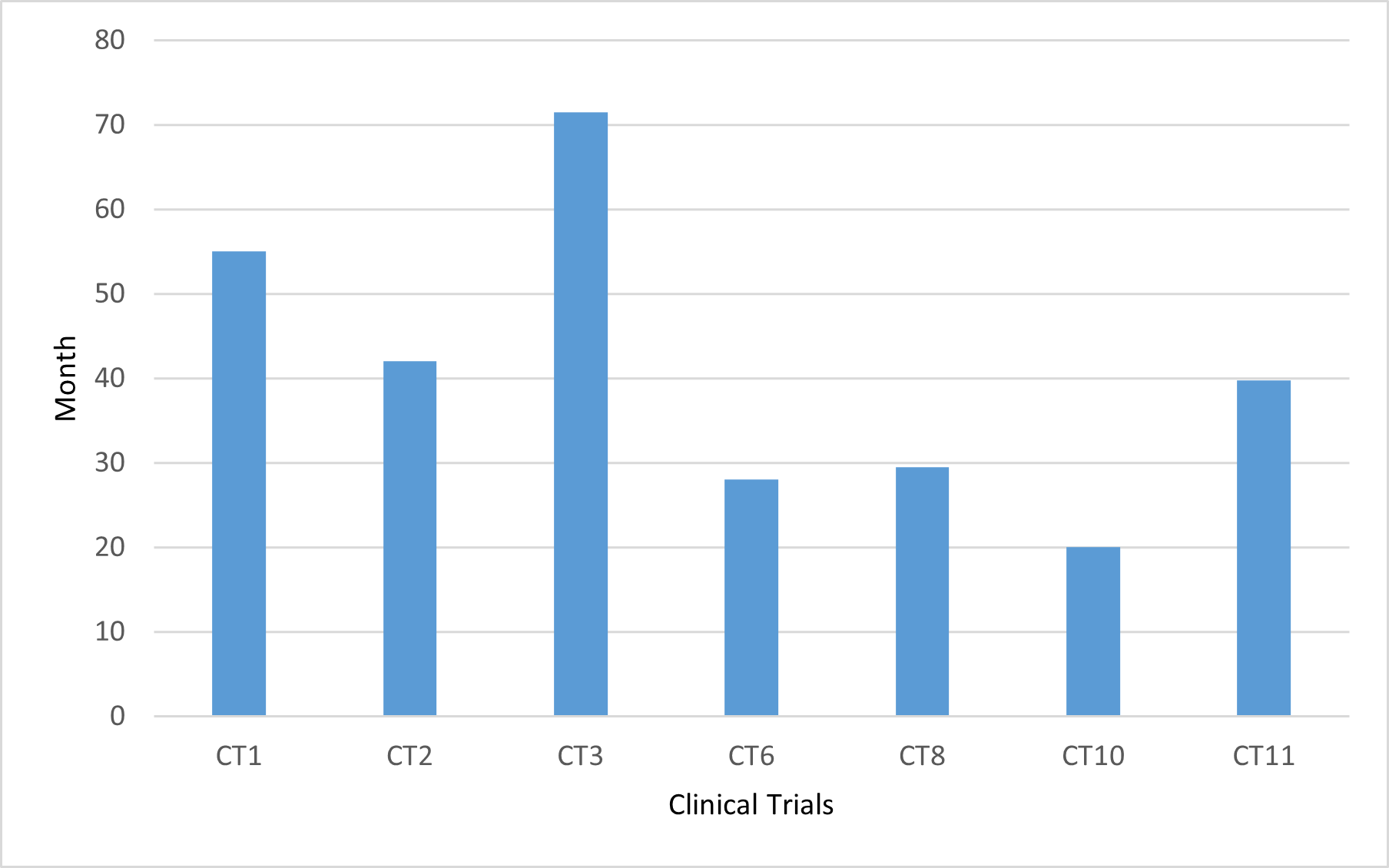
Our data indicate an average time lapse of 40.8 months between the completion of a CT and its results becoming available in clinical databases or the scientific literature, with figures ranging from 20 to 71 months (Figure 2).
Discussion
The development of a healthcare solution is initially related to scientific discoveries that constitute the first phase of the innovation process and lead to a large volume of publications in scientific journals (Targino, 2001Targino, M. G. Comunicação científica na sociedade tecnológica: periódicos electrônicos em discussão. Comunicação e Sociedade, v. 3, p. 93-112, 2001. Doi: https://doi.org/10.17231/comsoc.3(2001).1317.
https://doi.org/10.17231/comsoc.3(2001)....
). As innovations come to technological maturity, research in the medical field can be protected by patents. In addition, before products come to market, research results can be disclosed on platforms designed to record CTs and the pipelines of sponsoring companies (Pimenta, 2017Pimenta, F. P. A patente como fonte de informação (des)necessária para a Biotecnologia em Saúde. Transinformação, v. 29, n. 3, p. 323-332, 2017. Doi: https://doi.org/10.1590/2318-08892017000300009.
https://doi.org/10.1590/2318-08892017000...
; Ellwood; Williams; Egan, 2020Ellwood, P.; Williams, C.; Egan, J. Crossing the valley of death: Five underlying innovation processes. Technovation, v. 109, n. 102162, p. 1-11, 2020. Doi: https://doi.org/10.1016/j.technovation.2020.102162.
https://doi.org/10.1016/j.technovation.2...
).
Although scientific journals are the primary means for publishing CTs, there are gaps in the information available in SA databases (Shrivastava; Shrivastava; Ramasamy, 2018Shrivastava, S.R.; Shrivastava, P. S.; Ramasamy, J. Emphasizing the necessity to register and report the results of all clinical trials: World Health Organization. Medical Journal of Dr. D.Y. Patil Vidyapeeth, v. 11, n. 1, p. 81-82, 2018. Doi: https://doi.org/ 10.4103/MJDRDYPU.MJDRDYPU_105_17.
https://doi.org/10.4103/MJDRDYPU.MJDRDYP...
). Similarly, the evaluated CT databases are incomplete. While the information contained in SA titles is useful for identifying CTs, our data indicate that the metadata related to the sponsor’s name, study phase, and intervention’s name are the identifiers that are most reliably reported in the scientific literature. The latter metadata therefore proved to be the most effective way to track CTs published in the scientific literature.
We highlight the challenge of linking scientific publications to the corresponding CT. This difficulty is due to identifying information of the CT being spread throughout the article, hindering the search for a non-specialist reader. In addition, the citation of the “Main ID” is sometimes completely lacking in scientific publications. Often (55.5%), a secondary identifier provided by the company sponsoring the CT is used instead (Table 2).
The WHO recommends the use of a “Secondary ID” for studies with the same purpose that must be registered on several databases to comply with specific local legislation (World Health Organization, 2018World Health Organization. International standards for clinical trial registries: the registration of all interventional trials is a scientific, ethical and moral responsibility, version 3.0, 2018. Disponível em: https://apps.who.int/iris/handle/10665/274994. Acesso em: 5 set. 2021.
https://doi.org/10665/274994...
). However, despite this guidance, the mere presence of a “Secondary ID” does not ensure that a CT is accurately cited in a SA if there is no “Main ID” citation. The absence of the “Main ID” must always be questioned in scientific publications since it jeopardizes the mission of the clinical record, which is to allow comprehensive access to information.
The non-availability of results for most of the completed CTs in the clinical databases consulted is a significant bottleneck for the publication and transparency of information, especially given the phenomenon of selective publication of results or non-publication of negative results (Sayão; Sales; Felipe, 2021Sayão, L. F.; Sales, L. F.; Felipe, C. B. M. Invisible science: publication of negative research results. Transinformação, v. 33, p. e200009, 2021. Doi: https://doi.org/10.1590/2318-0889202133e200009.
https://doi.org/10.1590/2318-0889202133e...
). Many authors have highlighted the problem of selective publication, when only studies with positive or statistically significant results are published, while those with negative or non-significant results remain undisclosed (Page; Mckenzie; Higgins, 2018Page, M. J.; Mckenzie, J. E.; Higgins, J. P. T. Tools for assessing risk of reporting biases in studies and syntheses of studies: a systematic review. BMJ Open, v. 8, n. 3, p. e019703, 2018. Doi: https://doi.org/10.1136/ bmjopen-2017-019703.
https://doi.org/10.1136/ bmjopen-2017-01...
; Borysowski; Wnukiewicz-Kozłowska; Górski, 2020Borysowski, J.; Wnukiewicz-Kozłowska, A.; Górski, A. Legal regulations, ethical guidelines and recent policies to increase transparency of clinical trials. British Journal of Clinical Pharmacology, v. 86, n. 4, p. 679-686, 2020. Doi: https://doi.org/10.1111/bcp.14223.
https://doi.org/10.1111/bcp.14223...
).
In addition, it should be noted that in several cases, results were published an average of three years from the end of the CT. This indicates a considerable time lag for communication. This data is consistent with studies showing that the results of up to 50% of CTs may not be available using any means (Hopewell et al., 2001Hopewell, S. et al. Time to publication for results of clinical trials. In: The Cochrane Collaboration (ed.). Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd, 2001. p. MR000011. Doi: https://doi.org/10.1002/14651858.MR000011.
https://doi.org/10.1002/14651858.MR00001...
; Chan et al., 2014Chan, A.-W. et al. Increasing value and reducing waste: addressing inaccessible research. The Lancet, v. 383, n. 9913, p. 257-266, 2014. Doi: https://doi.org/10.1016/S0140-6736(13)62296-5.
https://doi.org/10.1016/S0140-6736(13)62...
; Schmucker et al., 2014Schmucker, C. et al. Extent of Non-Publication in Cohorts of Studies Approved by Research Ethics Committees or Included in Trial Registries. PLoS ONE, v. 9, n. 12, p. e114023, 2014. Doi: https://doi.org/10.1371/journal.pone.0114023.
https://doi.org/10.1371/journal.pone.011...
). Although legal regulations in the European Union and the US impose an obligation to include summarized CT results in their respective registry, compliance with these regulations has generally been lacking (Prayle; Hurley; Smyth, 2012Prayle, A. P.; Hurley, M. N.; Smyth, A. R. Compliance with mandatory reporting of clinical trial results on ClinicalTrials.gov: cross sectional study. BMJ, v. 344, p. d7373-d7373, 2012. Doi: https://doi.org/10.1136/bmj.d7373.
https://doi.org/10.1136/bmj.d7373...
; Chen et al., 2016Chen, R. et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ, v. 352, n. i.637, p.1-8, 2016. Doi: https://doi.org/10.1136/bmj.i637.
https://doi.org/10.1136/bmj.i637...
; Goldacre et al., 2018Goldacre, B. et al. Compliance with requirement to report results on the EU Clinical Trials Register: cohort study and web resource. BMJ, v. 362, n. k3218, p. 1-10, 2018. Doi: https://doi.org/10.1136/bmj.k3218.
https://doi.org/10.1136/bmj.k3218...
). Our data reveal failures in the publication of results from CTs that have already been completed. However, it is difficult to determine whether these failures result from selective publication or merely an excessive delay in disclosure.
The failure to publish CT results is underscored by the fact that the results of six studies (67% of all studies with published results) are only available in CT databases, with no published results in the scientific literature. As such, these databases represent a potential source of information, sometimes unique, about technological innovations in healthcare.
No scientific publications reporting clinical data for GSK3186899 and DNDI-0690 were found, which clearly shows that CT databases are the only way to obtain information on these technologies. Records in CT databases generally provide information on their experimental design and development, providing essential scientific data on the corresponding technology. As such, data from CT records play a fundamental role in providing information to the scientific community on new healthcare solutions since these records often hold information that can only be found in these databases (Viergever et al., 2014Viergever, R. F. et al. The quality of registration of clinical trials: still a problem. PLoS ONE, v. 9, n. 1, p. e84727, 2014. Doi: https://doi.org/10.1371/journal.pone.0084727.
https://doi.org/10.1371/journal.pone.008...
). The information in these databases can be used to indicate technologies in development that may be directed to overcome current scientific issues.
It is clear that researchers are generally influenced almost exclusively by SAs and only rarely seek other information sources (Pimenta, 2017Pimenta, F. P. A patente como fonte de informação (des)necessária para a Biotecnologia em Saúde. Transinformação, v. 29, n. 3, p. 323-332, 2017. Doi: https://doi.org/10.1590/2318-08892017000300009.
https://doi.org/10.1590/2318-08892017000...
). This leads to a tendency to ignore other useful sources when planning and conducting a scientific study, leading to duplicated research and investments. Meanwhile, encouraging the scientific community to seek other information sources can help increase the quality and efficiency of scientific efforts.
Conclusion
This study demonstrates the potential of CT databases as a source of important information for the development of technological solutions. However, our results highlight the challenge of accurately identifying links between scientific publications and the corresponding CT records since the metadata are not identical and can require manual analysis by an expert professional.
This study sheds light on three major obstacles facing the scientific community and the public with respect to access to information and transparency in clinical studies: (i) the lack of correlation between CT records and scientific publications, (ii) the considerable time lag between the conclusion of a CT and the publication of results, and (iii) the non-availability of results from completed CTs in any of the databases consulted.
Acknowledgments
We thank Dr. Cicera Henrique da Silva, full professor at Fiocruz, for a critical review of this article.
References
- Borysowski, J.; Wnukiewicz-Kozłowska, A.; Górski, A. Legal regulations, ethical guidelines and recent policies to increase transparency of clinical trials. British Journal of Clinical Pharmacology, v. 86, n. 4, p. 679-686, 2020. Doi: https://doi.org/10.1111/bcp.14223.
» https://doi.org/10.1111/bcp.14223 - Brasil. Resolução RDC n° 39, de 5 de junho de 2008. Regulamento para a realização de pesquisa clínica [S.l]: Anvisa, 2008. Disponivel em: Disponivel em: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2008/res0039_05_06_2008.html Acesso: 20 maio, 2021.
» https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2008/res0039_05_06_2008.html - Brasil. Resolução RDC nº 36, de 27 junho de 2012. Altera a RDC nº 39, de 05 de junho de 2008 [S.l]: Anvisa, 2012. Disponível em: Disponível em: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2012/rdc0036_27_06_2012.html Acesso: 20 maio, 2021.
» https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2012/rdc0036_27_06_2012.html - Chan, A.-W. et al Increasing value and reducing waste: addressing inaccessible research. The Lancet, v. 383, n. 9913, p. 257-266, 2014. Doi: https://doi.org/10.1016/S0140-6736(13)62296-5.
» https://doi.org/10.1016/S0140-6736(13)62296-5 - Chen, R. et al Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ, v. 352, n. i.637, p.1-8, 2016. Doi: https://doi.org/10.1136/bmj.i637.
» https://doi.org/10.1136/bmj.i637 - Ellwood, P.; Williams, C.; Egan, J. Crossing the valley of death: Five underlying innovation processes. Technovation, v. 109, n. 102162, p. 1-11, 2020. Doi: https://doi.org/10.1016/j.technovation.2020.102162.
» https://doi.org/10.1016/j.technovation.2020.102162 - Goldacre, B. et al Compliance with requirement to report results on the EU Clinical Trials Register: cohort study and web resource. BMJ, v. 362, n. k3218, p. 1-10, 2018. Doi: https://doi.org/10.1136/bmj.k3218.
» https://doi.org/10.1136/bmj.k3218 - Gouy, C. M. L.; Porto, T. F.; Penido, C. Avaliação de ensaios clínicos no Brasil: histórico e atualidades. Revista Bioética, v. 26, n. 3, p. 350-359, 2018. Doi: https://doi.org/10.1590/1983-80422018263254.
» https://doi.org/10.1590/1983-80422018263254 - Hopewell, S. et al Time to publication for results of clinical trials. In: The Cochrane Collaboration (ed.). Cochrane Database of Systematic Reviews Chichester, UK: John Wiley & Sons, Ltd, 2001. p. MR000011. Doi: https://doi.org/10.1002/14651858.MR000011.
» https://doi.org/10.1002/14651858.MR000011 - Page, M. J.; Mckenzie, J. E.; Higgins, J. P. T. Tools for assessing risk of reporting biases in studies and syntheses of studies: a systematic review. BMJ Open, v. 8, n. 3, p. e019703, 2018. Doi: https://doi.org/10.1136/ bmjopen-2017-019703.
» https://doi.org/10.1136/ bmjopen-2017-019703 - Pimenta, F. P. A patente como fonte de informação (des)necessária para a Biotecnologia em Saúde. Transinformação, v. 29, n. 3, p. 323-332, 2017. Doi: https://doi.org/10.1590/2318-08892017000300009.
» https://doi.org/10.1590/2318-08892017000300009 - Prayle, A. P.; Hurley, M. N.; Smyth, A. R. Compliance with mandatory reporting of clinical trial results on ClinicalTrials.gov: cross sectional study. BMJ, v. 344, p. d7373-d7373, 2012. Doi: https://doi.org/10.1136/bmj.d7373.
» https://doi.org/10.1136/bmj.d7373 - Quental, C.; Salles Filho, S. Ensaios clínicos: capacitação nacional para avaliação de medicamentos e vacinas. Revista Brasileira de Epidemiologia, v. 9, n. 4, p. 408-424, 2006. Doi: https://doi.org/10.1590/S1415-790X2006000400002.
» https://doi.org/10.1590/S1415-790X2006000400002 - Sayão, L. F.; Sales, L. F.; Felipe, C. B. M. Invisible science: publication of negative research results. Transinformação, v. 33, p. e200009, 2021. Doi: https://doi.org/10.1590/2318-0889202133e200009.
» https://doi.org/10.1590/2318-0889202133e200009 - Schmucker, C. et al Extent of Non-Publication in Cohorts of Studies Approved by Research Ethics Committees or Included in Trial Registries. PLoS ONE, v. 9, n. 12, p. e114023, 2014. Doi: https://doi.org/10.1371/journal.pone.0114023.
» https://doi.org/10.1371/journal.pone.0114023 - Shrivastava, S.R.; Shrivastava, P. S.; Ramasamy, J. Emphasizing the necessity to register and report the results of all clinical trials: World Health Organization. Medical Journal of Dr. D.Y. Patil Vidyapeeth, v. 11, n. 1, p. 81-82, 2018. Doi: https://doi.org/ 10.4103/MJDRDYPU.MJDRDYPU_105_17.
» https://doi.org/10.4103/MJDRDYPU.MJDRDYPU_105_17 - Targino, M. G. Comunicação científica na sociedade tecnológica: periódicos electrônicos em discussão. Comunicação e Sociedade, v. 3, p. 93-112, 2001. Doi: https://doi.org/10.17231/comsoc.3(2001).1317.
» https://doi.org/10.17231/comsoc.3(2001).1317 - Tse, T.; Fain, K. M.; Zarin, D. A. How to avoid common problems when using ClinicalTrials.gov in research: 10 issues to consider. BMJ, v. 361, n. k1452, p. 1-8, 2018. Doi: https://doi.org/10.1136/bmj.k1452.
» https://doi.org/10.1136/bmj.k1452 - Van Raan, A. F. J. Scientometrics: state-of-the- art. Scientometrics, v. 38, n. 1, p. 205-218, 1997.
- Vanti, A. P. Da bibliometria à webometria: uma exploração conceitual dos mecanismos utilizados para medir o registro da informação e a difusão do conhecimento. Ciência da Informação, v. 31, n. 2, p. 369-379, 2002. Doi: https://doi.org/10.1590/S0100-19652002000200016.
» https://doi.org/10.1590/S0100-19652002000200016 - Viergever, R. F. et al The quality of registration of clinical trials: still a problem. PLoS ONE, v. 9, n. 1, p. e84727, 2014. Doi: https://doi.org/10.1371/journal.pone.0084727.
» https://doi.org/10.1371/journal.pone.0084727 - World Health Organization. International standards for clinical trial registries: the registration of all interventional trials is a scientific, ethical and moral responsibility, version 3.0, 2018. Disponível em: https://apps.who.int/iris/handle/10665/274994. Acesso em: 5 set. 2021.
» https://doi.org/10665/274994
Como citar este artigo/How to cite this article
-
2
Trigueiros, B. A. F. S.; Ávila, A. R.; Pimenta, F. P. An examination of the use of clinical trials as a source of information in scientific research. Transinformação, v. 34, e210065, 2022. https://doi.org/10.1590/2318-0889202234e210065
Publication Dates
-
Publication in this collection
08 July 2022 -
Date of issue
2022
History
-
Received
09 Sept 2021 -
Accepted
18 Nov 2021




 Note: A: State of completion of the clinical trial; B: Publication means of clinical trials results; C: Publication of clinical trails information (no results); The slice represented as Indeterminate means lack of information in the metadata indicating the completion of the study.Source: Elaborated by the authors (2021)
Note: A: State of completion of the clinical trial; B: Publication means of clinical trials results; C: Publication of clinical trails information (no results); The slice represented as Indeterminate means lack of information in the metadata indicating the completion of the study.Source: Elaborated by the authors (2021)
 Source: Prepared by the authors (2021).
Source: Prepared by the authors (2021).