Abstracts
In this study the ozonized olive and sunflower oils are chemical and microbiologically compared. These oils were introduced into a reactor with bubbling ozone gas in a water bath at room temperature until they were solidified. The peroxide, acidity and iodine values along with antimicrobial activity were determined. Ozonization effects on the fatty acid composition of these oils were analyzed using Gas-Liquid Chromatographic Technique. An increase in peroxidation and acidity values was observed in both oils but they were higher in ozonized sunflower oil. Iodine value was zero in ozonized olive oil whereas in ozonized sunflower was 8.8 g Iodine per 100 g. The antimicrobial activity was similar for both ozonized oils except for Minimum Bactericidal Concentrations of Pseudomona aeruginosa. Composition of fatty acids in both ozonized oils showed gradual decrease in unsaturated fatty acids (C18:1, C18:2) with gradual increase in ozone doses.
ozone; ozonized sunflower oil; ozonized olive oil; peroxide value; antimicrobial activity; Gas-Liquid Chromatography
Neste estudo, óleos de oliva e girassol ozonizados foram comparados química e microbilogicamente. Estes óleos foram introduzidos em um reator com gás ozônio borbulhante, em banho-maria a temperatura ambiente, até solidificação. O teor de peróxido, de iodo e o grau de acidez foram determinados juntamente com a atividade antimicrobiana. Os efeitos da ozonização na composição dos ácidos graxos desses óleos foram analisados usando-se a técnica de Cromatografia Gás-Líquido. Um aumento nos valores de peroxidação e de acidez foi observado em ambos os óleos, mas foram maiores no óleo de girassol ozonizado. O teor de iodo obtido no azeite de oliva ozonizado foi zero, enquanto no óleo de girassol ozonizado foi de 8,8 g de iodo per 100 g. A atividade antimicrobiana foi similar para os dois óleos ozonizados, com exceção da Concentração Mínima Bactericida de Pseudomona Aruginosa. A composição dos ácidos graxos nos dois óleos ozonizados mostrou um decréscimo gradual de ácidos graxos insaturados (C18:1, C18:2), com o aumento gradual da ozonização.
ARTICLE
Comparative study of ozonized olive oil and ozonized sunflower oil
Maritza F. Díaz* * e-mail: maritza.diaz@cnic.edu.cu , I; Rebeca HernándezI; Goitybell MartínezI; Genny VidalI; Magali GómezI; Harold FernándezI; Rafael GarcésII
IDepartment of Ozonized Substances, Ozone Research Center, National Center for Scientific Research, P.O. Box 6412 Havana, Cuba
IIInstituto de la Grasa, CSIC, Sevilla, Spain
ABSTRACT
In this study the ozonized olive and sunflower oils are chemical and microbiologically compared. These oils were introduced into a reactor with bubbling ozone gas in a water bath at room temperature until they were solidified. The peroxide, acidity and iodine values along with antimicrobial activity were determined. Ozonization effects on the fatty acid composition of these oils were analyzed using Gas-Liquid Chromatographic Technique. An increase in peroxidation and acidity values was observed in both oils but they were higher in ozonized sunflower oil. Iodine value was zero in ozonized olive oil whereas in ozonized sunflower was 8.8 g Iodine per 100 g. The antimicrobial activity was similar for both ozonized oils except for Minimum Bactericidal Concentrations of Pseudomona aeruginosa. Composition of fatty acids in both ozonized oils showed gradual decrease in unsaturated fatty acids (C18:1, C18:2) with gradual increase in ozone doses.
Keywords: ozone, ozonized sunflower oil, ozonized olive oil, peroxide value, antimicrobial activity, Gas-Liquid Chromatography
RESUMO
Neste estudo, óleos de oliva e girassol ozonizados foram comparados química e microbilogicamente. Estes óleos foram introduzidos em um reator com gás ozônio borbulhante, em banho-maria a temperatura ambiente, até solidificação. O teor de peróxido, de iodo e o grau de acidez foram determinados juntamente com a atividade antimicrobiana. Os efeitos da ozonização na composição dos ácidos graxos desses óleos foram analisados usando-se a técnica de Cromatografia Gás-Líquido. Um aumento nos valores de peroxidação e de acidez foi observado em ambos os óleos, mas foram maiores no óleo de girassol ozonizado. O teor de iodo obtido no azeite de oliva ozonizado foi zero, enquanto no óleo de girassol ozonizado foi de 8,8 g de iodo per 100 g. A atividade antimicrobiana foi similar para os dois óleos ozonizados, com exceção da Concentração Mínima Bactericida de Pseudomona Aruginosa. A composição dos ácidos graxos nos dois óleos ozonizados mostrou um decréscimo gradual de ácidos graxos insaturados (C18:1, C18:2), com o aumento gradual da ozonização.
Introduction
The characterization of vegetable oils has been the subject of academic study for over 200 years. In the latter half of last century, and additional stimulus has been the growth of chemistry and biochemistry and the desire to understand the mechanisms leading to the wide variety of natural products and their function.1
The olive oil is obtained from the fruit of the olive tree and the sunflower oil is obtained from the seed of the sunflower flower. Both oils contain different fatty acid composition, olive oil presents a high proportion of oleic acid (65-85 %) and sunflower oil is rich in linoleic (48-74 %) and oleic acids (14-39 %).2
The reaction of ozone with these vegetable oils occurs almost exclusively with the carbon-carbon double bonds present in unsaturated fatty acids.3 This reaction produces several oxygenated compounds such as hydroperoxides, ozonides, aldehydes, peroxides, diperoxides and polyperoxides.3-7 These oxygenated compounds could be also responsible for the wide biological activity of ozonized vegetable oils. The yield of oxygenated compounds from unsaturated oils depends on reaction conditions necessary, as the type of medium where the reaction takes place, the presence of additives, the reaction temperature, the type of reactor, the agitation of the reaction mixture, the applied ozone doses, etc.3,8-10
The knowledge of the physicochemical properties of ozonized vegetable oils has a great importance for its characterization and identification. Analytical methods as peroxide, acidity, and iodine values are used to follow up the ozonization process and for determining the quality of ozonized vegetable oils.11-15 The most routine approach to the analysis of fatty acids in the organic compounds is Gas-Liquid Chromatography (GLC), which has been used for analysis of saturated and unsaturated fatty acids in vegetable oils.16-18
As a natural preparation, ozonized oil is now available in several countries, but information with regards to the chemical data, standard preparations and antimicrobial activity is limited. However, ozonized sunflower oil (OLEOZON®) from Cuba has been tested and it found to have valuable antimicrobial activity against bacteria, virus and fungi.19-22 At the University Hospital pharmacy of Siena, they make your own preparation by bubbling ozone in pure olive oil for at least 30 min in a cooled bath. In other countries the pure olive oil is ozonized by two days until it solidifies.23 Both oils are widely used for their therapeutic effects. For this reason it is the objective in this work to compare chemical composition and microbiological activity of the ozonized olive and sunflower oils using different volumetric assays, Gas-Liquid Chromatographic Technique (GLC) and agar dilution and macro dilution methods with different microorganisms.
Experimental
Solvents and reagents
Glacial acetic acid, chloroform, potassium iodide, sodium, thiosulfate, starch, ethanol, ether, potassium hydroxide, phenolphthalein, iodine bromide, toluene, dimetoxipropane from MERCK (Germany). Edible sunflower and olive oils were obtained from Ideal registered trademark, Argentina and Borges Oils registered trademark, Spain, respectively.
General ozonization procedure
A mixture of 80 mL of sunflower oil and 8 mL of water was introduced into a bubbling reactor where ozone reaction took place in water bath at room temperature 25 ºC. The ozonization was allowed to continue for 8.05 hours and 2 samples were taken at different applied ozone doses (54.6 and 246.8 mg g-1). The same ozonization reactions were obtained using a mixture of 80 mL of olive oil and 8 mL of water, which was continued for 5.73 hours and 2 samples were taken at different applied ozone doses (34.9 and 177.0 mg g-1). The ozone doses were chosen to achieve peroxide values ranged between 700 and 800 mmol-equiv. kg-1 (sample 1) and up to solidification complete (sample 2).
Ozone generation
Ozone was generated by passing oxygen through a Trailigaz Labo model 12-02 ozone generator at a fixed voltage (170 V), and a constant flow rate of 30 L h-1. The ozone initial concentration (75.2 mg L-1) was determined by Anseros Ozomat equipment.
Peroxide value
Peroxide value is the number that expresses, in miliequivalents of active oxygen, the quantity of peroxide contained in 1 000 g of the substance.11
Acid value
Acid value is the number of mg of potassium hydroxide required to neutralize the free acids in 1.0 g of the substance.12
Iodine value
Iodine value of a substance is the weight of iodine absorbed by 100 parts by weight of the substance.13
Gas-Liquid Chromatography (GLC)
Unsaturated and saturated fatty acids of untreated and ozonized sunflower and olive oils samples were analyzed. For GLC analysis of fatty acids esterified, methyl ester derivatives were first prepared by transesterification as described by Garcés and Mancha.24
Analysis were performed in a DB-17HT capillary column (15 m x 0.25 mm ID, film thickness 0.15 µm), with a flame ionization detector at 280 °C. The carrier gas was hydrogen at 1 mL min-1 and a pressure of 50 kPa. The column temperature was programmed from 100 to 200 °C at 8 °C min-1. Injection volume was 5 µL. A model Hewlett Packard 5890 chromatographic system was used for the analysis. External fatty acid standards (Sigma-Aldrich Chemical Company) were used to identify components.
Antimicrobial activity
The antimicrobial activities from the ozonized sunflower oil with different peroxides values on bacterial and yeast strains were determined. Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 10536, Pseudomonas aeruginosa ATCC 27853 and Bacillus subtilis ATCC 6633 were chosen. Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal Concentrations (MBCs) by agar dilution and macro dilution methods were based on National Committee for Clinical Laboratory Standards.25,26
Statistical analysis
Three experiences were carried out. All data were expressed as mean and standard deviation.
Results and Discussion
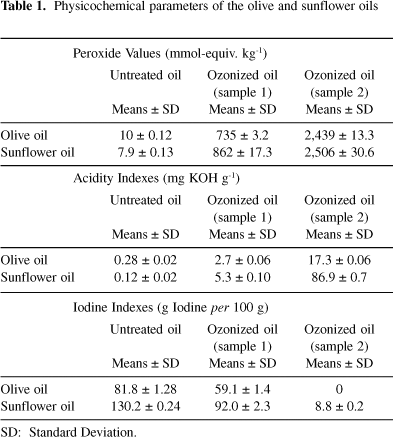
Table 1 shows the peroxides, acid and iodine values of the two untreated and ozonized oils studied. During ozonization reaction, an increase of peroxide and acid values was obtained, whereas a decrease of iodine values was observed.
The reaction of ozone with vegetable oil occurs almost exclusively with the carbon-carbon double bonds present in unsaturated fatty acids.3,15 Ozonized olive and sunflower oils (sample 1) reached viscosity value of 160 and 185 mPas, respectively, while that the sample 2 reached to solidification complete. In both oil samples an increase of peroxide values has been observed due to formation of peroxidic substances when ozone reacts with unsaturated compounds through the known Criegee mechanism.27 In sample 2, both ozonized oils showed maximum peroxide values of 2,439 and 2,506 mmol-equiv. kg-1. This behavior might be due to formation of polymeric peroxides which are responsible for viscous mass achieved in both ozonized oils.
An increase in peroxidation and acidity values was observed in both oils but it was higher in ozonized sunflower oil. In sample 1 (ozonized olive and sunflower oils), where an increase of peroxide values occurs rapidly up to 34.9 and 54.6 mg g-1 of applied ozone dosage, acid values have a discrete increase from 0.28 to 2.7 mg KOH g-1 and 0.12 to 5.3 mg KOH g-1, respectively. In the sample 2, acids values increase up to 17.3 and 86.9 mg KOH g-1 respectively, which represent an increase of 6.4 and 16.4 fold with regards to sample 1 acid values. These results can be explained by the decomposition of all the peroxidic compounds present in the different equilibriums, into the same reaction system.7 One example of these is the formation of carboxylic acid from peroxidic compounds.6 Besides, sunflower oil has a higher proportion of unsaturated fatty acid than olive oil, led to very complex ozonized system where the peroxidic compound decomposition to acid is very high.
Iodine value showed a decrease in relation to applied ozone dosage (Table 1). It is well known that this value is a measure of double bond content in oils,13 principally oleic and linoleic acids. Ozone reaction with the vegetable oils which have high levels of unsaturated fatty acids, led to rapid decrease of iodine values.3 Iodine value was zero in (sample 2) ozonized olive oil whereas in (sample 2) ozonized sunflower oil was 8.8 g iodine per 100 g, this is indicative that all unsaturated groups in olive oil reacted with ozone, but not in sunflower oil.
Unsaturated fatty acids in untreated olive oil are rich in oleic acid which present one unsaturated in C9, while the sunflower oil has major content of linoleic acid which present two unsaturated in C9 and C12, respectively,16 so olive oil has less double bond for ozone reaction.
Ozonization effects on the fatty acids composition of these oils were analyzed using Gas-Liquid Chromatographic Technique (GLC) (Table 2). Composition of fatty acids in both ozonized oils showed gradual decrease in unsaturated fatty acids (C18:1, C18:2) with gradual increase in ozone doses. In sunflower oil the ozonization reaction occurs through linoleic acid while in olive oil occurs through oleic acid.
These results are indicative that there are double bonds for ozone reaction in each system. It does not coincide with results obtained by (sample 2) ozonized olive oil using iodine value determination (Table 1), because when an applied ozone dosage of 177.0 mg g-1 is achieved, double bounds are not detected by this assay but gas chromatography quantifies them. This experiment demonstrates that iodine value determination is not exact for this measurement. When applied ozone dosage is elevated, polymerizations for condensation of peroxides that have been formed for ozonization reaction occur7 and the high viscosity observed in the system obstruct the access to double bond of iodine bromide reagent.
Data for antimicrobial activity of ozonized olive and sunflower oils with different peroxide values are presented in Table 3. The antimicrobial activity was similar for both ozonized oils except for Minimum Bactericidal Concentrations of Pseudomona aeruginosa ATCC 27853. The ozonized sunflower oil at low peroxide value had better antimicrobial activity against of Pseudomona aeruginosa while ozonized olive oil was better to high peroxide value. This research indicates that at higher peroxide values, higher ozonized olive and sunflower oils antimicrobial activity power. Also, ozonized olive and sunflower oils present similar germicidal effect.
In other study21 the activity in vitro of ozonized sunflower oil OLEOZON® on Staphylococcus aureus ATCC 25923 was studied. The value of Minimum Inhibitory Concentrations was 9.5 mg mL-1 and the value of Minimum Bactericidal Concentrations was 356 mg mL-1. These results were better in this study (Table 3), which can be due to changes in the analyzed ATCC strain; different peroxide values in the oils and other ozonization procedure were used.
Research efforts in the field of ozonized vegetable oil chemistry over the years; have been concerned with the oxygenized products elucidation that could be related with their germicidal effect.15,28 These results taken together demonstrate that when peroxide values have a notable increase the produced oxygenized compounds are responsible for the germicidal effect of these oils.
Conclusions
An increase in peroxide and acid values was obtained in both oils but they were higher in ozonized sunflower oil.
When peroxide values are elevated, the ozonized oils have high viscosity that obstructs the access to double bond of iodine bromide reagent. For this reason the iodine value determination is not exact for this measurement.
The ozonized olive and sunflower oils have similar antimicrobial activity against Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 10536 and Bacillus subtilis ATCC 6633, except against Pseudomona aeruginosa ATCC 27853, where sunflower oil at low peroxide value had better antimicrobial activity, while ozonized olive oil was better to high peroxide value.
Received: May 25, 2005
Published on the web: February 24, 2006
- 1. Hilditch, T.P.; Williams, P.N.; The Chemical Constitution of Natural Fats, 4th ed., Chapman & Hall: London, 1964.
- 2. Gunstone, F.D.; Harwood, J.L.; Padley, F.B.; The Lipid Handbook Occurrence and Characteristics of Oils and Fats, 2nd ed., Chapman & Hall: London, 1994.
- 3. Bailey, P.; Ozonolysis of Olefins: Introduction, Initial Ozone Attack and Adduct; The Peroxides Products. Ozonization in Organic Chemistry, Academic Press: New York, 1978, ch. 2.
- 4. Rebrovic, L.; J. Am. Oil Chem. Soc. 1992, 69, 159.
- 5. Pryor, W.A.; Squadrito, G.L.; Friedman, M.; Free Radic. Biol. Med. 1995, 19, 935.
- 6. Díaz, M.; Álvarez, I.; Vélez, H.; Hernández, F.; Ledea, O.; Molerio, J.; Bol. Soc. Chil. Quim. 1997, 42, 349.
- 7. Ledea, O.; Ph. D. Thesis, National Center for Scientific Research, Havana City, 2003.
- 8. Pryor, W.A.; Wu, M.; Chem. Res. Toxicol. 1992, 5, 505.
- 9. Diaz, M.; Hernández, F.; Ledea, O.; Gavín, J.A.S.; Moleiro, J.; Ozone-Sci. Eng. 2003, 25, 121.
- 10. Ledea, O.; Díaz, M.; Molerio, J.; Jardines, D.; Rosado, A.; Correa, T.; Rev. CENIC Ciencias Quim. 2003, 34, 1.
- 11. British Pharmacopoeia, Appendix XF, IA, IB. Peroxide value, 2000.
- 12. British Pharmacopoeia, Appendix XB, IA, IB. Acid value, 2000.
- 13. British Pharmacopoeia, Appendix XE, IA, IB. Iodine value, 2000.
- 14. Hernández, R.; Martínez, G.; Díaz, M.; Rev. CENIC Ciencias Quim. 2004, 35, 159.
- 15. Díaz, M.; Núñez, N.; Quincose, D.; Díaz, W.; Hernández, F.; Ozone-Sci. Eng. 2005, 27, 1.
- 16. Firestone, D.; Reina, R.J. In Food Authentication; Ashurst, P.R.; Dennis, M.J., eds.; Chapman and Hall: London, 1996.
- 17. Firestone, D.; Physical and Chemical Characteristics of Oils, Fats, and Waxes, Copyright by AOCS Press: London, 1999.
- 18. Panreac Química S.A.; Aceites y Grasas, Métodos analíticos en alimentaria, Métodos oficiales de Análisis, Centre Telematic Editorial SRL: Barcelona, 1999.
- 19. Lezcano, I.; Nuñez, N.; Gutierrez, M.; Molerio, J.; Regüeiferos, M.G.; Díaz, W.; Rev. CENIC, Ciencias Biol. 1996, 27, 46.
- 20. Lezcano, I.; Molerio, J.; Regüeiferos, M.G.; Contreras, R.; Roura, G.; Díaz, W.; Rev. CENIC, Ciencias Biol. 1998, 29, 209.
- 21. Lezcano, I.; Nuñez, N.; Espino, M.; Gómez, M.; Ozone-Sci. Eng. 2000, 22, 207.
- 22. Sechi, L.A.; Lezcano, I.; Nuñez, N.; Espim, M.; Dupre, I.; Pinna, A.; J. Appl. Microbiol. 2001, 90, 279.
- 23. Bocci, V.; Oxygen-Ozone Therapy. A Critical Evaluation, Kluwer Academia Publishers: Dordrecht, The Netherlands, 2002.
- 24. Garcés, R.; Mancha, M.; Anal. Biochem. 1993, 211, 139.
- 25. NCCLS; Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Standard 1, 7, 3rd ed., 1992.
- 26. NCCLS; Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically: Approved Standard 13, 25, 3rd ed., 1993.
- 27. Criegee, R.; Mechanism of Ozonolisis, 14th ed., Angewandte Chemie Int: England, 1975.
- 28. Díaz, M.; Lezcano, I.; Molerio, J.; Hernández, F.; Ozone-Sci. Eng. 2001, 23, 35.
Publication Dates
-
Publication in this collection
17 Apr 2006 -
Date of issue
Apr 2006
History
-
Received
25 May 2005