Abstracts
The cytotoxicities of five nor-²-lapachone-based 1,2,3-triazoles and the precursor azidonaphthoquinone were assayed against six neoplasic cancer cell lines: SF-295 (central nervous system), HCT-8 (colon), MDAMB-435 (melanoma), HL-60 (leukaemia), PC-3 (prostate) and B-16 (murine melanoma). IC50 values ranging from 0.43 to 9.48 µM were obtained. 3-(4-(1-hydroxycyclohexyl)-1H-1,2,3-triazol-1yl)-2,2-dimethylnaphtho[1,2-b]furan-4,5-dione proved highly cytotoxic to MDAMB-435, with IC50 even lower than the one from doxorubicin (positive control). In an attempt to correlate physicochemical parameters (reduction potentials and calculated log P) with cytotoxic activity, electrochemical studies were conducted in acetate buffer, pH 4.5, using a vitreous carbon electrode and calculated log P values were obtained. Despite the absence of a structural conjugative interaction between the two systems (quinone and triazole), the heterocyclic group was found to influence the voltammetric behaviour, as indicated by anodic shifts in the reduction potentials. No correlation was found between EpIc and cytotoxicity. In contrast, a comparison of EpIc with previously reported trypanocidal activities reconfirmed the trend for higher activity coupled with better quinone electrophilicity (> EpIc).A leading term in the correlation of cytoxicity, despite the absence of a linear correlation, was the calculated log P: the lower the lipophilicity, the lower the cytoxicity (> IC50).
lapachones; cytotoxicity; reduction potentials; clog P; naphthoquinone-triazoles; trypanocidal agents
A atividade citotóxica de cinco derivados 1,2,3-triazólicos da nor-²-lapachona e do precursor azidonaftoquinona, foi avaliada contra seis linhagens de células neoplásicas: SF-295 (sistema nervoso central), HCT-8 (cólon), MDAMB-435 (melanoma), HL-60 (leucemia), PC-3 (próstata) e B-16 (melanoma murino). Valores de IC50 entre 0,43 e 9,48 µM foram obtidos. A 3-(4-(1-hidróxicicloexil)-1H-1,2,3-triazol-1-il)-2,2-dimetilnaftol[1,2-b]furan-4,5-dione apresentou-se altamente citotóxica contra MDAMB-435, com IC50 menor do que o encontrado para doxorubicina (controle positivo). Na tentativa de obter correlação entre parâmetros físico-químicos (potencial de redução e clog P) e atividade citotóxica, estudos eletroquímicos foram realizados em tampão acetato, pH 4,5, em eletrodo de carbono vítreo, e valores de log P calculados (clog P). Apesar da ausência de interação estrutural conjugativa entre o sistema quinônico e o anel triazólico, observou-se a influência do anel heterocíclico no comportamento voltamétrico, com deslocamento anódico dos potenciais de redução. Não se verificou correlação entre os parâmetros eletroquímicos (EpIc) e a atividade citotóxica. Por outro lado, a comparação de EpIc com atividades tripanocidas, já descritas, reafirmou a tendência de maior atividade biológica para quinonas mais eletrofílicas (> EpIc). Apesar da ausência de correlação direta, foi possível verificar que clog P influencia a atividade citotóxica: quanto menor a lipofilia (< clog P), menor a citotoxicidade (> IC50).
ARTICLE
Cytotoxic, trypanocidal activities and physicochemical parameters of nor- β -lapachone-based 1,2,3-triazoles
Eufrânio N. da Silva JúniorI; Maria Aline B. F. de MouraII; Antonio V. PintoIII; Maria do Carmo F. R. PintoIII; Maria Cecília B. V. de SouzaI; Ana J. AraújoIV; Claudia PessoaIV; Letícia V. Costa-LotufoIV; Raquel C. MontenegroIV; Manoel Odorico de MoraesIV; Vitor F. FerreiraI, * * e-mail: mofg@qui.ufal.br; cegvito@vm.uff.br ; Marilia O. F. GoulartII, * * e-mail: mofg@qui.ufal.br; cegvito@vm.uff.br
IDepartamento de Química Orgânica, Instituto de Química, Universidade Federal Fluminense, Campus do Valonguinho, 24020-141 Niterói-RJ, Brazil
II Instituto de Química e Biotecnologia, Universidade Federal de Alagoas, Tabuleiro do Martins, 57072-970 Maceió-AL, Brazil
IIINúcleo de Pesquisas de Produtos Naturais, Universidade Federal do Rio de Janeiro, Cidade Universitária, 21944-970 Rio de Janeiro-RJ, Brazil
IVDepartamento de Fisiologia e Farmacologia, Universidade Federal do Ceará, Campus do Porangabussu, 60430-270 Fortaleza-CE, Brazil
ABSTRACT
The cytotoxicities of five nor-β-lapachone-based 1,2,3-triazoles and the precursor azidonaphthoquinone were assayed against six neoplasic cancer cell lines: SF-295 (central nervous system), HCT-8 (colon), MDAMB-435 (melanoma), HL-60 (leukaemia), PC-3 (prostate) and B-16 (murine melanoma). IC50 values ranging from 0.43 to 9.48 µM were obtained. 3-(4-(1-hydroxycyclohexyl)-1H-1,2,3-triazol-1yl)-2,2-dimethylnaphtho[1,2-b]furan-4,5-dione proved highly cytotoxic to MDAMB-435, with IC50 even lower than the one from doxorubicin (positive control). In an attempt to correlate physicochemical parameters (reduction potentials and calculated log P) with cytotoxic activity, electrochemical studies were conducted in acetate buffer, pH 4.5, using a vitreous carbon electrode and calculated log P values were obtained. Despite the absence of a structural conjugative interaction between the two systems (quinone and triazole), the heterocyclic group was found to influence the voltammetric behaviour, as indicated by anodic shifts in the reduction potentials. No correlation was found between EpIc and cytotoxicity. In contrast, a comparison of EpIc with previously reported trypanocidal activities reconfirmed the trend for higher activity coupled with better quinone electrophilicity (> EpIc).A leading term in the correlation of cytoxicity, despite the absence of a linear correlation, was the calculated log P: the lower the lipophilicity, the lower the cytoxicity (> IC50).
Keywords: lapachones, cytotoxicity, reduction potentials, clog P, naphthoquinone-triazoles, trypanocidal agents
RESUMO
A atividade citotóxica de cinco derivados 1,2,3-triazólicos da nor-β-lapachona e do precursor azidonaftoquinona, foi avaliada contra seis linhagens de células neoplásicas: SF-295 (sistema nervoso central), HCT-8 (cólon), MDAMB-435 (melanoma), HL-60 (leucemia), PC-3 (próstata) e B-16 (melanoma murino). Valores de IC50 entre 0,43 e 9,48 µM foram obtidos. A 3-(4-(1-hidróxicicloexil)-1H-1,2,3-triazol-1-il)-2,2-dimetilnaftol[1,2-b]furan-4,5-dione apresentou-se altamente citotóxica contra MDAMB-435, com IC50 menor do que o encontrado para doxorubicina (controle positivo). Na tentativa de obter correlação entre parâmetros físico-químicos (potencial de redução e clog P) e atividade citotóxica, estudos eletroquímicos foram realizados em tampão acetato, pH 4,5, em eletrodo de carbono vítreo, e valores de log P calculados (clog P). Apesar da ausência de interação estrutural conjugativa entre o sistema quinônico e o anel triazólico, observou-se a influência do anel heterocíclico no comportamento voltamétrico, com deslocamento anódico dos potenciais de redução. Não se verificou correlação entre os parâmetros eletroquímicos (EpIc) e a atividade citotóxica. Por outro lado, a comparação de EpIc com atividades tripanocidas, já descritas, reafirmou a tendência de maior atividade biológica para quinonas mais eletrofílicas (> EpIc). Apesar da ausência de correlação direta, foi possível verificar que clog P influencia a atividade citotóxica: quanto menor a lipofilia (< clog P), menor a citotoxicidade (> IC50).
Introduction
Cancer is the second leading cause of death worldwide after cardiovascular diseases. The antitumor potential of quinones has been known for more than three decades, ever since the seminal studies conducted by the National Cancer Institute, NCI-USA, in which a number of synthetic and natural quinones were assayed and showed anticancer activity.1 Since then, interest in this subject has grown exponentially.2-6
Quinones play a major role as bioreductive drugs. Indeed, they are reactive oxygen species (ROS) enhancers and redox catalysts.6-11 A striking feature of quinone chemistry is the ease of reduction and therefore the ability to act as oxidizing or dehydrogenating agents, the driving force being the formation of a fully aromatic system.6-7,9,11 In this regard, quinones are known to play a pivotal role in energy metabolism and in many other key biochemical processes, mainly in chemotherapy, which uses redox cycling drugs.9,11
The electrochemical properties of quinone compounds are obviously very important for their bioreductive activation to either the semiquinone or hydroquinone.9,11 There are several examples of correlations between electrochemical potentials and biological activities in this class of compounds. For example, a definite correlation has been found between redox potentials and the inhibitory effects of naphthoquinones on Epstein-Barr virus early antigen activation12-14 and their cytotoxicity.15,16 In other cases, this correlation has not been evinced.17,18
There are also several examples of quinone activity outside the field of cancer,19 e.g.,inChagas'disease,where a correlation has been found between trypanocidal activity and redox potential.9,11,20
Chagas' disease is a long-term debilitating disease caused by the flagellate protozoan Trypanosoma cruzi, which is mainly transmitted by triatomine insects or by blood transfusion. However, several routes of transmission that do not involve insect vectors have also been described, such as transmission viablood products or transplantation of infected organs, and vertical transmission. It is one of the most serious endemic parasitic diseases of Latin America, with a social and economic impact far outweighing the combined effects of other parasitic diseases.21 At present, the number of people infected with Chagas' disease worldwide is estimated to be about 10-12 million. The process of urbanization in Latin America and migratory population movements from endemic countries have led to the disease being diagnosed in non-endemic areas.22
A special feature of T. cruziis its unique sensitivity to the action of intracellular generators of H2O2. T. cruzipossesses an original redox defence system based upon trypanothione and trypanothione reductase, a NADPH-dependent flavoprotein, which regenerates trypanothione from its oxidised form (disulphide form). It lacks catalase and glutatione peroxidase and is therefore considerably more sensitive to H2O2-induced oxidative stress than its biological hosts. To date, Chagas' disease has defied all attempts to develop an efficient chemotherapy.21 Despite the recognition of the importance of redox cyclers as potent trypanocidal agents, few reports have shown a possible correlation between redox potentials of quinones and trypanocidal activities.20
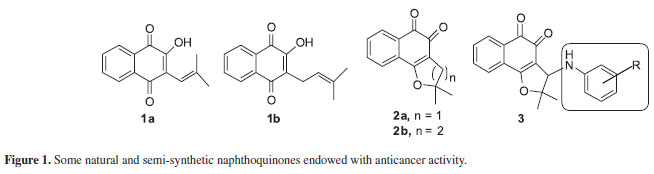
Since the finding that lapachol (1b), a natural naphthoquinone, has antitumor activity against carcinoma Walker 256,23 several structural modifications have been made24 in order to find compounds with new, enhanced or more selective biological activities.25-30 An important naphthoquinone obtained from lapachol(1b) is β-lapachone (2b)9 (Figure 1), which is currently under multiple Phase II clinical trials.31
Nor-β-lapachone (2a) also has anticancer activity against SF-295 (central nervous system), MDAMB-435 (melanoma), and HL-60 (leukaemia) within the range of 0.3-1.75 µM.32 Our group recently demonstrated that the introduction of arylamine groups (Figurea 1, 3) in the dihydrofuran ring of 2aenhanced the anticancer activity of this substance against six cancer cell lines with the IC50 below 1.5 µM.32
1,2,3-Triazoles are an important class of compounds possessing a variety of biological activities,33 e.g., as antiplatelet agents,34 as dopamine D2 receptor ligands related to schizophrenia,35 and as anticonvulsant,36 anti-inflammatory,37 anti-allergic,38-41 antiviral42-45 and antimicrobial agents.46-51
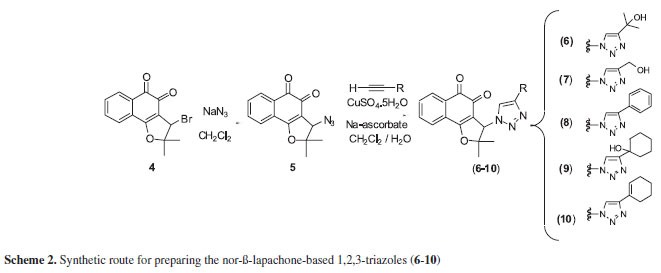
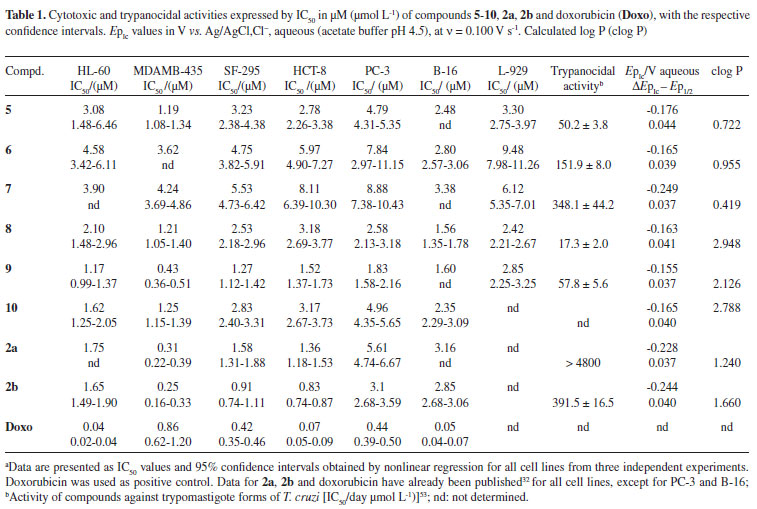
Since triazoles and naphthoquinones are important compounds with independent pharmacological activity, it would be relevant to produce some compounds by molecular hybridization using these moieties. The use of this approach in the design of new drugs has proved useful in modifying the selectivity profile and in reducing undesirable side effects.52 Additionally, thisapproach has proved successful for obtaining trypanocidal compounds (Scheme 1).53 All synthesized naphthoquinoidal 1,2,3-triazoles proved more active than the original quinones against the infective bloodstream trypomastigote form of T. cruzi, with IC50/day values in the range of 17 to 359 µM, the apolar phenyl substituted triazole 8being the most active compound (Table 1).53
Based on an investigation of additional activities for this new class of compounds, this communication reports on the cytotoxic activities against six neoplasic cancer cell lines: SF-295 (central nervous system), HCT-8 (colon), MDAMB-435 (melanoma), HL-60 (leukaemia), PC-3 (prostate) and B-16 (murine melanoma) and against one normal cell, the murine fibroblast L-929, of azidoquinone 5and of the naphthoquinones containing a 1,2,3-triazole ring in the C-3 position of the dihydrofuran ring of 2a, named 6-10.An analysis was also made of their haemolytic activity, assayed with erythrocyte suspensions, in order to contribute to the clarification of the mechanism of biological action.
Due to the importance of electrochemical studies of quinones in biological mechanisms of action,9,11 voltammetric parameters (EpIc) are also presented, aiming to correlate biological and physicochemical parameters. The experiments were conducted in a protic medium (to resemble a hydrophilic environment). Additionally, based on the same rationale,9,11 previously reported data on trypanocidal activity53 are compared with measured reduction potentials.
Biological activity towards a live host involves a complex outcome that is not usually dominated by only one parameter. Generally, various physicochemical properties impact the pharmacokinetic and metabolic fate of a biologically active compound; hence, a thorough understanding of such characteristics is crucial in studies of modes of action.54 In drug metabolism and pharmacokinetics, one of the most important parameters is lipophilicity, adequately described by experimentally obtained log P,54 or by a calculated one (clog P)46; therefore, in the present case, we also report clog P for all the compounds.
Results and Discussion
Chemistry
The compounds were synthesized by the Huisgen reaction55 using copper catalysis.As shown in Scheme 2, the syntheses of 6-10were carried out from the azidoquinone 5by 1,3-dipolar cycloaddition.56 The 3-bromo-nor-β-lapachone (4), easily obtained from nor-lapachol (1a),57 is the precursor of 5, obtained so far by substitution with sodium azide. The synthesis and characterization of triazolic compounds (6-10) have already been reported,53 except for compound 10, whose spectroscopic data are included in the experimental section.
Biology
Compounds 5-10were tested in vitroagainst six cancer cell lines and a normal murine fibroblast L-929, in comparison with the quinones 2a, 2band doxorubicin, the positive control, through an MTT assay.58 Concentrations that induce 50% inhibition of cell growth (IC50) in µM are reported in Table 1. Based on their activity, the compounds were classified against at least two cancer cell lines as highly active (IC50 < 2 µM), moderately active (2 µM < IC50 < 10 µM), or inactive (IC50 > 10 µM).
The great majority of the compounds are strongly or moderately cytotoxic against all cancer cell lines with IC50 in the range of 0.43 to 9.48 µM, although they are less cytotoxic than standards 2a, 2band doxorubicin, with few exceptions (see compound 9, Table 1). The most sensitive cancer cell lines are MDAMB-435 and B-16, as already evidenced for 2a, 2band arylaminonaphthoquinones,32 unlike doxorubicin, which is more active against HL-60, HCT-8 and B-16. Compound 5(IC50 = 1.19 µM) was selectively active against MDAMB-435. Among the triazolic naphthoquinones, compound 9showedthe highest activity against cancer cells (IC50 ranging from 0.43 up to 1.83 µM) with the strongest activity against MDAMB-435. Additionally, it showed high selectivity compared with other cancer cells (2-4 × more selective) as well as towards normal cells (L-929, IC50 = 2.85 µM) (Table 1). The cytoxicity of compound 9for melanoma cells is similar to that of the original quinones (2aand 2b) and higher than doxorubicin. Towards HL-60, 9is more active (IC50 = 1.17 µM) than the original quinones 2aand 2b. Compound 8showed higher cytotoxic activity than compounds 6and 7. The presence of aromatic substituent (for 8) and non-aromatic (9and 10) rings on the triazole moiety seems relevant for activity, possibly indicative of lipophilic effects. The absence of haemolytic activity (EC50 > 508.35 µM) suggests that the cytotoxicity of the compounds was not related to membrane damage of mouse erythrocytes.
Electrochemistry and clog P
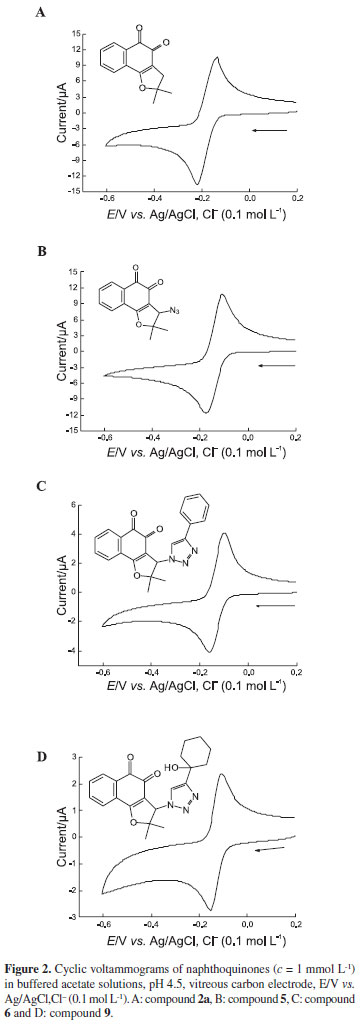
The importance of the data on electron transfer involved in biological activities of quinones prompted us to conduct electrochemical studies of the majority of quinones, using cyclic voltammetry, ina protic(acetate buffer, pH4.5) medium. A relevant fact is that this paper offers the very first description of the electrochemistry of compounds 2a and 5-10.
In this study, we used the potential of the first reduction wave (EpIc), which gives a quantitative measure of ease of reduction of an oxidant or electron acceptor, for purposes of comparison. Figures 2A-D display cyclic voltammograms (CV) of compounds 5, 2a, 6and 9, respectively, as representatives of dihydrofuran naphthoquinones (5, 2a) and triazolic quinones (6, 9), which are typical of well-behaved quinones. The CVs show a reversible, diffusional and bielectronic pair of reduction waves [(EpIc - Ep1/2)experimental= 37-44 mV, (EpIc-Ep1/2)theoretical~ 30 mV], leading to the formation of the respective hydroquinones, with participation of two protons.9,11
In this case, EpIc values range from -0.155V (compound 9) up to -0.249 V (compound 7) vs.Ag/AgCl,Cl-(0.1 mol L-1), all the values representing easily reducible compounds. The ease of reduction in the series, expressed by EpIc (Table 1), is: 9 > 10 ~ 8 ~ 6 > 5 > 2a > 2b ~ 7.
Despite absence of a structural conjugative interaction between the two systems (quinone and triazole) due to the presence of a spacer (Csp3), the heterocyclic group influences the voltammetric behaviour, showing evidence of anodic shifts in the reduction potentials. This fact has been observed in several instances, e.g., the reduction of 2-methyl-5-nitroimidazole substituted at the N1-ethyl side chain with halogens.59 In the present case, it is possible that the electrogenerated hydroquinone is stabilized by hydrogen bonding with the heterocyclic nitrogen, which would favour reduction.
Although there is no direct correlation between these values and cytotoxicity, all the reduction potentials are in the region that allows the enzymatic reduction of quinones and further reaction with oxygen, explaining the presence of cytotoxicity for the great majority of assayed quinones.9,11 Banasri and coworkers18 also investigated the redox behaviour of aminoquinones and compared them with their cytotoxicity activities, but found no evidence of a correlation. Evidently the electrochemical parameters of quinonoids, albeit essential for cellular event analysis, do not always show a quantitative correlation with their cytotoxic properties. In fact, many other factors such as lipophilicity, membrane permeability, site-specific binding, etc., need critical consideration in order to rationalize the relationship of structural modulation and cytotoxicity.54
Log P values were calculated (Table 1) for all derivatives by semi-empirical methods. The values for the less cytotoxic compounds 5-7were the lowest in the series. The more active compounds 8-10showed the highest clog P values, even compared with references, 2aand 2b. Partitioning can be related to either absorption or permeation. It is reasonable to suppose that those compounds can be better absorbed and permeated in the cell of the organism. As in the present case, the lipophilicity of catechols has been shown to enhance cytotoxicity.60 The lipophilicity of quinones is similar to that of the parent catechols. This means that the transport of both catechols and quinones into and out of the cell is similar, so there is no significant differentiation between reduced and oxidized forms with respect to transport through the cellular membrane.61
As mentioned in the introduction, it is worthy comparing electrochemical results and trypanocidal activities. The IC50 order shown in Table 1 is: 8 > 5 > 9 > 6 > 7 > 2b > > 2a.
With regard to this activity, despite the impossibility of establishing a direct correlation due to the small number of compounds, it was observed that the more electrophilic compounds are better trypanocidal agents.
Conclusions
The nor-β-lapachone-based 1,2,3-triazoles were generally cytotoxic against the cancer cell lines tested, with IC50 below 5 µM. The results demonstrated that these triazoles are a promising addition to the arsenal of anticancer drugs. An enhancement of the activity between the triazole and 1,2-naphthoquinone moieties was demonstrated in the case of compound 9. The synthetic compounds 8-10appear to be very interesting as new prototypes that could be exploited for the preparation of analogues, opening prospects for the development of new anticancer drugs, despite the fact that the two standard naphthoquinones, nor-β-lapachone and β-lapachone and doxorubicin were, generally, more potent. As for the calculated clog P values, the data (Table 1) allowed for the discrimination between highly and moderately cytotoxic compounds. Higher lipophilicity values (clog P) were associated with better cytotoxicity. Despite the importance of electrochemical data, in this case, no correlation was found between EpIc and cytotoxicity. In contrast, the comparison of EpIc with previously reported trypanocidal activities reconfirmed the trend for higher activity coupled with better quinone electrophilicity (> EpIc).
Experimental
Chemical synthesis
Melting points were obtained on a Reichert micro hot stage and are uncorrected. Analytical grade solvents were used. The solvents were previously purified as described in the literature.62 Column chromatography was performed on silica gel (Acros Organics 0.035-0.070 mm, pore diameter about 6 nm). Infrared spectra were recorded on a Perkin-Elmer FT-IR Spectrometer. 1H and 13C NMR were recorded at room temperature using aVarian Unity Plus 300 spectrometer, in the aforementioned solvents, with TMS as internal standard. Chemical shifts (δ) are given in ppm and coupling constants (J) in hertz.
Synthetic procedures
Nor-lapachol (2-hydroxy-3-(2-methyl-propenyl)-[1,4]-naphthoquinone); β-lapachone (2,2-dimethyl-3,4-dihydro2H-benzo[h]chromene-5,6-dione) (2b); nor-β-lapachone (2,3-dihydro-2,2-dimethylnaphtho[1,2-b]furan-4,5-dione) (2a) and 3-bromo-nor-β-lapachone (3-bromo-2,3-dihydro2,2-dimethylnaphtho[1,2-b]furan-4,5-dione) (4), were prepared from lapachol 1b by standard procedures.63,57 The nor-β-lapachone-based 1,2,3-triazoles (6-10) were prepared from the azidoquinone (5), as described in the literature.53
3-(4-Cyclohex-1-enyl-1,2,3-triazol-1-yl)-2,2-dimethyl-2,3dihydro-naphtho[1,2-b]furan-4,5-dione (10)
The reaction of the 3-azido-2,2-dimethyl-2,3-dihydronaphtho[1,2-b]furan-4,5-dione (6) (299 mg, 1.1 mmol) in 13.4 mL of CH2Cl2/H2O (1:1), followed by the addition of CuSO4∙5H2O (12.5 mg, 0.05 mmol), sodium ascorbate (29.5 mg, 0.14 mmol) and prop-2-in-1-ol (106 mg, 1 mmol) resulted in product 10, (345 mg, 0.92 mmol, 92% yield) a yellow solid (mp 189-190 ºC). 1H NMR (300 MHz, CDCl3) δ: 8.18 (1H, ddd, J7.3, 1.7, 0.6 Hz), 7.82-7.71 (3H, m), 7.31 (1H, s), 5.95 (1H, s), 2.34-2.29 (2H, m), 2.20-2.13 (2H, m), 1.75-1.62 (5H, m), 1.75 (3H, s), 1.20 (3H, s); 13C NMR (75 MHz, DMSO-d6) δ: 20.9 (CH3), 22.0 (CH2), 22.2 (CH2), 25.1 (CH2), 26.1 (CH2), 27.5 (CH3), 66.5 (CH), 95.8 (C0), 111.3 (C0), 117.6 (CH), 125.3 (CH), 125.3 (CH),125.4 (CH), 126.6 (C0), 126.8 (C0), 129.7 (CH), 131.4 (C0), 133.1 (CH), 134.6 (CH), 170.9 (C0), 174.3 (C=O),179.9 (C=O); IR (film) ν max/cm-1: 1656 (C=O), 1620 (C=O).
Anticancer assay
Cytotoxicity against cancer cell lines: compounds 5-10were tested for cytotoxic activity against six cancer cell lines in concentrations ranging from 25-18.57 µmol L-1 in the following cell lines SF-295 (central nervous system), HCT-8 (colon), MDAMB-435 (melanoma), HL-60 (leukaemia), PC-3 (prostate), B-16 (murine melanoma) and one normal cell L-929 (murine fibroblast), all supplied by the National Institute of Health, Bethesda, Maryland.All the cell lines were stored in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mol L-1 glutamine, 100 U/mL penicillin and 100 µg mL-1 streptomycin at 37 ºC with 5% CO2. Each compound was dissolved in DMSO and diluted with water to a concentration of 1 mg mL-1. They were then incubated with the cells for 72 h. The negative control received the same amount of DMSO (0.005% in the highest concentration). Doxorubicin (17-1.0 µmol L-1) was used as a positive control. The cell viability was determined by reduction of the yellow dye 3-(4,5-dimethyl-2-thiazol)2,5-diphenyl-2H-tetrazolium bromide (MTT) to a blue formazan product, as described by Mosmann.58
Cell membrane disruption
The tests were performed in 96-well plates using a 2% mouse erythrocyte suspension in 0.85% NaCl containing 10 mmol L-1 CaCl2, following the method described by Jimenez et al.64 The compounds diluted as mentioned above were tested at concentrations ranging from 1.5 to 200 µg mL-1.After 30 min of incubation at room temperature and centrifugation, the supernatant was removed and the released haemoglobin was measured spectrophotometrically at 540 nm. DMSO was used as a negative control and Triton X-100 (1%) was used as a positive control.After incubation at room temperature for 30 min and centrifugation, the supernatant was removed and the released haemoglobin was measured spectrophotometrically at 540 nm. ED50 is the calculated dose that effectively induced lysis in 50% of the Triton X-100.
Electrochemistry
The electrochemical experiments of cyclic voltammetry (CV) were performed using an Autolab (Echo-Chemie, Utrecht, Netherlands) PGSTAT 20. The working electrode was a BAS (Bioanalytical Systems, West. Lafayette, IN, USA) GC electrode of 3 mm diameter, the counter electrode was a platinum coil, and the reference electrode was Ag|AgCl,Cl- (0.1 mol L-1), and all were contained in a one-compartment electrochemical cell with 10 mL capacity. In the CV experiments, the scan rate varied from 0.020 to 2 V s-1, while the reported parameters were determined at a rate of 0.100 V s-1. It was necessary to degas the cell with a nitrogen flow for reduction studies. All the experiments were conducted at room temperature (25 ± 1ºC).
Aqueous acetate buffer solutions (0.2 mol L-1; pH 4.5) were used in all the experiments and were prepared from analytical grade reagents and purified water (conductivity < 0.1 µS cm-1) obtained from a Millipore (Bedford, MA, USA) Milli-Q system. A Marconi (Piracicaba, Brazil) pH-meter with a combined glass electrode (model MAPA200; series 0113992) was used to measure the buffer pH.
Lipophilicity calculation (clog P)
Theoretical parameters of lipophilicity (clog P) were calculated (Table 1) for all the derivatives using the semi-empirical methods: AM1, using the Villar method included in the Spartan package, and OSIRIS Property Explorer (Thomas Sander, Actelion Pharmaceuticals Ltd) freely available on the web site http://www.organic-chemistry. org/prog/peo/. These programs were used to calculate the theoretical log P based on the experimentally determined partition coefficients between n-octanol and water of commercially available drugs.
Acknowledgments
This work was supported by CNPq (National Research Council of Brazil), within the framework of the Instituto do Milênio/CNPq-INOFAR, MCT/CNPq/MS/Neoplasias, RENORBIO, BNB, as well as by CAPES, FAPERJ and FAPEAL (Brazil).
Supplementary Information
Available free of charge at http://jbcs.org.br, as PDF file.
Received: November 12, 2008
Web Release Date: April 3, 2009
Supplementary Information
Figure S1 - Click to enlarge
- 1. Driscoll, J. S.; Cancer Chemother. Rep. 21974, 4, 3.
- 2. Pardee, A. B.; Li, Y-Z.; Li, C-J.; Curr. Cancer Drug Targets2002, 2, 227.
- 3. Kongkathip, N.; Kongkathip, B.; Siripong, P.; Sangma, C.; Luangkamin, S.; Niyomdecha, M.; Pattanapa, S.; Piyaviriyagul, S.; Kongsaeree, P.; Bioorg. Med. Chem 2003, 11, 3179.
- 4. da Silva Júnior, E. N.; de Souza, M. C. B. V.; Fernandes, M. C.; Menna-Barreto, R. F. S.; Pinto, A. V.; Pinto, M. C. F. R.; Lopes, F. A.; de Simone, C. A.; Andrade, C. K. Z.; Ferreira, V. F.; de Castro, S. L.; Bioorg. Med. Chem 2008, 16, 5030.
- 5. da Silva, M. N.; Ferreira, V. F.; de Souza, M. C. B. V.; Quim. Nova2003, 26, 407.
- 6. Asche, C.; Mini-Rev. Med. Chem.2005, 5, 449.
- 7. Goulart, M. O. F.; Lima, N. M. F.; Santana, A. E. G.; Ferraz, P. A. L.; Cavalcanti, J. C. M.; Liwo, A.; Falkowsky, P.; Ossowsky, T.; J. Electroanal. Chem.2004, 566, 25.
- 8. Monks, T. J.; Jones, D. C.; Curr. Drug Metab 2002, 3, 425.
- 9. Hillard, E. A.; de Abreu, F. C.; Ferreira, D. C. M.; Jaouen, G.; Goulart, M. O. F.; Amatore, C.; Chem. Commun 2008, 23, 2612.
- 10. Bolton, J. L.; Trush, M.; Penning, T.; Dryhurst, G.; Monks, T. J.; Chem. Res. Toxicol.2000, 13, 135.
- 11. De Abreu, F. C.; Ferraz, P. A. M.; Goulart, M. O. F.; J. Braz. Chem Soc 2002, 13, 19.
- 12. Koyama, J.; Morita, I.; Kobayashi, N.; Osakai, T.; Cancer Lett. (Shannon, Irel.)2004, 212, 1.
- 13. Koyama, J.; Morita, I.; Tagahara, K.; Osakai, T.; Hotta, H.; Yang, M. X.; Mukaianaka, T.; Nishino, H.; Tokuda, H.; Chem. Pharm. Bull.2001, 49, 1214.
- 14. Koyama, J.; Morita, I.; Kobayashi, N.; Osakai, T.; Hotta, H.; Takayasu, J.; Nishino, H.; Tokuda, H.; Cancer Lett. (Shannon, Irel.)2003, 201, 25.
- 15. Crawford, P. W.; Gross, J.; Lawson, K.; Cheng, C. C.; Dong, Q.; Liu, D. F.; Luo, Y. L.; Szczepankiewicz, B. G.; Heathcock, C. H.; J. Electrochem. Soc.1997, 144, 3710.
- 16. Pan, A-S.; Gonzalez, H.; Mol. Pharmacol.1990, 37, 966.
- 17. Wu, H-Q.; Huang, Z-S.; Bu, X-Z.; Shen, Y-D.; Zhang, Z-L.; Xie, B-F.; Liu, Z-C.; Gu, L-Q.; Chan, A. S. C.; Eur. J. Med. Chem.2005, 40, 1341.
- 18. Das Sarma, M.; Gosh, R.; Patra, A.; Hazra, B.; Eur. J. Med. Chem.2008, 43, 1878.
- 19. Lima, N. M. F.; Correia, C. S.; Leon, L. L.; Machado, G. M. C.; Madeira, M. F.; Santana, A. E. G.; Goulart, M. O. F.; Mem. Inst. Oswaldo Cruz2004, 99, 757.
- 20. Goulart, M. O. F.; Freitas, L. R.; Tonholo, J.; de Abreu, F. C.; Raslan, D. S.; Starling, S.; Zani, C. L.; Oliveira, A. B.; Chiari, E.; Bioorg. Med. Chem. Lett.1997, 7, 2043.
- 21. Lockman, J. W.; Hamilton, A. D.; Curr. Med. Chem.2005, 12, 945.
- 22. Gascón, J.; Albajar, P.; Cañas E.; Flores, M.; Prat J. G. I.; Herrera, R. N.; Lafuente, C. A.; Luciardi, H. L.; Moncayo, A.; Molina,L.; Muñoz, J.; Puente, S.; Sanz G.; Treviño, B.; Sergio-Salles, X.; Rev. Esp. Cardiol.2007, 60, 285.
- 23. Rao, K. V.; McBride, T. J.; Oleson, J. J.; Cancer Res 1968, 28, 1952.
- 24. Neves-Pinto, C.; Dantas, A. P.; Moura, K. C. G.; Emery, F. S.; Polequevitch, P. F.; Pinto, M. C. F. R.; de Castro, S. L.; Pinto, A. V.; ArzneimForsch/DrugRes2000, 50, 1120.
- 25. Pérez-Sacau, E.; Díaz-Peñate, R.G.; Estévez-Braun, A.; Ravelo, A. G.; Garcia-Castellano, J. M.; Pardo, L.; Campillo, M.; J. Med. Chem 2007, 50, 696.
- 26. Ferreira, V. F.; Jorqueira, A.; Souza, A. M. T.; da Silva, M. N.; de Souza, M. C. B. V.; Gouvêa, R. M.; Rodrigues, C. R.; Pinto, A. V.; Castro, H. C.; Santos, D. O.; Araújo, H. P.; Bourguignon, S. C.; Bioorg. Med. Chem.2006, 14, 5459.
- 27. Pinto, A. V.; Menna-Barreto, R. F. S.; de Castro, S. L.; Recent Progress in Medicinal Plants, Studium Press: Houston, 2006, v. 16, p. 109.
- 28. Neves-Pinto, C.; Malta, V. R. S.; Pinto, M. C. F. R.; Santos, R. H. A.; de Castro, S. L.; Pinto, A. V.; J. Med. Chem.2002, 45, 2112.
- 29. Silva, R. S. F.; Costa, E. M.; Trindade, U. L. T.; Teixeira, D. V.; Pinto, M. C. F. R.; Santos, G. L.; Malta, V. R. S.; de Simone, C. A.; Pinto, A. V.; de Castro, S. L.; Eur. J. Med. Chem 2006, 41, 526.
- 30. Barbosa, T. P.; Camara, C. A.; Silva, T. M. S.; Martins, R. M.; Pinto, A. C.; Vargas, M. D.; Bioorg. Med. Chem 2005, 13, 6464.
- 31. Bey,E.A.;Bentle,M.S.;Reinicke,K.E.;Dong,Y.;Yang,C.-R.; Girard, L.; Minna, J. D.; Bornmann, W. G.; Gao, J.; Boothman, D. A.; Proc. Natl. Acad. Sci. USA2007, 104, 11832.
- 32. Da Silva Júnior, E. N.; de Souza, M. C. B. V.; Pinto, A. V.; Pinto, M. C. F. R.; Goulart, M. O. F.; Pessoa, C.; Costa-Lotufo, L.; Montenegro, R. C.; Moraes, M. O.; Ferreira, V. F.; Bioorg. Med. Chem 2007, 15, 7035.
- 33. Kacprzak, K.; Synlett2005, 943.
- 34. Menegatti, R.; Cunha, A. C.; Ferreira, V. F.; Perreira, E. F. R.; El-Nabawi, A.; Eldefrawi, A.; Albuquerque, T. E. X.; Neves, G.; Rates, S. M. K.; Fraga, C. A. M.; Barreiro, E. J.; Bioorg. Med. Chem.2003, 11, 4807.
- 35. Cunha, A. C.; Figueiredo, J. M.; Tributino, J.; Miranda, A. L.; Castro, H. C.; Zingali, R. B.; Fraga, C. A.; de Souza, M. C. B. V.; Ferreira, V. F.; Barreiro, E. J.; Bioorg. Med. Chem.2003, 11, 2051.
- 36. Kelley, J. L.; Koble, C. S.; Davis, R. G.; McLean, E. W.; Soroko, F. E.; Cooper, B. R.; J. Med. Chem 1995, 38, 4131.
- 37. Biagi, G.; Dell'Omodarme, G.; Ferretti, M.; Giorgi, I.; Livi, O.; Scartoni, V.;Il Farmaco1990, 45, 1181.
- 38. Biagi, G.; Dell'Omodarme, G.; Ferretti, M.; Giorgi, I.; Livi, O.; Scartoni, V.; Il Farmaco1992, 47, 335.
- 39. Biagi, G.; Ferretti, M.; Giorgi, I.; Livi, O.; Scartoni, V.; Lucacchini, A.; Il Farmaco1993, 48, 1159.
- 40. Buckle, D. R.; Rockell, C. J.; Smith, H.; Spicer, B. A.; J. Med. Chem.1984, 27, 223.
- 41. Buckle, D. R.; Rockell, C. J.; Smith, H.; Spicer, B. A.; J. Med. Chem.1986, 29, 2262.
- 42. San-Felix, A.; Velazquez, S.; Perez-Perez, M. J.; Balzarini, J.; De, C. E.; Camarasa, M. J.; J. Med. Chem 1994, 37, 453.
- 43. Velazquez,S.;Alvarez,R.;Perez,C.;Gago,F.;De,C.E.;Balzarini, J.; Camarasa, M. J.; Antivir. Chem. Chemother 1998, 9, 481.
- 44. Al-Masoudi,N.A.;Al-Soud,Y.A.;Abdul-Zahra,A.; Heteroatom Chem.2004, 15, 380.
- 45. Da Silva, F. C.; de Souza, M. C. B V.; Frugulhetti, I. I. P.; Castro, H. C.; Souza, S. L. O.; de Souza, T. M L.; Rodrigues, D. Q.; Souza, A. M. T.; Abreu, P. A.; Passamani, F.; Rodrigues, C. R.; Ferreira, V. F.; Eur. J. Med. Chem.2009, 44, 373.
- 46. Costa, M. S.; Boechat, N.; Rangel, E. A.; da Silva, F. C.; de Souza, A. M. T.; Rodrigues, C. R.; Castro, H. C.; Júnior, N. I.; Lourenço, M. C. S.; Wardell, S. M. S. V.; Ferreira, V. F.; Bioorg. Med. Chem 2006, 14, 8644.
- 47. Gallardo, H.; Conte, G.; Bryk, F.; Lourenço, M. C. S.; Costa, M. S.; Ferreira, V. F.; J. Braz. Chem. Soc.2007, 18, 1285.
- 48. Holla, B. S.; Mahalinga, M.; Karthikeyan, M. S.; Poojary, B.; Akberali, P. M.; Kumari, N. S.; Eur. J. Med. Chem 2005, 40, 1173.
- 49. Dabak, K.; Sezer, O.; Akar, A.; Anac, O.; Eur. J. Med. Chem.2003, 38, 215.
- 50. Mares, D.; Romagnoli, C.; Andreotti, E.; Manfrini, M.; Vicentini, C. B.; J. Agric. Food Chem.2004, 52, 2003.
- 51. Willner, D.; Jelenevsky, A. M.; Cheney, L. C.; J. Med. Chem 1972, 15, 948.
- 52. Viegas-Junior, C.; Danuello, A.; da S. Bolzani, V.; Barreiro, E. J.; Fraga, C. A. M.; Curr. Med. Chem 2007, 14, 1829.
- 53. da Silva Júnior, E. N.; Souza, M. C. B. V.; Pinto, A. V.; Pinto, M. C. F. R.; Ferreira, V. F.; Menna-Barreto, R. F. S.; Silva, R. S. F.; Teixeira, D. V.; de Simone, C. A.; de Castro, S. L.; Eur J. Med. Chem 2008, 43, 1774.
- 54. Van de Waterbeemd, H. V.; Gifford, E.; Nat. Rev. Drug Discovery2003, 2, 192.
- 55. Huisgen, R. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., ed., Wiley: New York, 1984.
- 56. Rostovtsev, V. V.; Green, G. L.; Fokin, V. V.; Sharpless, K. B.; Angew. Chem., Int. Ed.2002, 41, 2596.
- 57. Pinto, A. V.; Pinto, M. C. F. R.; de Oliveira, C. G. T.; An. Acad. Bras. Cienc.1982, 54, 107.
- 58. Mosmann, T.; J. Immunol. Methods1983, 65, 55.
- 59. Cavalcanti, J. C. M.; de Abreu, F. C.; Oliveira, N. V.; de Moura, M. A. B. F.; Chaves, J. G.; Alves, R. J.; Bertinaria, M.; Fruttero, R.; Goulart. M. O. F.; Bioelectrochemistry2004, 63, 353.
- 60. Lam, L. K.; Garg, P. K.; Swanson, S. M.; Pezzuto, J. M.; J. Pharm. Sci.1988, 77, 393.
- 61. Chichirau, A.; Flueraru, M.; Chepeleva, L. L.; Wright, J. S.; Willmore, W. G.; Durst, T.; Hussain, H. H.; Charron, M.; Free Rad. Biol. Med.2005, 38, 344.
- 62. Ferreira, V. F.; Quim. Nova1992, 15, 348.
- 63. Fieser, L. F.; Fieser, M.; J. Am. Chem. Soc.1948, 70, 3215.
- 64. Jimenez, P. C.; Fortier, S. C.; Lotufo, T. M. C.; Pessoa, C.; Moraes, M. E. A.; Moraes, M. O.; Costa-Lotufo, L. V.; J. Exp. Mar. Biol. Ecol.2003, 287, 93.
Publication Dates
-
Publication in this collection
10 June 2009 -
Date of issue
2009
History
-
Accepted
03 Apr 2009 -
Received
12 Nov 2008